Abstract
The purpose of this study was to evaluate the activity of single-agent bevacizumab in patients with recurrent anaplastic glioma and assess correlative advanced imaging parameters. Patients with recurrent anaplastic glioma were treated with bevacizumab 10 mg/kg every 2 weeks. Complete patient evaluations were repeated every 4 weeks. Correlative dynamic contrast-enhanced MR and 18fluorodeoxyglucose PET imaging studies were obtained to evaluate physiologic changes in tumor and tumor vasculature at time points including baseline, 96 h after the first dose, and after the first 4 weeks of therapy. Median overall survival was 12 months (95% confidence interval [CI]: 6.08–22.8). Median progression-free survival was 2.93 months (95% CI: 2.01–4.93), and 6-month progression-free survival was 20.9% (95% CI: 10.3%–42.5%). Thirteen (43%) patients achieved a partial response. The most common grade ≥3 treatment-related toxicities were hypertension, hypophosphatemia, and thromboembolism. Single-agent bevacizumab produces significant radiographic response in patients with recurrent anaplastic glioma but did not meet the 6-month progression-free survival endpoint. Early change in enhancing tumor volume at 4 days after start of therapy was the most significant prognostic factor for overall and progression-free survival.
Keywords: anaplastic glioma, bevacizumab, FDG, perfusion MRI
Bevacizumab was the first new drug labeled for gliomas in a decade after it received accelerated approval by the FDA in May 2009 based on improved response rates relative to historical controls for recurrent glioblastoma. This provisional approval expedited patient access to an effective therapy for one of the most lethal and aggressive cancers.
Bevacizumab is a logical drug to study in glioblastoma multiforme (GBM), which harbors the trademark histological feature of tumor neovasculature, although a survival benefit from bevacizumab has yet to be demonstrated in GBM. While clinical trials for initial therapy of GBM with bevacizumab are under way to investigate survival effects, patients receiving the drug as second-line treatment are gaining clinical benefit in terms of tumor response, decreased dependency on corticosteroids, and improvement in symptoms of disease.1,2
The role of bevacizumab in the treatment of WHO grade III gliomas is less clear. The available data for patients with anaplastic gliomas are derived largely from retrospective studies that demonstrate response rates from 15% to 79%, median progression-free survival from 5.0 to 13.4 months, and median overall survival from 6.8 to 12.6 months.3–9 Interpretation of these data is complicated because the studies often mix WHO grade III and IV tumors and describe a variety of combinations with other cytotoxic drugs such as irinotecan, carboplatin, and temozolomide.
The National Cancer Institute (NCI) trial that supported approval of bevacizumab in recurrent GBM was designed with patient enrollment stratified by histology to account for the differences in natural histories of grade III and grade IV tumors. Here, we report results of the anaplastic glioma arm of that study, as well as corollary neuroimaging studies using dynamic contrast-enhanced (DCE)–MRI and 18fluorodeoxyglucose (FDG) PET as biomarkers of treatment response and patient outcomes.
Patients and Methods
Eligibility Criteria
Patients ≥18 years old with histologically confirmed anaplastic glioma, recurrent following standard external beam fractionated radiotherapy and temozolomide chemotherapy, were enrolled. Eligible histologies included anaplastic astrocytoma, anaplastic oligodendroglioma, anaplastic oligoastrocytoma, and malignant astrocytoma not otherwise specified. Patients were required to have a KPS of ≥60%, normal metabolic and end-organ function, and an estimated survival of at least 2 months. Competent patients or their designated powers of attorney/health care proxies were required to sign informed consent for this NCI Institutional Review Board–approved trial. There were no limits on the number of prior therapies. There was a minimum washout period of 2 or 4 weeks required after molecularly targeted or cytotoxic agents, respectively. Patients had to be at least 4 weeks from completion of radiation therapy. Patients also had to be on a stable dose of corticosteroids for at least 5 days prior to obtaining their baseline MRI scan within 14 days of enrollment. Patients with acute intracranial hemorrhage determined by non–contrast-enhanced CT scan were ineligible, as were patients therapeutically anticoagulated.
Treatment and Patient Assessment
Patients were treated with bevacizumab 10 mg/kg every 14 days on a 28-day cycle. Dose delays were allowed for reversible and preventable toxicity. All patients underwent a perfusion MRI scan at baseline, within 4 days of the first bevacizumab infusion, and then at 4-week intervals. An FDG PET scan was performed at baseline and at the end of the first 4-week cycle. Blood counts were obtained every 2 weeks. A full metabolic screen, history, and physical and neurological exams were performed prior to each cycle. MRI scans were assessed by 2 independent reviewers (T.K., J.B.) using both qualitative Levin criteria and measured Macdonald criteria.10,11 Both criteria were modified by requiring that patients be on a stable or decreasing dose of corticosteroids and that the amount of fluid-attenuated inversion recovery (FLAIR) abnormality be stable or improved for a scan to be scored as partial response (PR) or stable disease (SD), consistent with the more contemporary Response Assessment in Neuro-Oncology criteria.12 A finding of disease progression by either Levin or Macdonald criteria was sufficient to terminate treatment, as was a determination of clinical progression in the absence of radiographic progression.
Study Design
Progression-free survival at 6 months (PFS6) was the primary endpoint of this study. Using Kaplan–Meier methodology, we estimated PFS6 and associated 95% confidence intervals (CIs). Unless the date of progression after removal from study was known, patients removed from study for toxicity were censored at the later of either the last evaluation on study or the start date of alternate therapy without disease progression. The study was designed to distinguish between a PFS6 of 30% and 55%. The historical value for comparison (i.e., ineffective rate 30%) was based on a large group of patients from 8 previous phase II studies in which none of the treatments was considered particularly effective.13 Target accrual was 32 patients. Bevacizumab would be considered effective if at least 13 patients had not progressed by 6 months. This gives a probability of 0.89 of concluding that the agent is effective if the PFS6 is 50%, and a probability of 0.13 of concluding that the agent is effective if the PFS6 is 30%. The maximal width of a 95% CI is 0.35. The expected 95% CI for PFS6 of 30% and 50% would be (0.14, 0.46) and (0.33, 0.67), respectively. Secondary endpoints were MRI and FDG PET response rates, and exploratory analyses of how those responses correlated with survival.
DCE-MRI
DCE-MRI scans obtained at baseline, within the first 96 h of starting treatment, and at the end of the first 4-week cycle were evaluated as potential early imaging biomarkers of bevacizumab efficacy. Thirty sequential 3D T1-weighted spoiled gradient-recalled (1.5-T GE Signa) or T1-weighted fast field echo (3-T Philips Acheiva) slabs covering the tumor were obtained every 20 s for 10 min, with resolution ∼1 × 1 × 5 mm3. Infusion of contrast (0.1 mmol/kg gadolinium–diethylenetriamine penta-acetic acid or gadoteridol at 0.3 mL/s) began after the fifth scan. Whole brain post-contrast 3D T1 volumes (∼1 × 1 × 1 mm3 resolution) were obtained. Time series were motion corrected (3D+time dataset registration, Analysis of Functional Neuroimages (AFNI), http://afni.nimh.nih.gov/14). A vascular input function was generated from the venous sinuses. Parametric maps of Ktrans (transfer constant from the vessel to the tissue), and fractional plasma volume (fpv) were computed by a nonlinear least squares fit of the signal intensity curves to the generalized kinetic model (DEMRI3 model, 3dNLfim program, AFNI). The baseline 3D T1 was used as a reference to which the other 3D T1 and all sets of parametric maps were rigidly coregistered (Fast Linear Image Registration Tool, http://www.fmrib.ox.ac.uk). The enhancing tumor volume (ETV) was roughly outlined by hand and refined using an expectation maximization algorithm (MEDx, Medical Numerics, http://medx.sensor.com/15). Within the ETV, the median of the top 100 voxels for Ktrans and fpv was computed for the 3 timepoints. The mean difference of each MRI parameter between Signa and Philips scanners at each time point was tested, and there was no significant effect of scanner type on results.
FDG PET
A baseline FDG PET was performed within 4 weeks of treatment start, and a follow-up FDG PET was performed at the end of the first 4-week cycle. Prior to PET imaging, patients were instructed to fast at least 6 h. Injection of FDG was not performed if there was evidence of hypoglycemia (serum glucose less than 200 mg/dL as measured on day of scan). Unit doses of FDG were supplied by Cardinal Health and calibrated to provide the required activity at the time of injection. FDG uptake was measured with a PET/CT scanner (Discovery ST 16 slice CT, GE Medical Systems) starting 1 h following intravenous injection of approximately 370 MBq FDG. Counts were acquired over 15 min with the scanner in 2D acquisition mode, and images were reconstructed to obtain 47 3.27-mm sections. Each section was reconstructed to a matrix of 128 × 128 with 2 mm × 2 mm pixel size. CT performed using helical acquisition, 140 kVP, 115 mA (56 mAs per rotation) was used for attenuation correction. Filters were applied to match the 7 mm full width half maximum resolution of the PET image. The whole-brain PET images were then segmented to white matter, grey matter, and tumor using the coregistered 3D T1 images. Within the ETV determined by the baseline or 4-week 3D T1 MRI, the median standard uptake value of the top 100 voxels was computed and normalized against the mean of all voxels in the grey matter.
Results
Patient Characteristics
Twenty-one men and 10 women with recurrent anaplastic gliomas were accrued and treated at the National Institutes of Health Clinical Center between January 2006 and February 2009. The majority of patients (68%) had anaplastic astrocytoma; 19% had anaplastic oligoastrocytoma; and 13% had anaplastic oligodendroglioma. The 1p and 19q deletion status was known for 7 of the 10 patients with oligodendroglial features; only 3 patients had codeletion. The median age was 44 years (range 24–66), median KPS was 90% (range 70–100%), and patients had received a median of 2 prior chemotherapy regimens before treatment with bevacizumab (range 0–7). At study entry, 58% of patients were on steroids.
Patient Outcomes
The most common grade ≥3 treatment-related toxicities were hypertension, hypophosphatemia, and thromoembolic events (Table 1). The toxicity profile observed in this study was similar to that of other experiences with single-agent bevacizumab in glioma patients.
Table 1.
Bevacizumab-related adverse events
| Common Toxicity Criteria Term | Grade |
N = 31 | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Total | |
| Hypertension | 1 | 4 | 5 | 10 | |
| Proteinuria | 8 | 1 | 9 | ||
| Epistaxis | 6 | 2 | 8 | ||
| Headache | 5 | 1 | 1 | 7 | |
| Thrombocytopenia | 6 | 1 | 7 | ||
| Hypophosphatemia | 4 | 2 | 6 | ||
| Fatigue | 3 | 3 | 6 | ||
| Elevated aspartate aminotransferase | 5 | 5 | |||
| Anemia | 4 | 4 | |||
| Rash/desquamation | 1 | 1 | 1 | 3 | |
| Hyperbilirubinemia | 3 | 3 | |||
| Thrombosis/thrombus/embolism | 2 | 2 | |||
| Hyperuricemia | 1 | 1 | 2 | ||
| Hemorrhage, rectal | 2 | 1 | 2 | ||
| Lymphopenia | 1 | 1 | 2 | ||
| Hypermagnesemia | 2 | 2 | |||
| Hypomagnesemia | 2 | 2 | |||
| Nausea | 2 | 2 | |||
| Rash: acneiform | 2 | 2 | |||
| Hand foot syndrome | 1 | 1 | |||
| Retinopathy | 1 | 1 | |||
| Hyponatremia | 1 | 1 | |||
| Hypoalbuminemia | 1 | 1 | |||
| Hypercalcemia | 1 | 1 | |||
| Confusion | 1 | 1 | |||
| Creatinine | 1 | 1 | |||
| Diarrhea | 1 | 1 | |||
| Edema: limb | 1 | 1 | |||
| Hemoglobinuria | 1 | 1 | |||
| Hemorrhage, upper respiratory | 1 | 1 | |||
| Myalgia | 1 | 1 | |||
| Petechiae | 1 | 1 | |||
| Hyperkalemia | 1 | 1 | |||
| Skin breakdown/decubitus ulcer | 1 | 1 | |||
| Vomiting | 1 | 1 | |||
| Wound complication, non-infectious | 1 | 1 | |||
Median overall survival was 12 months (95% CI: 6.08–22.8). Median progression-free survival was 2.93 months (95% CI: 2.01–4.93), and PFS6 was 20.9% (95% CI: 10.3%–42.5%).
Thirty patients were evaluable for radiographic response. The overall response rate based on Levin criteria was 67% (20 partial responses) whereas the response rate based on Macdonald criteria was 43% (13 partial responses). Average duration of response in these patients was 9.07 months (standard deviation ± 7.27). Eight patients (26%) had stable disease for greater than 2 months of therapy. A typical bevacizumab-mediated radiographic response is shown in Figure 1. At progression, all but 2 patients had an increase in contrast enhancement: 1 who came off study for clinical progression with stable radiographic disease, and 1 who had progression of only FLAIR signal abnormality consistent with infiltrating tumor.
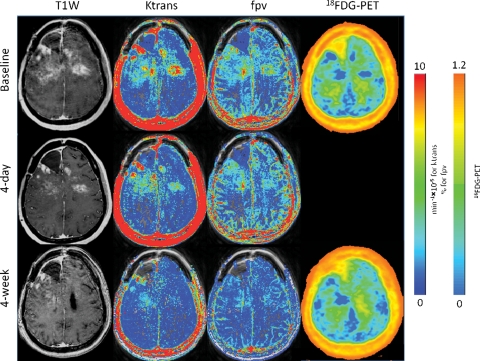
Fig. 1.
Sample radiographic response by DCE-MRI and FDG PET comparing baseline scans to 4-day (4D) and 4-week (4W) studies across different parameters. Each parameter was evaluated using the median of the top 100 voxels within the area determined by the T1 enhancing tumor volume (ETV) for a given time point. At 4 days, the patient had a 57% reduction in ETV, 56% reduction in Ktrans, and 1% reduction in fpv; at 4 weeks compared with baseline, ETV decreased by 89%, Ktrans by 84%, fpv by 80%, and PET standard uptake value increased by 11%.
Patients appeared to receive clinical benefit from treatment, with 12/18 (67%) on corticosteroids at the start of treatment able to decrease their requirement for corticosteroids by an average dose reduction of 71%. Additionally, 15 patients (48%) had improved neurological symptoms with treatment.
Imaging Correlates
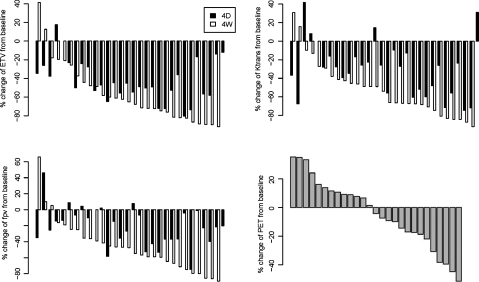
We evaluated MRI parameters (ETV, Ktrans, fpv) at baseline, 4 days (4D), and 4 weeks (4W) after start of therapy. Because of the skewness of the distributions of these parameter values, a linear mixed effect model and a linear model were fitted on log-transformed data. Mean ETV was significantly decreased by 43.5% at 4D (P < .0001) and by 56.6% at 4W (P < .0001). Mean Ktrans was significantly decreased by 30.8% at 4D (P = .0001) and by 51.9% at 4W (P < .0001). Mean fpv was also significantly decreased by 21.4% at 4D (P = .0082) and by 45.9% at 4W (P < .0001). Finally, mean FDG PET standard uptake value was decreased by 4.06% at 4W but the change was not significant (P = .1749). Figure 2 demonstrates the range of percent change of these parameters across the entire study group, where the vast majority of patients had some decrease in ETV, Ktrans, and fpv at the early imaging timepoints. Age was negatively associated with baseline fpv (P = .044). Its negative association with Ktrans was borderline significant (P = .055), and the effect of age on baseline ETV and PET was not significant (P = .346 and 0.6349, respectively). Age was not associated with percentage change of these MRI parameter values at 4D and 4W, or of PET at 4W.
Fig. 2.
Plots show data for all patients sorted by percentage change from baseline to 4W evaluation across imaging parameters: ETV, Ktrans, fpv, and FDG PET standard uptake value (SUV). The majority of patients had a decrease in the MRI parameters at 4D and 4W from treatment start, demonstrating vascular permeability and tumor perfusion effects of bevacizumab. The average decrease in FDG PET SUV was small and not significant.
The relationship between survival and MRI and PET baseline parameter values and the change from baseline (Δ) at 4D and 4W were evaluated. ▵ETV4D, ▵ETV4W, ▵Ktrans4D, ▵Ktrans4W, ▵fpv4D, ▵fpv4W, and ▵PET4W were calculated by subtracting the baseline value from the later 4D or 4W timepoint. Therefore, a more negative value (or smaller value) indicates a larger decrease in the parameter over time. Other clinical factors, such as age, KPS, and gender, were also included in a univariate Cox proportional hazards model, where the hazard risk (HR) is the relative risk of progression or death for a unit change in the continuous variable (Table 2).
Table 2.
Estimated hazard ratios (HRs) from a univariate Cox proportional hazards model
| Variable | Death |
Progression |
||
|---|---|---|---|---|
| HR | P | HR | P | |
| Age/10 | 1.05 | .77 | 0.82 | .2739 |
| Gender (male) | 0.46 | .1031 | 0.54 | .13 |
| KPS | 0.97 | .1666 | 0.99 | .567 |
| ETV at baseline | 1.47 | .0793 | 1.14 | .4390 |
| ETV at 4D | 1.82 | .0031 | 1.34 | .0597 |
| ETV at 4W | 1.44 | .0455 | 1.30 | .0787 |
| ▵ETV4D | 1.89 | <.0001 | 2.22 | <.0001 |
| ▵ETV4W | 1.14 | .1020 | 1.28 | .0047 |
| Ktrans at baseline | 1.15 | .4713 | 1.09 | .6038 |
| Ktrans at 4D | 1.11 | .6726 | 1.09 | .6725 |
| Ktrans at 4W | 1.46 | .1464 | 1.20 | .3928 |
| ▵Ktrans4D | 0.79 | .3762 | 0.86 | .5533 |
| ▵Ktrans4W | 1.08 | .6757 | 1.08 | .6929 |
| fpv at baseline | 0.98 | .9422 | 0.89 | .6909 |
| fpv at 4D | 1.01 | .9682 | 0.97 | .8976 |
| fpv at 4W | 1.06 | .8390 | 0.94 | .7943 |
| ▵fpv4D | 1.04 | .7555 | 1.02 | .8759 |
| ▵fpv4W | 1.03 | .7688 | 1.01 | .9376 |
| PET at baseline | 2.34 | .0769 | 1.52 | .3099 |
| PET at 4W | 3.46 | .0316 | 1.86 | .1664 |
| ▵PET4W | 0.97 | .8686 | 1.06 | .7338 |
Enhancing tumor volume (ETV), Ktrans, fpv, and FDG-PET intake values were log-transformed. HRs for ▵ETV4D, ▵ETV4W, Ktrans4D, ▵Ktrans4W, ▵fpv4D, ▵fpv4W, and ▵PET4W correspond to relative risk of death or progression for a ≥25% increase in these parameter values. HRs for other covariates correspond to relative risk for one-unit increase of the continuous variable.
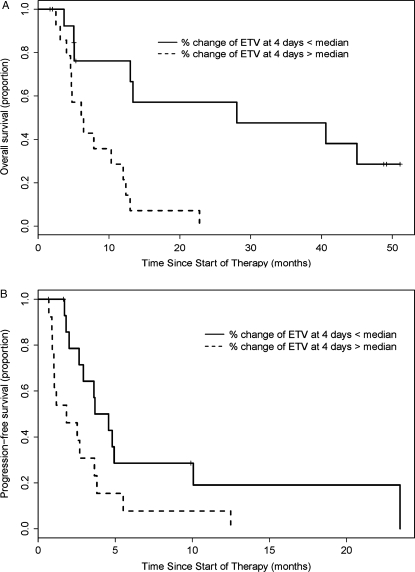
For risk of death, ETV (log-transformed) at 4D, 4W, change in ETV at 4D (ΔETV4D), and PET (log-transformed) at 4W were significant at the 0.05 level. When these factors were included in the multivariate analysis, only ΔETV4D was significant (P < .01). Figure 3A shows Kaplan–Meier estimates of overall survival for individuals above and below the median of percentage change of ETV at 4D (−48.9%). The log-rank tests comparing the 2 Kaplan–Meier curves were significant (P = .0008). The median survival for individuals above and below the median of percentage change of ETV at 4D was 6.25 and 28.04 months, respectively. Baseline FDG PET at 4W was also significantly associated with risk of death with the highest HR of any parameter, 3.46 (P = .0316).
Fig. 3.
The only significant factor in the multivariate analysis was % change of ETV at 4D. Kaplan–Meier estimates for patients with survival above and below the median % change in ETV (−48.6%) were significantly different for both overall survival (P = .0008) and progression-free survival (P = .0296).
The percentage change of ETV at both 4D and at 4W was significantly associated with disease progression on therapy. The median progression-free survival for individuals above and below the median of percentage change of ETV at 4D was 1.84 and 4.12 months, respectively (log rank test P = .0296); at 4W, 1.81 and 4.57 months, respectively (log rank test P = .0042). When these factors were included in the multivariate analysis, only ΔETV4D was significant (P < .001). Figure 3B shows Kaplan–Meier estimates for progression-free survival of individuals above and below the median of percentage change of ETV at 4D (−48.9%).
Discussion
We observed a PFS6 of 20.9% (95% CI: 10.3%–42.5%) for recurrent anaplastic glioma patients treated with single-agent bevacizumab, which was not significantly different from the historical control described by Wong et al. in 1999.13 However, the 43% response rate observed compares favorably with the historical response rate of 14%.13 A more contemporary historical control was published by Lamborn et al.16 that reported a response rate of only 9% for recurrent anaplastic gliomas, excluding regimens containing temozolomide. Despite the rather disappointing effects on PFS6, bevacizumab monotherapy did appear to have a significant clinical benefit for patients in terms of radiographic response, decreased dependency on steroids, and improvement of neurological symptoms, as is seen in patients with glioblastoma.
Few prospective studies using bevacizumab have separated analyses for grade III and grade IV gliomas. Desjardins et al.17 reported a trial of bevacizumab in combination with irinotecan in 36 patients with recurrent anaplastic glioma using 2 different dosing regimens of bevacizumab. PFS6 was 55% (95% CI: 36%–70%) and the response rate was 61%. While these results suggest an advantage of this regimen over historical control, a direct comparison with the current study cannot be made due to differences in the treatment regimens. While irinotecan does not seem to add marginal benefit to bevacizumab monotherapy for recurrent glioblastoma, combination with cytotoxic therapy may be beneficial for grade III tumors more sensitive to standard chemotherapy. Controlled studies with a logical cytotoxic pairing would be required to determine this.
The GBM arm of this trial was previously reported, where advanced age was, paradoxically, a positive prognostic factor for progression-free survival.2 This was not observed for the patients with anaplastic tumors. Grade III gliomas, more commonly than GBM, have a prominent degree of nonenhancing infiltrative disease. Therefore, effects on vascular permeability and tumor blood volume may not correlate well with disease progression. Additionally, patients with grade III tumors tend to be younger, where an effect at extremes of age may not be evaluable in this small cohort of patients (age range, 24–66 years).
In this study, significant changes in vascular permeability (Ktrans) and tumor blood volume (fpv) were observed after treatment with bevacizumab. These effects on perfusion MRI have previously been observed with bevacizumab and other vascular endothelial growth factor–directed agents, although the clinical relevance of fpv has been demonstrated primarily in non-glioma studies to date.18–21 Interestingly, early change in enhancing tumor volume was the most significantly predictive variable for patient outcome. Historically established prognostic factors such as patient age, KPS, and baseline tumor volume were not as correlated with overall nor progression-free survival in the univariate analysis. This finding may imply that tumor characteristics are more important than patient characteristics when the effectiveness of bevacizumab as therapy is considered.
Early imaging with MRI using a quantitative approach to evaluate contrast enhancement may provide a biomarker to help stratify patients for different therapeutic approaches or future clinical trials. The only parameter to have any significant correlation with overall and progression-free survival in the multivariate analysis was ΔETV4D. In fact, subjective radiographic response (PR), as determined by Levin criteria at 4D (35% patients), was not a significant variable for survival (P = .52). ΔETV4D may be a more sensitive predictor of outcome, since the majority of patients experienced at least some early decrease in ETV. This finding could potentially have clinical implications, where patients without significant change in ΔETV4D within the first few days of bevacizumab therapy may better benefit from the addition of another agent. A ≥ 25% change from baseline for all imaging parameters was used for the Cox proportional hazards analysis, but the optimal threshold for ΔETV4D that predicts improved survival is not determined.
Historical controls for patients with recurrent anaplastic glioma demonstrate median overall survival of 9 to 11.8 months and median progression-free survival 2 to 6 months.13,16 When patients in this study are stratified by ΔETV4D, those below the median ΔETV4D (larger decreases in enhancing tumor volume) had a dramatically improved overall survival relative to historical controls (28.04 mo). Of the 31 patients in this study, 6 had overall survival greater than 24 months. All 6 of these patients had ΔETV4D below the median; 5 of them had overall survival >40 months. None had oligodendroglial tumor. At study entry, 3 patients had some portion of their heterogeneously enhancing mass exhibit features of necrosis by FDG PET and/or perfusion MRI. However, hypermetabolism observed with FDG PET, and increased cerebral blood volume on perfusion MRI, indicated viable tumor in some portion of the enhancing disease for all 6 patients. These patients were treated with bevacizumab for their second or greater recurrence of disease and received salvage chemotherapy after coming off study therapy; 5 were retreated with bevacizumab-containing regimens at some point. Patients with ΔETV4D below the median did not have a particularly prolonged median progression-free survival (4.12 mo). Therefore, it is difficult to determine whether the long-term survival observed in this small subset of patients is attributable to bevacizumab therapy. However, it appears that ΔETV4D in response to single-agent bevacizumab may be able to identify patients with an alternative natural history of disease.
In conclusion, the single-agent bevacizumab experience in anaplastic glioma mirrors the benefits observed for recurrent GBM patients in terms of improved radiographic response and clinical benefit to patients. However, there does not appear to be an advantage in progression-free survival compared with historical controls. Combination therapy may be superior for these patients, and prospective trials should be performed to investigate this possibility. Future studies of bevacizumab in malignant glioma should separate analyses for patients with grade III tumors, since outcomes do not exactly mirror those for GBM. Early changes in ETV within the first few days of therapy may be a useful marker of which patients with recurrent anaplastic glioma will benefit the most from single-agent bevacizumab.
Conflict of interest statement. None declared.
Funding
National Cancer Institute Intramural Research Program.
Acknowledgments
Data were previously presented at the Joint Meeting of the Society for Neuro-Oncology and AANS/CNS Section on Tumors, October 2009, New Orleans, LA.
References
- 1.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. doi:10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 2.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. doi:10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali SA, McHayleh WM, Ahmad A, et al. Bevacizumab and irinotecan therapy in glioblastoma multiforme: a series of 13 cases. J Neurosurg. 2008;109(2):268–272. doi: 10.3171/JNS/2008/109/8/0268. [DOI] [PubMed] [Google Scholar]
- 4.Gil Gil MJ, Sr, Martinez-Garcia M, Reynes G, et al. Combination of bevacizumab plus irinotecan in recurrent malignant gliomas (MG): A retrospective study of efficacy and safety. ASCO Meeting Abstracts. 2008;26(15 suppl):13011. [Google Scholar]
- 5.Kang T, Jin T, Peereboom D. Irinotecan and bevacizumab in progressive primary brain tumors: The Cleveland Clinic experience. ASCO Meeting Abstracts. 2007;25(18 suppl):2077. [Google Scholar]
- 6.Narayana A, Chheang S, Knopp E, et al. Comparing cerebral blood volume and vascular permeability measurements with tumor volume measurements following anti-angiogenesis therapy in recurrent gliomas. ASCO Meeting Abstracts. 2007;25(18 suppl):2030. [Google Scholar]
- 7.Norden AD, Drappatz J, Muzikansky A, et al. An exploratory survival analysis of anti-angiogenic therapy for recurrent malignant glioma. J Neurooncol. 2009;92(2):149–155. doi: 10.1007/s11060-008-9745-8. doi:10.1007/s11060-008-9745-8. [DOI] [PubMed] [Google Scholar]
- 8.Poulsen HS, Grunnet K, Sorensen M, et al. Bevacizumab plus irinotecan in the treatment patients with progressive recurrent malignant brain tumours. Acta Oncol. 2009;48(1):52–58. doi: 10.1080/02841860802537924. doi:10.1080/02841860802537924. [DOI] [PubMed] [Google Scholar]
- 9.Zuniga RM, Torcuator R, Jain R, et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009;91(3):329–336. doi: 10.1007/s11060-008-9718-y. doi:10.1007/s11060-008-9718-y. [DOI] [PubMed] [Google Scholar]
- 10.Levin VA, Crafts DC, Norman DM, Hoffer PB, Spire JP, Wilson CB. Criteria for evaluating patients undergoing chemotherapy for malignant brain tumors. J Neurosurg. 1977;47(3):329–335. doi: 10.3171/jns.1977.47.3.0329. [DOI] [PubMed] [Google Scholar]
- 11.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 12.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 13.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 14.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. doi:10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 15.Solomon J, Butman JA, Sood A. Segmentation of brain tumors in 4D MR images using the hidden Markov model. Comput Methods Programs Biomed. 2006;84(2–3):76–85. doi: 10.1016/j.cmpb.2006.09.007. doi:10.1016/j.cmpb.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10(2):162–170. doi: 10.1215/15228517-2007-062. doi:10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desjardins A, Reardon DA, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent WHO grade 3 malignant gliomas. Clin Cancer Res. 2008;14(21):7068–7073. doi: 10.1158/1078-0432.CCR-08-0260. doi:10.1158/1078-0432.CCR-08-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desjardins A, Barboriak DP, Herndon JE, II, et al. Effect of bevacizumab (BEV) and irinotecan (CPT-11) on dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in glioblastoma (GBM) patients. ASCO Meeting Abstracts. 2008;26(15 suppl):2026. [Google Scholar]
- 19.Sorensen AG, Batchelor TT, Zhang WT, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69(13):5296–5300. doi: 10.1158/0008-5472.CAN-09-0814. doi:10.1158/0008-5472.CAN-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell CL, O'Connor JP, Roberts C, et al. A two-part Phase II study of cediranib in patients with advanced solid tumours: the effect of food on single-dose pharmacokinetics and an evaluation of safety, efficacy and imaging pharmacodynamics. Cancer Chemother Pharmacol. doi: 10.1007/s00280-010-1534-3. Epub 2010 Dec. [DOI] [PubMed] [Google Scholar]
- 21.Notohamiprodjo M, Sourbron S, Staehler M, et al. Measuring perfusion and permeability in renal cell carcinoma with dynamic contrast-enhanced MRI: a pilot study. J Magn Reson Imaging. 2010;31(2):490–501. doi: 10.1002/jmri.22028. [DOI] [PubMed] [Google Scholar]