Abstract
Kin selection predicts that helpers in cooperative systems should preferentially aid relatives to maximize fitness. In family-based groups, this can be accomplished simply by assisting all group members. In more complex societies, where large numbers of kin and non-kin regularly interact, more sophisticated kin-recognition mechanisms are needed. Bell miners (Manorina melanophrys) are just such a system where individuals regularly interact with both kin and non-kin within large colonies. Despite this complexity, individual helpers of both sexes facultatively work harder when provisioning the young of closer genetic relatedness. We investigated the mechanism by which such adaptive discrimination occurs by assessing genetic kinship influences on the structure of more than 1900 provisioning vocalizations of 185 miners. These ‘mew’ calls showed a significant, positive linear increase in call similarity with increasing genetic relatedness, most especially in comparisons between male helpers and the breeding male. Furthermore, individual helping effort was more heavily influenced by call similarity to breeding males than to genetic relatedness, as predicted if call similarity is indeed the rule-of-thumb used to discriminate kin in this system. Individual mew call structure appeared to be inflexible and innate, providing an effective mechanism by which helpers can assess their relatedness to any individual. This provides, to our knowledge, the first example of a mechanism for fine-scale kin discrimination in a complex avian society.
Keywords: Manorina melanophrys, kin selection, individual recognition, cooperative breeding, sociality, vocalizations
1. Introduction
A variety of hypotheses exist to explain the apparent altruism of cooperative helping behaviour, but kin selection appears to have gathered the most support thus far (reviews in [1–3]). Aiding kin may offset any costs associated with helping via indirect fitness benefits [4], and relatedness has been shown to be important in cooperative helping among invertebrates, vertebrates and even amoebae (e.g. [5–9]). Despite this, surprisingly few studies have isolated the mechanism(s) by which kin favouritism occurs, particularly among vertebrates. A better understanding of the mechanisms that mediate how cooperative behaviour occurs would, however, yield greater insight into why cooperation might be favoured between individuals. For example, spatial limits on cooperative interactions may be relevant, if environmental characteristics limit distances over which a kinship signal can be propagated. Conversely, if recognition mechanisms are relatively coarse or even absent, helpers may fail to provide differential aid that would have yielded greater indirect fitness benefits [10].
Discrimination of kin from non-kin may occur via olfactory, visual and/or acoustic cues [11]. Among avian systems, recognition mechanisms outside of kin recognition in social group contexts have typically involved acoustic signals [12–14]. Acoustic cues and/or signals of kinship also appear the most likely modality for kin recognition in social groups of birds, with brood mates [15] or young and older male relatives [16] having similar calls. By learning call structure and building a ‘template’ against which to compare calls in later life, discrimination between kin and non-kin can be achieved, analogous to many systems of song learning [11].
Kin-recognition studies in social groups of birds have thus far involved only relatively simple family structures where young encounter only kin during the early stages of their development. This provides a ‘window’ where kin-recognition templates can be formulated with little risk of error [1,15]. Indeed, kinship cues may not even be necessary as many cooperative bird societies are characterized by high levels of kin structure, with ‘helpers’ often being past offspring of a breeding pair that have delayed dispersal (e.g. [2,17]). In such scenarios, the simple rule-of-thumb ‘assist any group member’ would suffice for obtaining indirect benefits [18]. Despite this bias in research focus, kin selection is just as likely to be important in fostering cooperative behaviour among individuals in so-called ‘complex societies’, where both kin and non-kin frequently interact. Even if population viscosity creates large between-group differences in relatedness, favouring indirect fitness benefits via ‘helping any group member’, the potential benefits of recognizing and preferentially aiding kin within complex social groups may still foster strong kin-recognition mechanisms [4,9,10], independent of any additional direct fitness benefits from sources such as group augmentation [19].
In complex societies, a potential stumbling block to kin recognition arises from a reduced, or even absent, opportunity to form association-based templates, as young interact with both related and unrelated group members from an early age. In this scenario, young could construct an effective call template by ‘weighting’ their template according to their exposure to the given calls, if relatives assist broods at a greater frequency than non-kin (e.g. [20]). Alternatively, call structure could have evolved independently of any learning process as an innate property of the individual genotype (sensu ‘greenbeards’; [21,22]) if, for example, calls simply reflect inherited differences in vocal tract morphology. If the different genes responsible for call recognition ability and vocal trait expression are closely linked, then kin discrimination could in theory flourish in complex societies under these conditions [21]. Identifying types of kin-recognition mechanism(s) present within cooperative systems therefore provides important information concerning how and why the underlying kinship structure has selected for the pattern of individual helper effort observed.
The cooperatively breeding bell miner (Manorina melanophrys) is an excellent model system for examining these questions. Miners live in highly social colonies that may comprise several hundred individuals [23]. Breeding is obligately cooperative, with helpers of both sexes provisioning at multiple, concurrent nests [24–27]. Nestlings are attended by 8–10 helpers throughout most of the nestling period [26,28], of which approximately one-third are unrelated to the broods that they provision [20]. Substantial costly helper investment and adaptive adjustment of effort according to brood kinship argue against recognition errors predominating in this system [20], with bell miners providing one of the best examples of fine-scale facultative adjustment of helping effort according to kinship ([20; see also [29]).
The mechanism by which this remarkable kin discrimination occurs in bell miners is unknown, although individually distinct provisioning ‘mew’ calls given by attendants appear a likely candidate [25,30,31]. These vocalizations are given as birds visit the nest, stimulating begging and increasing prey transfer efficacy. Mew calls also occur when leaving the nest and, intriguingly, are more often given by both helpers and breeding males if another individual is in the nest area [27,31]. Mew calls therefore appear to provide an excellent mechanism through which kinship might be signalled and, whatever their function, they must provide significant adaptive benefits given that nest predation in this system increases substantially with all acoustic signals around nests [32]. To test whether kinship influences mew call structure and therefore helping in bell miners, we examined detailed acoustic recordings collected during several years of research into helping behaviour in this system, with the aim of ascertaining if mew calls: (i) are individually consistent across nests, (ii) encode fine-scale information concerning relatedness, (iii) are adjusted according to calls heard as a nestling or adult, and (iv) are used as a cue for adjusting individual helping effort.
2. Methods
(a). Study populations and molecular analyses
Data were collected between October 2004 and December 2006 from two bell miner colonies near Melbourne, Australia (La Trobe: 37°42′58″ S, 145°03′20″ E; St Andrews: 37°35′09″ S, 145°15′41″ E). Prior to observation, colony members were captured with mist nets, colour banded and ca 70 µl of blood collected from the alar vein prior to storage in 70 per cent ethanol. Samples were sexed and genotyped at six loci [33]. Relatedness was assessed using Kingroup v. 2 [34], which calculated the likelihood of helpers being either related (primary hypothesis r = 0.5, null hypothesis r = 0) or unrelated (primary hypothesis r = 0, null hypothesis of r = 0.5) to both members of the breeding pair, based on the log-likelihood ratio required to exclude 95 per cent of 1000 simulated pairwise comparisons. This process enabled us to statistically standardize the relationships generated from microsatellite markers, taking into account the proportion of variance in relatedness likely to have resulted from individuals sharing an allele owing to inheritance versus chance (i.e. the expected distributions based on allele frequencies across the population as a whole [34]). This process yielded relatedness (r) values that closely matched the known putative relationships [26]. Helpers in avian systems appear to have little information on direct relatedness to broods [18], and as such we examined relatedness relative to the breeding pair as a proxy for genetic relatedness to the brood for each helper. As indirect benefits could accrue via either maternal, paternal or both lines (extra-pair young are rare; [35]), relatedness relative to both the breeding female and the breeding male was examined.
(b). Vocalization recordings
Breeding pairs were identified genetically and by observing 33 nests (11 from La Trobe; 22 from St Andrews) within 24 h of hatching, as helpers rarely attend nests during this period [28]. Provisioning behaviour was observed intensively between 6 and 9 days post-hatch, after which helping effort does not increase with chick age, regardless of sex or relatedness [20,28]. The identity of each uniquely colour-banded individual was documented with a telescope (Kowa TS662) from a hide greater than 10 m away and/or analysis of video footage from cameras placed greater than 3 m from nests (Sony CCD-TR1100E or DCR-TRY265E). These distances do not cause disturbance effects in any behaviour discussed here [36]. Mew calls were recorded with a lavalier microphone (Sony ECM77B, Japan), placed 20 cm below nests, connected to a Marantz PMD670 recorder (Tokyo, Japan: uncompressed PCM, 48 kHz, 16 bits). A single mew call from each bird's nest visit was extracted and saved as a .wav file using Raven 1.3 (Cornell, USA) giving a maximum of five calls/bird/context at each nest with a high signal : background noise ratio. A total of 1981 calls were collected, with 1138 mew calls given in the context of feeding chicks (n = 185 individuals) and 843 (n = 114) as helpers left the nest area. On average, we recorded 4 ± 0.1 s.e. calls per individual (n = 280) given in the context of feeding nestlings, and 3.5 ± 0.1 s.e. calls from birds (n = 243) as they left the nest area.
(c). Spectrographic cross-correlation
Spectrographic cross-correlation (SPCC) involves the comparison of two signals over time by ‘sliding’ them past each other and obtaining the peak correlation score [37]. Values typically range between 0, indicating orthogonal signals, through to 1 for identical signals [38]. We used Sample Manager 3.2.0 (AudioPhile Engineering, USA) to add 5 ms of silence to the start and end of each vocalization, ensuring hop size (2.67 ms) included vocalizations [38]. Vocalizations were bandpass-filtered at 500–24 000 Hz (a suitable bandwidth [25]) and normalized using Raven, before spectrograms with a 512-point fast Fourier transform length (3 dB bandwidth 135 Hz), a Hann window function and 75 per cent overlap (grid resolution 2.67 ms) were constructed. Each spectrogram was then compared with every other (3 924 361 comparisons) using ‘biased’ normalization (to reduce the influence of outliers) in the linear batch correlator of Raven [37].
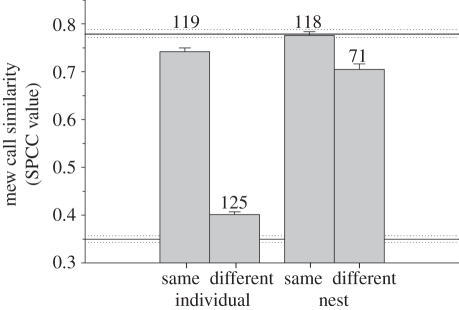
SPCC compares the entire specified bandwidth, and so random background noise tends to prevent SPCC values from reaching correlations of 1, while also positively influencing values via any similarities in background noise. Likewise, all mew calls have a somewhat similar structure that will always yield correlations greater than 0. To estimate these effects, we calculated the average SPCC values of different exemplars from the same individual at the same nest (0.78 ± 0.01; n = 118 individuals; maximum of five calls/bird) as an approximate upper limit in this study. Conversely, comparing exemplars from unrelated individuals across colonies provides the relative minimum SPCC of 0.35 ± 0.01 (n = 118; figure 1).
Figure 1.
Mew call similarity (spectrographic cross correlation (SPCC) coefficients) between exemplars recorded from different individuals within the same nest and for a given individual at the same versus different nests. Sample sizes (number of individuals) are given above each bar. Solid and dotted lines represent means ± s.e., respectively of effective minimum and maximum SPCC values for this study (see §2 for details).
(d). Statistical analyses
Data were analysed using linear mixed models. To examine the individuality of calls, we extracted correlations comparing vocalizations of attendants at the same nest (i.e. a within-nest approach), and tested for differences in mean SPCC values between exemplars from the same individual versus other birds, identified by the binomial factor same bird, with both bird and nest identity as random effects. A separate mixed model was then used to examine the consistency of individual vocalizations across nests, by assessing SPCC values that only compared calls from a given individual (i.e. a within-bird approach). Fixed effects in this model included the binomial effect same nest comparing exemplars from a given individual recorded at the same versus different nests, breeding status (breeder versus helper) and individual sex. Random effects were bird and nest identity.
Calls given ‘at the nest’ had a slightly, but consistently higher SPCC correlation value than those given as individuals ‘left the nest area’. Rather than indicating individuals give different calls across these contexts, this result appeared to be driven by lowering signal to noise ratios for calls recorded as individuals left nests. This was owing to individuals moving away from microphones during sampling and additional background noise created during flight. We initially included the significant effect of call context in all models; however, it did not interact with nor affect the significance of any other variables. Given this, we present data only for calls recorded at the nest for simplicity.
The role of relatedness was examined by restricting the dataset to comparisons involving birds attending the same nest, which is the level at which helper effort is adjusted [20]. Mixed models assessed SPCC values comparing the calls of helpers at a given nest with the calls of either: (i) the breeding female, or (ii) the breeding male. The covariate relatedness was fitted to examine direct comparisons of relatedness and call structure between a given helper and the breeding pair. The additional fixed effect sex and the random effect of bird identity were also fitted. Within- and between-subjects effects of relatedness were partitioned according to Van de Pol & Wright [39].
To investigate the evidence of learning shaping call structure, the similarity of vocalizations from known nestlings subsequently recorded in later life was contrasted with the calls of individuals that they had either first heard as: (i) a nestling, or (ii) as an adult (fixed effect: recording stage). Changes in adult calls were assessed over time by contrasting the calls of a focal bird with those it was associating with at nests when recordings were made versus those it cooperated with the previous year. Additional fixed effects in both analyses were: sex (of both focal and comparative birds), individual status, elapsed time between recordings (years), the covariate relatedness with the random effects of bird and nest identity. To avoid potential confounds, both of these analyses involved only comparisons of exemplars recorded across different nests.
The influence of body size on call structure was compared by measuring individual body mass, wing and head to bill length [28]. These were log-transformed before an unrotated, single component representing body size was extracted from principal component (PC) analysis, which explained 65 per cent of the variance. Residuals for each size measure, after controlling for the extracted component, were obtained via linear regression. Mixed models compared SPCC coefficients of calls between individuals relative to their difference in body size PC scores and residuals for each measure [40].
We assessed the relative predictive power of within-subject differences in both genetic relatedness and mew call similarity (relative to breeding females and breeding males) on within-individual variation in nestling provisioning effort of helpers (log-transformed visit rates: these have importantly been demonstrated to have no additional confounding variation from load sizes or prey types delivered per visit [20]). These analyses included individual sex as a factor and individual identity as a random effect. We used corrected Akaike's information criteria (AICc) [41] to determine the model with the best fit.
Backward sequential elimination of non-significant interaction terms was used to simplify all models. All two-way interactions were fitted and are presented when they contributed to the best-fit model. Analyses were carried out using IBM Statistics v. 19.0 (SPSS, Inc) applying two-tailed tests and a critical p-value of 0.05 throughout. Means are presented ±1 s.e.
3. Results
(a). Provisioning mew calls are individually distinctive
Calls recorded from the same individual were significantly more correlated than those from different individuals using a linear mixed model (F1,414 = 2777.67, p < 0.001; figure 1). This confirms earlier findings [25,30,31] that bell miners have individually distinctive mew call vocalizations.
(b). Individual mew calls are consistent across locations and breeding status
The similarity of mew calls from the same individual was influenced by whether vocalizations were recorded from the same versus different nests (F1,512 = 122.35, p < 0.001; figure 1). This result is most likely an effect of minor differences in acoustic environments on SPCC scores (ca 5%; based on mean differences; see §2), because the variance for calls recorded from the ‘same’ single nest and several ‘different’ nests are virtually identical; a result not consistent with what would be observed if individuals gave a different sounding call at each nest that they visited (figure 1). In addition, in this analysis, the calls of males were significantly more consistent within individuals (0.75 ± 0.005; n = 533) than those of females (0.73 ± 0.01; n = 82, F1,217 = 6.40, p = 0.012), although the small effect size here suggests that this is unlikely to be biologically relevant. No differences in call structure were detected as a given bird changed status from a helper to a breeder or vice versa (F1,611 = 1.51, p = 0.220). All effects were additive without significant interactions.
(c). Mew calls encode fine-scale relatedness information
No significant relationship was found between the influence of helper relatedness to breeding females and mew call similarity overall (table 1). When partitioned into within- and between-subject effects, the latter was marginally significant (table 1). This indicates that the more related helpers, on average, across all nests had more similar calls to the breeding females, perhaps reflecting younger helpers aiding their parents. There was no influence of helper sex on these relationships.
Table 1.
The results of linear mixed models comparing the SPCC coefficients of mew calls recorded from helpers and (i) breeding females (ii) breeding males. (Analyses are split into those examining raw data, with relatedness and sex as factors, or decomposed into the within- and between-subjects effects of relatedness. Estimates and error terms are provided for final models and significant terms are presented in bold. Random term fitted was bird identity (Wald Z/p-value). (i) 1.01, 0.312; (ii) 2.81, 0.005.)
| effect | (i) breeding female |
(ii) breeding male—male helpers only |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| est. | s.e. | F | d.f. | p | est. | s.e. | F | d.f. | p | |
| relatedness | 0.065 | 0.075 | 0.734 | 1,107 | 0.393 | 0.185 | 0.059 | 9.914 | 1,92 | 0.002 |
| sex | 0.067 | 0.039 | 2.916 | 1,123 | 0.090 | — | — | — | — | — |
| relatedness × sex | 1.651 | 1,130 | 0.201 | — | — | — | — | — | ||
| within- and between-subjects analyses | ||||||||||
| relatedness-within | −0.066 | 0.105 | 0.400 | 1,60 | 0.529 | 0.192 | 0.076 | 6.373 | 1,54 | 0.015 |
| relatedness-between | 0.211 | 0.093 | 5.190 | 1,118 | 0.025 | 0.175 | 0.087 | 4.014 | 1,91 | 0.048 |
| sex | 1.898 | 1,120 | 0.171 | — | — | — | — | — | ||
When helper calls were contrasted with those of the breeding males that helpers assisted, a significant interaction between helper sex and relatedness between birds existed (F1,132 = 5.077, p = 0.026). This arose through SPCC values of male, but not female, helpers rising positively with relatedness to breeding males. This interaction did not appear to be owing to the relative paucity of female helpers in our sample (n = 24), as comparable tests using 10 randomly selected samples of an equivalent number of male helpers lead to consistent positive slopes (mean = 0.23 ± 0.06) that were significant on six of 10 occasions.
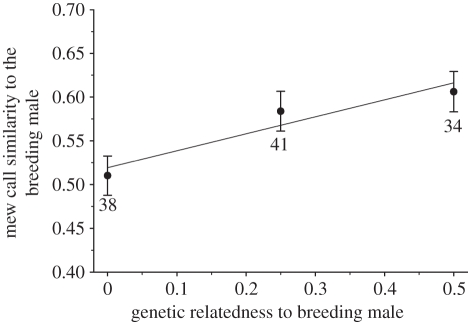
Among male helpers alone, there was a significant positive effect of relatedness on SPCC values (table 1 and figure 2). This relationship was then decomposed into between- and within-subject components, that is, either average relatedness and mew call similarity across all nests attended by a focal individual, or the relative difference between mean values and those recorded at a given nest, respectively. Both showed a significant positive relationship. The slopes of the two relationships did not differ (F1,109 = 0.02, p = 0.877). These results therefore clearly demonstrate that the mew calls of male helpers contain information concerning genetic relatedness to breeding males, with calls being more similar as relatedness increased.
Figure 2.
SPCC coefficients between the mew call exemplars of male helpers and the breeding males provisioning at the same nests relative to their difference in average genetic relatedness. The solid line represents best-fit (see table 1 for estimates), with means ± 1 s.e. presented for each relatedness category. Numbers indicate sample sizes.
(d). Nestlings do not learn their call
Among known age offspring, there was no evidence that nestlings learnt their calls. The eventual calls of nestlings when expressed post-fledging were no more similar to those of birds that fed them as nestlings (0.58 ± 0.01; n = 102 comparisons) than the calls of individuals they only associated with as adults (0.59 ± 0.02; n = 70; table 2).
Table 2.
The results of linear mixed models comparing the SPCC coefficients of mew calls recorded from (i) nestlings subsequently recorded as helpers and (ii) adults recorded across two adjacent seasons, with the calls of the birds heard in the initial versus next breeding season (recording stage). (Additional fixed effects: sex, relatedness between the birds being compared, the number of seasons between samples and all significant two-way interactions. A prefix of F indicates the focal bird (e.g. nestling), C the comparison bird, significant terms are presented in bold, estimates and error terms are provided for final models. Random terms fitted included nest and bird identity (Wald Z/p-value). (i) 1.45, 0.148; (ii) 3.18, 0.001.)
| effect | (i) known nestlings |
(ii) adults |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| est. | s.e. | F | d.f. | p | est. | s.e. | F | d.f. | p | |
| rec. stage | 0.009 | 0.203 | 0.002 | 1,4 | 0.965 | 0.005 | 0.021 | 0.046 | 1,235 | 0.830 |
| Fsex | 0.166 | 0.139 | 1.432 | 1,4 | 0.291 | 0.044 | 0.039 | 1.265 | 1,36 | 0.268 |
| Csex | 0.057 | 0.043 | 1.784 | 1,160 | 0.184 | 0.094 | 0.013 | 48.638 | 1,639 | <0.001 |
| relatedness | <0.001 | <0.001 | 0.348 | 1,160 | 0.556 | −0.044 | 0.044 | 0.799 | 1,656 | 0.372 |
| elapsed time | 0.159a; 0.177b | 0.415a; 0.226b | 1.539 | 2,8 | 0.271 | −0.047a | 0.023 | 4.208 | 1,165 | 0.042 |
a1 year difference.
b2 years difference.
(e). Adults do not exhibit call convergence with associates
Among adult helpers recorded over two consecutive years, there was no indication that their mew calls became more similar to those of associates at previous nests (0.52 ± 0.01, n = 456) versus associates at current nests (0.58 ± 0.01; n = 216; table 2), as expected if helpers converge on similar call structures within ‘coteries’ (i.e. groups of individuals who provision the same subset of nests within a bell miner colony [24]). As with previous results, male calls were more closely aligned when compared with female calls. The different seasons from which recordings were obtained had a small (ca 4%) influence based upon differences in means, probably reflecting subtle seasonal variation in ambient noise characteristics. Individual bell miners therefore appear to possess a relatively fixed individual mew call structure throughout their lives that reflects variation in genetic relatedness and, to a lesser extent, individual sex.
(f). No evident associations between mew call structure and body size measures
Given consistent sex differences in call similarity and sexual size dimorphism in this system [42], the role of body size on SPCC values was examined. Irrespective of whether comparisons were between the same versus different sexes, neither the body size PC, nor the residuals remaining for each measure after controlling for this body size PC, explained a significant proportion of variation in mew call similarity between individuals (table 3).
Table 3.
The results of linear mixed models comparing the SPCC coefficients of mew calls between pairs of birds according to their relative sex (same sex) and difference in a body size PC and the residuals from this body size PC for each of or the measurements of body mass, wing length and head to bill length. (Estimates and error terms are provided for final models with the random term bird identity (Wald Z = 6.96, p < 0.001).)
| parameter | est. | s.e. | F-ratio | d.f. | p |
|---|---|---|---|---|---|
| same sex | 0.001 | 0.003 | 0.270 | 1,111 | 0.605 |
| body size PC | 0.0005 | 0.005 | 0.010 | 1,133 | 0.919 |
| body mass | −72.65 | 54.02 | 1.809 | 1,166 | 0.180 |
| wing length | −127.31 | 93.55 | 1.852 | 1,16 | 0.175 |
| head to bill length | −213.42 | 158.32 | 1.817 | 1,166 | 0.179 |
(g). Mew call similarity predicts helper effort more reliably than genetic relatedness
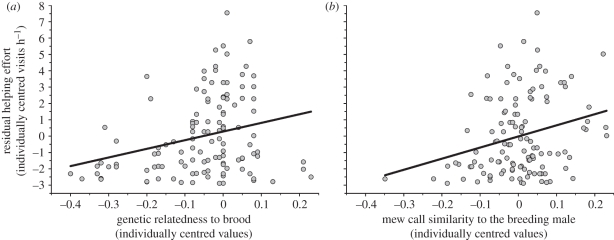
We used AICc values to compare the relative explanatory power of within-subject differences in either genetic relatedness or mew call similarity to: (i) the breeding female, or (ii) breeding male on within-individual variation in helper effort (nest visit rates; table 4). We anticipated that both genetic relatedness and mew call similarity would be important in explaining helper effort, but that mew calls as the proximate mechanism driving helper behaviour should explain relatively more variance in helping. While breeding female mew call similarity had little effect, both genetic relatedness and breeding male mew call similarity provided a significantly better fit than the basic model (ΔAICc over 2; table 4). However, a model including both sex (F1,75 = 0.444, p = 0.507) and mew call similarity to the breeding male (F1,89 = 10.564, p = 0.002) provided the best fit overall (figure 3).
Table 4.
Linear mixed model selection comparisons examining the relative importance of within-subject variation in genetic relatedness versus mew call similarity to the breeding female or male in explaining within-subject variation in helper effort. (Basic models include the intercept and sex, with individual identity as a random effect. Changes in AICc values are presented relative to the model receiving the most support (presented in bold). Results are shown for: (i) all helpers, or (ii) helpers excluding first-order relatives (that may have had putative information on relatedness to broods). Note that in (ii) all helpers were male, precluding sex being fitted to the basic model.)
| (i) all helpers |
(ii) excluding close relatives |
|||
|---|---|---|---|---|
| model factors | AICc | ΔAICc | AICc | ΔAICc |
| basic | 86.962 | 9.630 | 52.723 | 9.104 |
| basic + relatedness | 79.364 | 2.032 | 45.542 | 1.923 |
| basic + breeding female mew call SPCC | 88.066 | 10.344 | 52.675 | 9.056 |
| basic + breeding male mew call SPCC | 77.332 | 0 | 43.619 | 0 |
Figure 3.
Within-individual variation in helping effort (residual nest visit rates, controlling for individual identity and sex) explained by within-individual variation in: (a) genetic relatedness to the brood (best-fit line shown: y = 5.42x + 0.29); and (b) mew call similarity to the breeding male (best-fit line shown: y = 6.80x − 0.02). Both relationships are significant, however AICc values indicate that (b) provides the best fit (see table 3 for details).
This model assumes that no putative information (e.g. direct experience with and knowledge of their parents' identity) is available to the birds. This may not be the case, so to test this indirectly, we re-ran the above models after excluding all first-order relatives (r = 0.5; previous offspring or siblings of breeders, unfortunately all female helpers fitted into this category). Results were virtually identical. Similarity of male helper mew calls to those of the breeding males again provided the best predictive model (F1,51 = 9.84, p = 0.003) which was better than the one containing genetic relatedness (ΔAICc = 1.923; table 4). Therefore, individual helpers appear to have been adjusting their helping effort using call similarity relative to the breeding male as a proxy for genetic relatedness to broods.
4. Discussion
(a). Mew calls encode reliable information concerning individual identity
Using a more general method and a far larger sample than previous work [25,30], we have confirmed the existence of individually distinct mew calls in bell miners, which may well facilitate individual recognition at nests. Calls given by the same individual produced SPCC coefficients well over 70 per cent. This figure very favourably compares with individual or group-specific signatures in other cooperative species (e.g. [43]) and corresponds with the existence of individual-specific vocalizations in a congener [44].
(b). Mew calls reliably indicate relatedness between individuals
While recent analyses have demonstrated a gradual and positive effect of genetic relatedness on individual helper effort in male and female bell miners [20], the mechanism by which this fine-scale kin recognition occurs in such a genetically complex society was unknown. Here, we clearly demonstrate a relationship between the genetic relatedness of individuals and the similarity of their mew call structure (figure 2). This could therefore provide the information needed for a rule-of-thumb to facilitate fine-scale kin-based adjustments in helping effort documented by Wright et al. [20] via mew call similarity of helpers versus breeding males (figure 3; extra-pair fertilizations are rare [35]).
(c). Mew call similarity best explains individual adjustments in helping effort
We show that mew call similarity explained most of the variation in fine-scale individual facultative adjustments in helping effort (see [20]), which was better than the variation explained by simple genetic relatedness of helpers to the focal brood. This strongly suggests that mew call similarity was the behavioural mechanism used by helpers as a proxy for the all-important genetic relatedness to the brood. Further experimental evidence is perhaps now required to confirm this, such as the use of playbacks of mew calls from different (more or less related) breeders that would be predicted to affect the effort of different helpers at the nest.
Interestingly, it was mew call similarity between the helper and the breeding male, and not the breeding female, which was important, despite genetic benefits from maternal lineages being equally valuable. This makes perfect sense in a social system like the bell miner, where the majority of helpers are male and breeding females are always unrelated immigrants into given colonies. Breeding females also have a relatively short lifespan compared with the resident males. The only related helpers likely to be assisting breeding females would be their own offspring (who have putative information concerning relatedness [26]). Therefore, all fine-grained differences in helper relatedness to the brood would be contained in helper's more complex relatedness to the breeding male. Such a kin-recognition system using mew call similarities would also allow unfamiliar breeding males to be assessed. Indeed, even following the exclusion of close relatives (i.e. those with clear putative relatedness information) from these analyses, mew call similarity still provided a better predictor of helper effort than genetic relatedness. This mechanism would therefore enable helpers to maximize their indirect benefits across interactions with any colony member that they encounter throughout their lives in this complex system. By doing so, greater helping of relatives presumably provides substantial benefits via increases in nestling fitness (see [28]), and also helps outweigh any increases in nest predation associated with giving calls at the nest [32], a risk which appears to be further mitigated by helpers reducing call rates in the absence of likely audiences [27]. Together, these results demonstrate how even in complex social systems, kin recognition can shape the evolution of helping behaviour as a result of differences in genetic relatedness within social groups [4,9].
Kin recognition in the bell miner system is therefore clearly not simply an epiphenomenon of a social group recognition signal [6]. While group-specific calls exist in several cooperative [15,45] and non-cooperative species [14], membership of a coterie alone does not guarantee high levels of relatedness in bell miners [33]. Furthermore, a threshold-based signal to identify relatives from non-relatives, such as that used by long-tailed tits (Aegithalos caudatus) to facilitate aid to at the level of cousins and above [46], would also appear to be insufficient to maximize indirect benefits among bell miner helpers. The relationship between both relatedness and call similarity in this system suggests that even small changes in indirect benefits are critical to shaping helping effort in bell miners [20], and that mew call similarity among male helpers appears to be the kin recognition rule-of-thumb used to adjust helper effort in this system.
(d). Mew calls appear to be an innate rather than a learned signal
The mechanism behind this kin-recognition system based on mew call similarities remains unknown. No evidence of a period of call learning was found, contrary to other cooperative systems [15], suggesting that other mechanisms such as differences in vocal tract morphology may have driven these results [47]. Furthermore, consistent sex comparisons yielded higher correlation values for males when compared with females (figure 1). This is consistent with persistent sex influences on mew call structures in other bell miner populations [25]. While we could find no effect of several measures of body size on call similarity, the lack of a relationship here does not necessarily preclude sex-based differences in areas yet to be measured directly, such as vocal tract morphology. Indeed, body size can have little bearing on sound-producing organs in birds (e.g. [48]). As female bell miners help for only a relatively brief period before dispersing to seek breeding opportunities in other colonies [24], a more robust kin-recognition mechanism may have been selected for in male helpers that spend their entire life (ca 6–8 years) inside their natal colony. Males help at the nests of numerous different pairs, even after obtaining a breeding position themselves, and would frequently have little putative information on relatedness. Female helpers on the other hand appear to simply ‘follow the lead’ of known kin, assisting nests at a similar rate to one or both of their parents (McDonald & Wright 2011, unpublished data).
How individual bell miner helpers ‘know’ the structure of their own calls in order to make comparisons with breeders remains unknown, although one possibility might be that nestlings somehow model their future calls on a template heard from their fathers, analogous to the process in songbirds [11]. Unfortunately, we do not have sufficient data to properly test the validity of this suggestion here. Given that mew calls were phenotypically fixed throughout each individual's lifetime, there seems to be little potential for ‘cheating’ in this system. Parent miners could conceivably mimic the calls of unrelated helpers in order to falsely signal high relatedness, thereby ‘deceiving’ non-relatives into increasing their rate of help. However, given these calls are relatively loud and compact social groupings, any ‘false’ calls would probably be overheard by any and all other helpers, both related and unrelated alike, leading to little net benefit to parents. The phenotypically fixed nature of individual mew calls would therefore appear to be an evolutionarily stable strategy for honest communication for kin-biased helping in a complex social system.
(e). Conclusions
Bell miner colonies are relatively large for such a highly cooperative species, representing one of the more complex social systems in the animal kingdom. Despite this, relatively fine-scale differences in relatedness influence the degree of help individuals provide throughout their lives [20]. Here, we reveal the apparent mechanism by which these complex facultative adjustments occur, with a linear increase in the similarity of mew calls with increasing relatedness between individuals, providing the raw information needed for a kin-recognition mechanism based upon mew call structure. Consistent with mew call similarity being used as the rule-of-thumb for relatedness in this system, rather than direct assessment of genetic relatedness specifically, variation in helper effort was best explained by helper mew call similarity to the calls of breeding males, and not by genetic relatedness to broods per se. Bell miners thus represent one of the first clear examples of kin recognition to facilitate kin-directed variation in helping effort within large, complex social groups. Finally, it should be noted that this within-group kin discrimination in helping effort exists in addition to the baseline level of help provided to all group members, presumably as a result of between-group kin selection arising from relatedness differences between colonies [4,9,20].
Acknowledgements
Research was approved by the La Trobe University Animal Ethics committee (AEC01/19(L)/V2), the Department of Sustainability and Environment (license 10002082) and the Australian Bird and Bat Banding Scheme (A2259), who also provided leg bands.
Nick and Joan Hoogenraad and the La Trobe University Wildlife Reserve kindly allowed fieldwork on their land. Maria Pacheco and Luc te Marvelde assisted with fieldwork, while Joy Tripovich helped extract call exemplars. Christine Hayes and Andrew Cockburn provided facilities for and carried out molecular analyses. The editor Trevor Price and two anonymous reviewers provided insightful comments on an earlier draft of the manuscript. P.G.M. was funded by a Macquarie University Fellowship, and both P.G.M. and J.W. were funded by BBSRC grant (5/S19268) to J.W. while at the University of Wales, Bangor.
References
- 1.Emlen S. T. 1997. Predicting family dynamics in social vertebrates. In Behavioural ecology, an evolutionary approach (eds Krebs J. R., Davies N. B.), pp. 301–337 Oxford, UK: Blackwell Scientific Publications [Google Scholar]
- 2.Cockburn A. 1998. Evolution of helping behavior in cooperatively breeding birds. Ann. Rev. Ecol. Syst. 29, 141–177 10.1146/annurev.ecolsys.29.1.141 (doi:10.1146/annurev.ecolsys.29.1.141) [DOI] [Google Scholar]
- 3.Lehmann L., Keller L. 2006. The evolution of cooperation and altruism: a general framework and a classification of models. J. Evol. Biol. 19, 1365–1376 10.1111/j.1420-9101.2006.01119.x (doi:10.1111/j.1420-9101.2006.01119.x) [DOI] [PubMed] [Google Scholar]
- 4.Hamilton W. D. 1964. The genetical evolution of social behaviour. II. J. Theor. Biol. 7, 17–52 [DOI] [PubMed] [Google Scholar]
- 5.Queller D. C., Strassmann J. E. 1998. Kin selection and social insects. Bioscience 48, 165–175 10.2307/1313262 (doi:10.2307/1313262) [DOI] [Google Scholar]
- 6.Sherman P. W., Reeve H. K., Pfennig D. W. 1997. Recognition systems. In Behavioural ecology: an evolutionary approach (eds Krebs J. R., Davies N. B.), pp. 69–96 Oxford, UK: Blackwell Science [Google Scholar]
- 7.Griffin A. S., West S. A. 2003. Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science 302, 634–636 10.1126/science.1089402 (doi:10.1126/science.1089402) [DOI] [PubMed] [Google Scholar]
- 8.Mehdiabadi N. J., Jack C. N., Farnham T. T., Platt T. G., Kalla S. E., Shaulsky G., Queller D. C., Strassmann J. E. 2006. Kin preference in a social microbe. Nature 442, 881–882 10.1038/442881a (doi:10.1038/442881a) [DOI] [PubMed] [Google Scholar]
- 9.Cornwallis C. K., West S. A., Griffin A. S. 2009. Routes to indirect fitness in cooperatively breeding vertebrates: kin discrimination and limited dispersal. J. Evol. Biol. 22, 2445–2457 10.1111/j.1420-9101.2009.01853.x (doi:10.1111/j.1420-9101.2009.01853.x) [DOI] [PubMed] [Google Scholar]
- 10.Hatchwell B. J. 2010. Cryptic kin selection: kin structure in vertebrate populations and opportunities for kin-directed cooperation. Ethology 116, 203–216 10.1111/j.1439-0310.2009.01732.x (doi:10.1111/j.1439-0310.2009.01732.x) [DOI] [Google Scholar]
- 11.Komdeur J., Hatchwell B. J. 1999. Kin recognition: function and mechanism in avian societies. Trends Ecol. Evol. 14, 237–241 10.1016/S0169-5347(98)01573-0 (doi:10.1016/S0169-5347(98)01573-0) [DOI] [PubMed] [Google Scholar]
- 12.Jouventin P., Aubin T. 2002. Acoustic systems are adapted to breeding ecologies: individual recognition in nesting penguins. Anim. Behav. 64, 747–757 10.1006/anbe.2002.4002 (doi:10.1006/anbe.2002.4002) [DOI] [Google Scholar]
- 13.Stoddard P. K. 1996. Vocal recognition of neighbors by territorial passerines. In Ecology and evolution of acoustic communication in birds (eds Kroodsma D. E., Miller E. H.), pp. 356–374 Ithaca, NY: Cornell University Press [Google Scholar]
- 14.Mammen D. L., Nowicki S. 1981. Individual differences and within-flock convergence in chickadee calls. Behav. Ecol. Sociobiol. 9, 179–186 10.1007/BF00302935 (doi:10.1007/BF00302935) [DOI] [Google Scholar]
- 15.Sharp S. P., McGowan A., Wood M. J., Hatchwell B. J. 2005. Learned kin recognition cues in a social bird. Nature 434, 1127–1130 10.1038/nature03522 (doi:10.1038/nature03522) [DOI] [PubMed] [Google Scholar]
- 16.Payne R. B., Payne L. L., Rowley I. 1988. Kin and social relationships in splendid fairy-wrens: recognition by song in a cooperative bird. Anim. Behav. 36, 1341–1351 10.1016/S0003-3472(88)80203-3 (doi:10.1016/S0003-3472(88)80203-3) [DOI] [Google Scholar]
- 17.Komdeur J., Richardson D. S., Burke T. 2004. Experimental evidence that kin discrimination in the Seychelles warbler is based on association and not on genetic relatedness. Proc. R. Soc. Lond. B 271, 963–969 10.1098/rspb.2003.2665 (doi:10.1098/rspb.2003.2665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright J., Parker P. G., Lundy K. J. 1999. Relatedness and chick-feeding effort in the cooperatively breeding Arabian babbler. Anim. Behav. 58, 779–785 10.1006/anbe.1999.1204 (doi:10.1006/anbe.1999.1204) [DOI] [PubMed] [Google Scholar]
- 19.Kokko H., Johnstone R. A., Clutton-Brock T. H. 2001. The evolution of cooperative breeding through group augmentation. Proc. R. Soc. Lond. B 268, 187–196 10.1098/rspb.2000.1349 (doi:10.1098/rspb.2000.1349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright J., McDonald P. G., te Marvelde L., Kazem A. J. N., Bishop C. 2010. Helping effort increases with relatedness in bell miners, but ‘unrelated’ helpers of both sexes still provide substantial care. Proc. R. Soc. B 277, 437–445 10.1098/rspb.2009.1360 (doi:10.1098/rspb.2009.1360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansen V. A. A., Van Baalen M. 2006. Altruism through greenbeard chromodynamics. Nature 444, 663–666 10.1038/nature04387 (doi:10.1038/nature04387) [DOI] [PubMed] [Google Scholar]
- 22.Gardner A., West S. A. 2010. Greenbeards. Evolution 64, 25–38 10.1111/j.1558-5646.2009.00842.x (doi:10.1111/j.1558-5646.2009.00842.x) [DOI] [PubMed] [Google Scholar]
- 23.Clarke M. F., Fitz-Gerald G. F. 1994. Spatial organisation of the cooperatively breeding bell miner Manorina melanophrys. Emu 94, 96–105 10.1071/MU9940096 (doi:10.1071/MU9940096) [DOI] [Google Scholar]
- 24.Clarke M. F. 1989. The pattern of helping in the bell miner (Manorina melanophrys). Ethology 80, 292–306 10.1111/j.1439-0310.1989.tb00748.x (doi:10.1111/j.1439-0310.1989.tb00748.x) [DOI] [Google Scholar]
- 25.McDonald P. G., Heathcote C. F., Clarke M. F., Wright J., Kazem A. J. N. 2007. Provisioning calls of the cooperatively breeding bell miner Manorina melanophrys encode sufficient information for individual discrimination. J. Avian Biol. 38, 113–121 10.1111/j.2007.0908-8857.03753.x (doi:10.1111/j.2007.0908-8857.03753.x) [DOI] [Google Scholar]
- 26.McDonald P. G., Kazem A. J. N., Clarke M. F., Wright J. 2008. Helping as a signal: does removal of potential audiences alter helper behavior in the bell miner? Behav. Ecol. 19, 1047–1055 10.1093/beheco/arn062 (doi:10.1093/beheco/arn062) [DOI] [Google Scholar]
- 27.McDonald P. G., te Marvelde L., Kazem A. J. N., Wright J. 2008. Helping as a signal and the effect of a potential audience during provisioning visits in a cooperative bird. Anim. Behav. 75, 1319–1330 10.1016/j.anbehav.2007.09.005 (doi:10.1016/j.anbehav.2007.09.005) [DOI] [Google Scholar]
- 28.te Marvelde L., McDonald P. G., Kazem A. J. N., Wright J. 2009. Do helpers really help? Provisioning biomass and prey type effects on nestling growth in the cooperative bell miner. Anim. Behav. 77, 727–735 10.1016/j.anbehav.2008.12.008 (doi:10.1016/j.anbehav.2008.12.008) [DOI] [Google Scholar]
- 29.Nam K.-B., Simeoni M., Sharp S. P., Hatchwell B. J. 2010. Kinship affects investment by helpers in a cooperatively breeding bird. Proc. R. Soc. B 277, 3299–3306 10.1098/rspb.2010.0737 (doi:10.1098/rspb.2010.0737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heathcote C. F. 1989. The acoustic repertoire of the bell miner, Manorina melanophrys. Melbourne, Australia: University of Melbourne [Google Scholar]
- 31.McDonald P. G., Wright J. 2008. Provisioning vocalizations in cooperative bell miners: more than a simple stimulus for nestling begging? Auk 125, 670–678 10.1525/auk.2008.07127 (doi:10.1525/auk.2008.07127) [DOI] [Google Scholar]
- 32.McDonald P. G., Wilson D. R., Evans C. S. 2009. Nestling begging increases predation risk, regardless of parental defense or spectral characteristics. Behav. Ecol. 20, 821–829 10.1093/beheco/arp066 (doi:10.1093/beheco/arp066) [DOI] [Google Scholar]
- 33.Painter J. N., Crozier R. H., Poiani A., Robertson R. J., Clarke M. F. 2000. Complex social organization reflects genetic structure and relatedness in the cooperatively breeding bell miner, Manorina melanophrys. Mol. Ecol. 9, 1339–1347 10.1046/j.1365-294x.2000.01012.x (doi:10.1046/j.1365-294x.2000.01012.x) [DOI] [PubMed] [Google Scholar]
- 34.Konovalov D. A., Manning C., Henshaw M. T. 2004. Kingroup: a program for pedigree relationship reconstruction and kin group assignments using genetic markers. Mol. Ecol. Notes 4, 779–782 10.1111/j.1471-8286.2004.00796.x (doi:10.1111/j.1471-8286.2004.00796.x) [DOI] [Google Scholar]
- 35.Conrad K. F., Clarke M. F., Robertson R. J., Boag P. T. 1998. Paternity and the relatedness of helpers in the cooperatively breeding bell miner (Manorina melanophrys). Condor 100, 343–349 10.2307/1370275 (doi:10.2307/1370275) [DOI] [Google Scholar]
- 36.McDonald P. G., Kazem A. J. N., Wright J. 2007. A critical analysis of ‘false-feeding’ behavior in a cooperatively breeding bird: disturbance effects, satiated nestlings or deception? Behav. Ecol. Sociobiol. 61, 1623–1635 10.1007/s00265-007-0394-2 (doi:10.1007/s00265-007-0394-2) [DOI] [Google Scholar]
- 37.Charif R. A., Clark C. W., Fristrup K. M. 2004. Raven 1.2 User's Manual. Ithaca, NY: Cornell Laboratory of Ornithology [Google Scholar]
- 38.Khanna H., Gaunt S. L. L., McCallum D. A. 1997. Digital spectrographic cross-correlation: tests of sensitivity. Bioacoustics 7, 209–234 [Google Scholar]
- 39.Van de Pol M., Wright J. 2009. A simple method for distinguishing within- versus between-subject effects using mixed models. Anim. Behav. 77, 753–758 10.1016/j.anbehav.2008.11.006 (doi:10.1016/j.anbehav.2008.11.006) [DOI] [Google Scholar]
- 40.Jakob E. M., Marshall S. D., Uetz G. W. 1996. Estimating fitness: a comparison of body condition indices. Oikos 77, 61–67 10.2307/3545585 (doi:10.2307/3545585) [DOI] [Google Scholar]
- 41.Burnham K. P., Anderson D. R. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer [Google Scholar]
- 42.McDonald P. G., Ewen J., Wright J. 2010. Brood sex ratio does not affect helper effort in a cooperative bird, despite extreme sex-biased dispersal. Anim. Behav. 79, 243–250 10.1016/j.anbehav.2009.11.007 (doi:10.1016/j.anbehav.2009.11.007) [DOI] [Google Scholar]
- 43.Sharp S. P., Hatchwell B. J. 2006. Development of family specific contact calls in the long-tailed tit Aegithalos caudatus. Ibis 148, 649–656 10.1111/j.1474-919X.2006.00568.x (doi:10.1111/j.1474-919X.2006.00568.x) [DOI] [Google Scholar]
- 44.Kennedy R. A. W., Evans C. S., McDonald P. G. 2009. Individual distinctiveness in the mobbing call of a cooperative bird, the noisy miner Manorina melanocephala. J. Avian Biol. 40, 481–490 10.1111/j.1600-048X.2008.04682.x (doi:10.1111/j.1600-048X.2008.04682.x) [DOI] [Google Scholar]
- 45.Radford A. N. 2005. Group-specific vocal signatures and neighbour–stranger discrimination in the cooperatively breeding green woodhoopoe. Anim. Behav. 70, 1227–1234 10.1016/j.anbehav.2005.04.002 (doi:10.1016/j.anbehav.2005.04.002) [DOI] [Google Scholar]
- 46.Russell A. F., Hatchwell B. J. 2001. Experimental evidence for kin-biased helping in a cooperatively breeding vertebrate. Proc. R. Soc. Lond. B 268, 2169–2174 10.1098/rspb.2001.1790 (doi:10.1098/rspb.2001.1790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forstmeier W., Burger C., Temnow K., Deregnaucourt S. 2009. The genetic basis of zebra finch vocalizations. Evolution 63–8, 2114–2130 10.1111/j.1558-5646.2009.00688.x (doi:10.1111/j.1558-5646.2009.00688.x) [DOI] [PubMed] [Google Scholar]
- 48.Fitch W. T., Hauser M. D. 2002. Unpacking ‘honesty’: vertebrate vocal production and the evolution of acoustic signals. In Animal communication (eds Simmons A., Fay R. R., Popper A. N.), pp. 65–137 New York, NY: Springer [Google Scholar]