Abstract
Surgical management of bilateral vestibular schwannomas (VS) in neurofibromatosis type 2 (NF2) is often difficult, especially when both tumors threaten the brainstem. When the largest tumor has been removed, the management of the contralateral VS may become puzzling. To give new insights into the growth pattern of these tumors and to determine the best time point for treatment (surgery or medical treatment), we studied radiological growth in 11 VS (11 patients with NF2) over a long period (mean duration, 7.6 years), before and after removal of the contralateral tumor while both were threatening the brainstem. We used a quantitative approach of the radiological velocity of diametric expansion (VDE) on consecutive magnetic resonance images. Before first surgery, growth patterns of both tumors were similar in 9 of 11 cases. After the first surgery, VDE of the remaining VS was significantly elevated, compared with the preoperative period (2.5 ± 2.2 vs 4.4 ± 3.4 mm/year; P = .01, by Wilcoxon test). Decrease in hearing function was associated with increased postoperative growth in 3 cases. Growth pattern of coexisting intracranial meningiomas was not modified by VS surgery on the first side.
In conclusion, removal of a large VS in a patient with NF2 might induce an increase in the growth rate of the contralateral medium or large VS. This possibility should be integrated in NF2 patient management to adequately treat the second VS.
Keywords: brain tumor, meningioma, natural history, neurofibromatosis type 2, vestibular schwannoma
Neurofibromatosis type 2 (NF2) is a rare and dominantly transmitted genetic condition that affects 1 in 33, 000 persons at birth.1 Bilateral vestibular schwannomas (VSs) are the hallmark of the disease. These tumors cause progressive hearing loss and may also threaten life via progressive brainstem compression. Surgery remains the main treatment option used to achieve tumor control, but this more frequently leads to iatrogenic hearing loss than in sporadic cases.2,3 Alternatives to surgery have been proposed, including radiosurgery4 (use of which in NF2 remains controversial5) and chemotherapy with bevacizumab,6,7 although its exact place in the tumor management algorithm remains to be precisely defined.
A precise understanding of the natural history of NF2-related tumors is essential to guide therapeutic strategy and to design and interpret the results of ongoing clinical trials.5 Several longitudinal studies have already been conducted on NF2-related schwannomas.8–12 They concluded that VS growth rates are highly variable but tend to decrease with increasing age. In the present study, which included a large number of patients with NF2 with long-term follow-up, we decided to focus on the growth patterns of remaining intracranial tumors after the removal of a large VS in the case in which both VSs threaten the brainstem. In this particular case, a common initial strategy is to remove the largest tumor. Because ipsilateral hearing function is frequently lost before or after surgery, a conservative management is generally advocated for the contralateral tumor. If the tumor shows significant growth or affects the ipsilateral hearing to an unserviceable level, the second-side surgery is performed with an auditory brainstem implantation to rehabilitate the hearing function.13 The aim of our study was to determine whether the first VS growth and surgery influences the growth of the contralateral VS, residual hearing, and progression of other associated intracranial tumors. These data will potentially provide clues to a tailored decision making and future clinical trials in patients with NF2.
Material and Methods
We reviewed a retrospective series of patients with NF2 treated for bilateral VS in our NF2 center during the period 1995–2009. The local institutional review board approved this retrospective chart review. All patients provided written informed consent. Inclusion criteria were (1) presence of bilateral VS, with 1 large tumor and 1 medium-sized VS in contact with or compressing the brainstem at the time of the first surgery; (2) availability of a follow-up magnetic resonance image (MRI) before and after the first surgery. Patients were required to undergo ≥2 consecutive MRIs before and after the first surgery to be eligible for analysis of tumor growth.
According to the longitudinal studies,14 NF2-related VS growth curves are highly variable from one patient to another and may demonstrate many patterns, such as exponential or linear growth or even stability. Because a single pattern does not predominate, there is a common agreement that linear fit is a reliable method to determine tumor growth.14 Evaluation of tumor size is also controversial because some authors advocate the use of direct measures, such as mean tumor diameter (MTDs),8 largest tumor diameter, or tumor volume,15 whereas others employ the tumor doubling time (TDT),10,11 arguing that there is a significant relationship between the baseline tumor volume and subsequent growth rate.14 In our series, the comparison of 2 segments of the growth curve made it difficult to assess the TDT. Because MTDs at first surgery were homogeneous (1.8 ± 0.6 cm), we decided to use the MTD, similar to the box model. Because our study focuses on tumors with at least brainstem contact, we decided not to evaluate the intracanalicular portion of the tumors. Finally, linear measurements were preferred over volumetric evaluations because of the quality of films and the varying acquisition protocols. Tumor volumes were estimated by 1 investigator (M.P.) using the 3-diameters technique (V = [D1*D2*D3]/2), where D1 and D2 represent the greatest diameter perpendicular to the petrous ridge (D1) and the greatest tumor diameter perpendicular to D1 (D2) measured on an axial gadolinium enhanced T1-weighted image. D3 represents the vertical height measured on coronal gadolinium enhanced T1-weighted image. Tumor volumes were converted into a mean tumor diameter (MTD = [2*V]1/3).16 Velocity of diametric expansion (VDE) was then estimated from a linear regression of changes in MTD with time. The VDE was measured before and after first surgery in each patient. Meningioma sizes were also evaluated before and after the first VS surgery by single-dimension linear measurements. Analyses were performed using Graphpad Prism. Results were expressed as mean values, and a statistical significance level of P = .05 was used. The nonparametric Wilcoxon signed rank test was performed to compare tumors before and after surgery. Linear regression analysis of preoperative versus postoperative MTD time series evaluated the best-fit value of the slope and intercept. The analysis used correlation to quantify the degree to which the variables were related, and the F test was performed to test both the null hypothesis that the overall slope was 0 and whether the slopes were significantly different. Hearing function was assessed using the AAO-HNS classification.17 Serviceable hearing was defined as AAO-HNS classes A and B.
MIB-1 label index evaluation was performed using immunohistochemistry with the MIB-1 antibody (Dako) with an automatized technique (streptavidin peroxidase with an automated immunostainer [Benchmark; Ventana]) for each tumor in the 5 patients with NF2 operated on bilaterally. The MIB-1 label index was determined by photographing randomly 10 light microscopic fields at a magnification of ×40. Stained and unstained nuclei were counted to calculate the percentage of MIB-1–positive nuclei.
Results
Eleven patients were studied. Clinical data are summarized in Table 1. The mean age at onset was 19.6 years (median, 18 years; range, 11–32 years), and the mean age at first surgery was 22.8 years (median, 20.4 years; range, 13–38 years). The mean duration of follow-up was 7.6 years after onset (median, 6.9 years; range, 2.3–15.8 years) and 4.5 years after first surgery (median, 3.2 years; range, 0.8–14.6 years). The mean MTD of the resected tumor at surgery was 3.0 cm (median, 2.9 cm; range, 2.7–3.6 cm). Resection was gross total in all cases through the translabyrinthine approach, with no residual tumor noted on early postoperative MRIs and no relapse at the end of radiological follow-up.
Table 1.
Patients’ characteristics
| Patient | Age at first surgery, years | Hearing on first side at surgery (ABCD class) | Duration of radiological follow-up, years |
MTD of the resected schwannoma at surgery, cm | MTD of the contralateral schwannoma at surgery, cm | VDE evolution, mm/year |
Contralateral ear |
Surgery of contralateral schwannoma | Time to second surgery (years) | Mean Ki-67 (%) (±SD) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Since diagnosis | Since first surgery | Before first surgery | After first surgery | Hearing status at first surgery (ABCD class) | Hearing status at the end of follow-up (ABCD class) | First operated schwannoma | Controlateral schwannoma | |||||||
| 1 | 32 | D | 6.4 | 4.3 | 2.9 | 2.8 | 1.3 | 3.3 | A | A | No | — | — | — |
| 2 | 38 | B | 12.0 | 5.9 | 3.3 | 1.4 | 0.6 | −0.1 | C | D | No | — | — | — |
| 3 | 24 | D | 9.4 | 6.9 | 3.4 | 1.8 | 1.7 | 2.9 | D | D | No | — | — | — |
| 4 | 20 | A | 6.7 | 3.2 | 1.8 | 1.0 | 1.6 | 2.9 | A | A | No | — | — | — |
| 5 | 18 | D | 9.4 | 2.4 | 3.6 | 1.5 | 2.0 | 7.8 | A | D | Yes | 2.3 | 1.7 (±0.4) | 2.0 (±0.6) |
| 6 | 20 | D | 3.2 | 0.8 | 2.9 | 1.6 | 3.3 | 2.1 | D | D | No | — | — | — |
| 7 | 25 | D | 15.8 | 14.6 | 3.1 | 2.9 | 2.6 | 0.8 | A | D | No | — | — | — |
| 8 | 28 | D | 6.9 | 0.9 | 2.8 | 2.0 | 3.3 | 7.0 | B | C | Yes | 1.6 | 1.4 (±0.9) | 2.4 (±1.5) |
| 9 | 18 | D | 2.3 | 2.0 | 3.6 | 1.9 | 4.0 | 7.9 | D | D | Yes | 2.0 | 1.5 (±0.5) | 2.8 (±1.0) |
| 10 | 13 | A | 2.9 | 1.1 | 2.9 | 1.8 | 5.7 | 10.5 | A | B | Yes | 1.5 | 4.0 (±1.3) | 14.0 (±0.3) |
| 11 | 13 | D | 9.2 | 7.7 | 2.7 | 1.0 | 1.3 | 3.2 | A | D | Yes | 7.7 | 2.1 (±1.3) | 1.7 (±1.0) |
Abbreviations: MTD, mean tumor diameter; VDE, velocity of diametric expansion.
Preoperative VS Growth
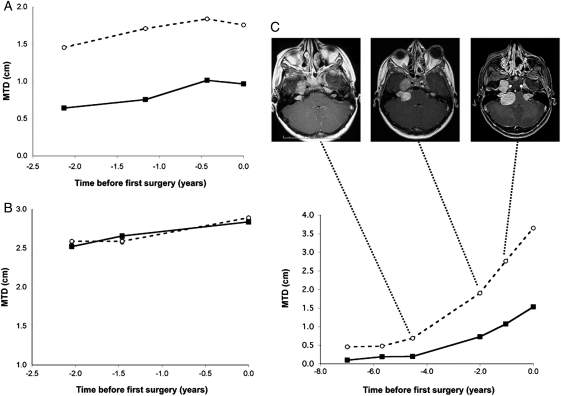
We first determined the growing patterns of both VSs before the first surgery in 11 patients. Two patterns of growth were observed. In the first group (n = 9), we observed that the size variation was linear and similar between both VSs. In 7 cases, the MTD of the first resected tumor was already higher than the contralateral one was at the beginning of follow-up (Fig. 1A). In the other 2 cases, ipsi- and contralateral VS sizes were similar from the beginning (Fig. 1B). In the second group (n = 2) with no correlation of VS growth rates, we were able to determine the time point at which growth curves started to progress further apart (Fig. 1C). This discrepancy was associated on MRI with a contact between the VS and another NF2-related tumor (petrous apex meningioma in case C and lower cranial nerve schwannoma in the other case).
Fig. 1.
Preoperative growth of bilateral vestibular schwannomas. Dotted lines and circles represent the growth curve of the first operated tumor. Plain lines and squares represent contralateral vestibular schwannoma (VS) growth. Abscission times correspond to the preoperative follow-up period, with time 0 being the time of the resection of the first VS (the largest). In 7 of 11 patients, mean tumor diameters (MTDs) differed at the beginning of follow-up, but growth rates were similar during follow-up (eg, patient 4 in A). In 2 patients, MTDs and growth rates were similar from the beginning to the end of follow-up (eg, patient 6 in B). In the last 2 patients, MTDs were similar at the beginning of follow-up, but growth rates varied during follow-up (eg, patient 5 in C). In case C, the contact between the right VS and the homolateral petrous ridge meningioma, having occurred between images 1 and 2, seems to have significantly triggered VS growth and may explain differential growth between the 2 VSs.
Pattern of Contralateral VS Growth After the First VS Removal
The mean MTD of the contralateral VS at surgery was 1.8 cm (median, 1.8 cm; range, 1.0–2.9 cm). The mean VDE before the first surgery was 2.5 mm/year (median, 2.0 mm/year; range, 0.6–5.7 mm/year). Growth patterns before surgery were mostly linearly increasing growths. After the first surgery, VDE increased in 8 of 11 patients and decreased in 3 patients. In 4 cases, the postsurgical VDE was at least 2 times higher than presurgical values. The mean postsurgical VDE was 4.4 mm/year (median, 3.2 mm/year; range, 0.1–10.5 mm/year). The increase in VDE was statistically significant compared with preoperative values in paired data analysis (P = .01, by Wilcoxon test).
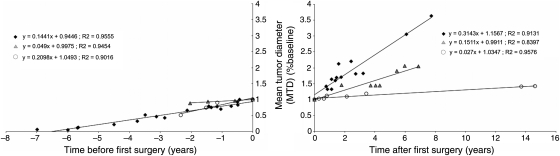
We then compared preoperative and postoperative patterns of growth for all 11 patients by regression analysis using the MTD at first surgery as the baseline MTD, and we determined pre- and postoperative relative VDE. We were able to identify 3 patterns of change in postsurgical relative VDE (Fig. 2). In group 1 (6 patients), relative VDE doubled after surgery (0.31 per year compared with 0.14 per year before the operation), and the difference was statistically significant (P < .0001) (Fig. 3). In group 2 (2 patients), the postoperative increase in relative VDE was less important (0.04 per year vs. 0.14 per year) and was not significant (P = .29). In group 3 (3 patients), relative VDE significantly decreased after the first surgery (0.02 per year compared with 0.2 per year before surgery; P = .0003917). Preoperative relative VDE were similar in all groups. Consequently, preoperative VDE did not seem to predict evolution in postoperative VDE in this group analysis. Growth patterns and regression analysis data for all patients are available in Supplementary Data (Fig. S1).
Fig. 2.
Changes in vestibular schwannoma (VS) growth rates after surgery for the first tumor. The origin of the timeline corresponds to the surgery of the first VS. Tumor size is expressed as a fraction of the baseline tumor mean tumor diameter (MTD), defined as the MTD of each tumor at the time of first surgery. Tumor groups as defined by regression analysis are represented as follows: group 1, black circles; group 2, gray triangles; and group 3, white circles. Slopes and R2 values are indicated.
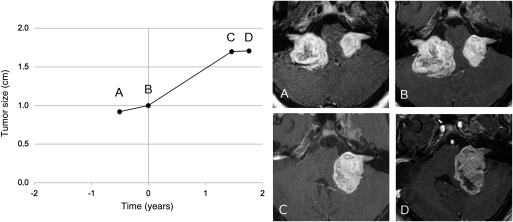
Fig. 3.
Progression of the radiological growth rate of the remaining vestibular schwannoma (VS) after resection of the contralateral tumor (Patient 9). Each point represents a magnetic resonance imaging examination (gadolinium-enhanced T1-weighted images) before and after removal of the first VS. Before surgery, the tumor grew with a radiological velocity of diametric expansion (VDE) of 4.0 mm/year. After surgery, the radiological VDE increased to 7.9 mm/year.
The remaining VS was operated on in 5 of 11 cases after a mean follow-up of 1.8 years (median, 1.8 years; range, 1.5–2.3 years) due to a rapid increase in tumor size, as shown by the doubling of the postoperative VDE for almost all these 5 patients (Table 1). When comparing patients with high increases in postoperative VDE (either with at least twice the preoperative value and/or second surgery due to rapid tumor growth) with others, we noticed that their mean age at diagnosis was lower (17.5 years vs 22.4 years), but this result did not reach statistical significance (P = .1, by Mann-Whitney U test).
The mean MIB-1 labeling index was 2.2% (median, 1.7%; range, 1.4%–2.1%) in the first operated VS compared with 4.6% (median, 2.4%; range, 1.8%–13.9%) in the contralateral tumor. The MIB-1 labeling index was higher in the second VS than in the first resected tumor in 4 of 5 patients (Table 1). However, on paired analysis, the MIB-1 labeling index was not statistically different in the second operated tumor versus the first one (Wilcoxon test).
Hearing
Hearing, as determined by the AAO-HNS classification, remained stable during follow-up in 5 patients, decreased while remaining serviceable in 1 patient, remained unserviceable in 1 patient, and became unserviceable in 4 patients (Table 1). Among the latter patients, 3 had a postoperative increase in VDE. Hearing deterioration was associated to an increased postoperative VDE defined as a doubling of preoperative values (P = .04, by χ2 test).
Tumor Burden
Among the 8 patients harboring meningiomas at the beginning of radiological follow-up, 2 had only 1 tumor and 6 harbored multiple meningiomas. No additional tumors were detected during follow-up for all patients but 1, for whom 5 new meningiomas were detected on follow-up examinations. In 4 patients, meningiomas presented a stable course of linear tumor progression without evident disruption in the tumors’ growth patterns related to the surgery of the first VS. The other 4 patients had stable tumors for which the size did not change over time. During follow-up, only 1 patient had to undergo an operation for a meningioma.
Discussion
Natural History
This study provided significant information of VS natural history in NF2. We observed an increase of VDE in the remaining VS after the removal of the first tumor when both compress the brainstem. This result was found on paired analysis but also for the whole cohort on regression analysis. However, this radiological observation was not associated with a statistically increased MIB-1 labeling index in the second tumor versus the first in the 5 patients with bilateral VS removal. In the literature, a high MIB-1 index has been described as an important factor related to the regrowth of sporadic VS after partial resection.18 However, this result may not apply to our study because we discuss cases of gross-total resections in patients with NF2 in whom the MIB-1 index is higher than in sporadic VS.19 The result must also be put into perspective with the small size of our cohort.
Previous studies of the natural history of VS in NF2 found that tumor growth rates were highly variable but decreased with increasing patient age. Ito et al.12 also suggested that, in patients with NF2, age at onset represents a more important factor than multiplicity of tumors for predicting the clinical course. Similarly, we demonstrate that an increase of postoperative VDE in the remaining VS is associated with young age at NF2 diagnosis and that global tumor burden does not seem to influence its growth. Previous studies have also pinpointed that growth rates, but not volumes, of left- and right-side VSs were significantly correlated.10–12 Conversely, in their cohort of 29 patients harboring 58 VS, Slattery et al.8 found a significant relationship between the sizes of left- and right-side VS at a precise time point (baseline and end of follow-up) but not between the growth rates of the tumors. In our cohort, we showed that, before the first surgery, VSs tended to have the same growth rate. We also postulate that, despite their intrinsic similar growth rate, bilateral VSs often present different sizes at diagnosis, depending on the timing of the second hit leading to biallelic inactivation of the NF2 gene (the first hit being the germline mutation).20 In 2 patients, we were able to show that the contact of a NF2-related tumor might be the cause of this discrepancy. This observation might explain why, in the study by Slattery and colleagues,8 VSs tended to be the same size at baseline, if evaluated early during growth, but presented a discrepancy in their growth patterns due to differences in their micro-environment. Because of the small size of our cohort, additional studies are mandatory to determine whether variations in growth rates of bilateral VS might be at least partly explained by the tumor microenvironment, especially other NF2-associated tumors.
There is no evidence in the literature that resection of the first VS influences the growth rate of the remaining contralateral tumor. Von Eckardstein et al.21 published 2 astonishing cases of spontaneous VS regression that occurred after resection of the contralateral tumor. In the first case, the maximum posterior fossa diameters of the tumors were 39 and 27 mm, respectively, which is similar to our cases, but in the other patient, tumors were smaller, and one of them was a recurrence. The major difference was the patients’ ages at diagnosis, which were 39 and 61 years. In our study, the most striking increases in postoperative VDE were seen in the youngest patients, and one may therefore postulate that, similar to basal tumor growth, postoperative tumor growth may be highly dependent on patient's age. Accordingly, Ito et al.12 showed that the only factor influencing tumor growth after treatment, either by partial surgery or radiosurgery, was the patient's age.
Growth Mechanism
One can speculate on 2 main mechanisms underlying these changes. On one hand, a pure mechanic effect could be suggested. Because both tumors compress the brainstem, the importance of the global tumor burden in the posterior fossa could slow down tumor growth. Therefore, surgery of one of the VSs might accelerate the growth rate of the remaining tumor as a result of the absence of counter-pressure. On the other hand, the role of paracrine factors has been proposed to explain growth of NF2-associated tumors. Pallini et al.22 described a patient who harbored bilateral VS and a parasellar meningioma, whose VS started to grow rapidly once the meningioma approached the ipsilateral schwannoma at the incisura. An epidermal growth factor-like molecule found in the patient's cerebrospinal fluid (CSF) suggested that an autocrine/paracrine mechanism might have triggered schwannoma growth. In our study, we showed that contact of 2 NF2-related tumors can indeed increase the growth rate of both tumors. We postulate that, after the first surgery, resection of a VS might release such growth factors in the CSF, explaining the observations in our study and the initial growth of both VS in the case report by von Eckardstein and colleagues.21 This mechanism might not be systemic, as shown by the absence of concomitant associated meningioma growth in our cases.
Management
Bilateral VS management in NF2 remains a difficult issue because tumor growth is hardly predictable and entails a risk of complete deafness. If both tumors are small at presentation, hearing-preserving surgery or conservative management may be advocated. But if patients present with large tumors that threaten the brainstem, decompressive surgery quickly becomes essential for the largest tumor in the less useful ear and management of the remaining VS may become difficult. In this study, we demonstrated that, if both VSs compress the brainstem, the tumor growth rate of the remaining VS increases after surgery of the contralateral tumor. This acceleration of growth of the second VS should be mentioned to the patient before surgery to prevent secondary disillusionment, because hearing difficulties heavily impact quality of life for patients with NF2, and social communication problems may outweigh any other consequences of the disease.23,24 Moreover, this accelerated growth could explain why radiosurgery is less effective in treating NF2 VS than for sporadic tumor, especially because younger patient age was found to be a predictor of poor tumor control in several studies.25–27 Our results underline the importance of regular radiological follow-up after the first side surgery (every 6 months). They incite focus on this particular time point in the natural history of VS in future clinical trials. This population should be analyzed by specific trials aiming to delay hearing loss in the remaining ear, especially in young patients.
Supplementary Material
Conflict of interest statement. None declared.
Funding
No funding supported this research.
Supplementary Material
Acknowledgments
We thank Dr. François Ducray for his helpful comments and suggestions on the manuscript.
References
- 1.Evans DG, Moran A, King A, Saeed S, Gurusinghe N, Ramsden R. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005;26(1):93–97. doi: 10.1097/00129492-200501000-00016. doi:10.1097/00129492-200501000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Brackmann DE, Fayad JN, Slattery WH, 3rd, et al. Early proactive management of vestibular schwannomas in neurofibromatosis type 2. Neurosurgery. 2001;49(2):274–280. doi: 10.1097/00006123-200108000-00007. discussion 280–273. [DOI] [PubMed] [Google Scholar]
- 3.Samii M, Matthies C, Tatagiba M. Management of vestibular schwannomas (acoustic neuromas): auditory and facial nerve function after resection of 120 vestibular schwannomas in patients with neurofibromatosis 2. Neurosurgery. 1997;40(4):696–705. doi: 10.1097/00006123-199704000-00007. discussion 705–696 doi:10.1097/00006123-199704000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Flickinger JC, Kondziolka D, Niranjan A, Lunsford LD. Results of acoustic neuroma radiosurgery: an analysis of 5 years' experience using current methods. J Neurosurg. 2001;94(1):1–6. doi: 10.3171/jns.2001.94.1.0001. [DOI] [PubMed] [Google Scholar]
- 5.Evans DG, Kalamarides M, Hunter-Schaedle K, et al. Consensus recommendations to accelerate clinical trials for neurofibromatosis type 2. Clin Cancer Res. 2009;15(16):5032–5039. doi: 10.1158/1078-0432.CCR-08-3011. doi:10.1158/1078-0432.CCR-08-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plotkin SR, Singh MA, O'Donnell CC, Harris GJ, McClatchey AI, Halpin C. Audiologic and radiographic response of NF2-related vestibular schwannoma to erlotinib therapy. Nat Clin Pract Oncol. 2008;5(8):487–491. doi: 10.1038/ncponc1157. doi:10.1038/ncponc1157. [DOI] [PubMed] [Google Scholar]
- 7.Mautner VF, Nguyen R, Kutta H, et al. Bevacizumab induces regression of vestibular schwannomas in patients with neurofibromatosis type 2. Neuro Oncol. 2010;12(1):14–18. doi: 10.1093/neuonc/nop010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slattery WH, 3rd, Fisher LM, Iqbal Z, Oppenhiemer M. Vestibular schwannoma growth rates in neurofibromatosis type 2 natural history consortium subjects. Otol Neurotol. 2004;25(5):811–817. doi: 10.1097/00129492-200409000-00027. doi:10.1097/00129492-200409000-00027. [DOI] [PubMed] [Google Scholar]
- 9.Abaza MM, Makariou E, Armstrong M, Lalwani AK. Growth rate characteristics of acoustic neuromas associated with neurofibromatosis type 2. Laryngoscope. 1996;106(6):694–699. doi: 10.1097/00005537-199606000-00007. doi:10.1097/00005537-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Baser ME, Makariou EV, Parry DM. Predictors of vestibular schwannoma growth in patients with neurofibromatosis type 2. J Neurosurg. 2002;96(2):217–222. doi: 10.3171/jns.2002.96.2.0217. [DOI] [PubMed] [Google Scholar]
- 11.Mautner VF, Baser ME, Thakkar SD, Feigen UM, Friedman JM, Kluwe L. Vestibular schwannoma growth in patients with neurofibromatosis type 2: a longitudinal study. J Neurosurg. 2002;96(2):223–228. doi: 10.3171/jns.2002.96.2.0223. [DOI] [PubMed] [Google Scholar]
- 12.Ito E, Saito K, Yatsuya H, Nagatani T, Otsuka G. Factors predicting growth of vestibular schwannoma in neurofibromatosis type 2. Neurosurg Rev. 2009;32(4):425–433. doi: 10.1007/s10143-009-0223-3. doi:10.1007/s10143-009-0223-3. [DOI] [PubMed] [Google Scholar]
- 13.Grayeli AB, Kalamarides M, Bouccara D, Ambert-Dahan E, Sterkers O. Auditory brainstem implant in neurofibromatosis type 2 and non-neurofibromatosis type 2 patients. Otol Neurotol. 2008;29(8):1140–1146. doi: 10.1097/MAO.0b013e31818b6238. doi:10.1097/MAO.0b013e31818b6238. [DOI] [PubMed] [Google Scholar]
- 14.Baser ME, Mautner VF, Parry DM, Evans DG. Methodological issues in longitudinal studies: vestibular schwannoma growth rates in neurofibromatosis 2. J Med Genet. 2005;42(12):903–906. doi: 10.1136/jmg.2005.031302. doi:10.1136/jmg.2005.031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris GJ, Plotkin SR, Maccollin M, et al. Three-dimensional volumetrics for tracking vestibular schwannoma growth in neurofibromatosis type II. Neurosurgery. 2008;62(6):1314–1319. doi: 10.1227/01.neu.0000333303.79931.83. discussion 1319–1320 doi:10.1227/01.neu.0000333303.79931.83. [DOI] [PubMed] [Google Scholar]
- 16.Mandonnet E, Delattre JY, Tanguy ML, et al. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol. 2003;53(4):524–528. doi: 10.1002/ana.10528. doi:10.1002/ana.10528. [DOI] [PubMed] [Google Scholar]
- 17.Kanzaki J, Tos M, Sanna M, Moffat DA, Monsell EM, Berliner KI. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol. 2003;24(4):642–648. doi: 10.1097/00129492-200307000-00019. discussion 648–649 doi:10.1097/00129492-200307000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda M, Oishi M, Hiraishi T, Natsumeda M, Fujii Y. Clinicopathological factors related to regrowth of vestibular schwannoma after incomplete resection. J Neurosurg. 2011;114(5):1224–1231. doi: 10.3171/2010.11.JNS101041. [DOI] [PubMed] [Google Scholar]
- 19.Saito K, Kato M, Susaki N, Nagatani T, Nagasaka T, Yoshida J. Expression of Ki-67 antigen and vascular endothelial growth factor in sporadic and neurofibromatosis type 2-associated schwannomas. Clin Neuropathol. 2003;22(1):30–34. [PubMed] [Google Scholar]
- 20.Hadfield KD, Smith MJ, Urquhart JE, et al. Rates of loss of heterozygosity and mitotic recombination in NF2 schwannomas, sporadic vestibular schwannomas and schwannomatosis schwannomas. Oncogene. 2010;29(47):6216–6221. doi: 10.1038/onc.2010.363. doi:10.1038/onc.2010.363. [DOI] [PubMed] [Google Scholar]
- 21.von Eckardstein KL, Beatty CW, Driscoll CL, Link MJ. Spontaneous regression of vestibular schwannomas after resection of contralateral tumor in neurofibromatosis type 2. J Neurosurg. 2010;112(1):158–162. doi: 10.3171/2009.5.JNS09240. [DOI] [PubMed] [Google Scholar]
- 22.Pallini R, Tancredi A, Casalbore P, et al. Neurofibromatosis type 2: growth stimulation of mixed acoustic schwannoma by concurrent adjacent meningioma: possible role of growth factors. Case report. J Neurosurg. 1998;89(1):149–154. doi: 10.3171/jns.1998.89.1.0149. [DOI] [PubMed] [Google Scholar]
- 23.Neary WJ, Hillier VF, Flute T, Stephens SD, Ramsden RT, Evans DG. The relationship between patients' perception of the effects of neurofibromatosis type 2 and the domains of the short form-36. Clin Otolaryngol. 2010;35(4):291–299. doi: 10.1111/j.1749-4486.2010.02176.x. doi:10.1111/j.1749-4486.2010.02176.x. [DOI] [PubMed] [Google Scholar]
- 24.Neary WJ, Hillier VF, Flute T, Stephens D, Ramsden RT, Evans DG. Use of a closed set questionnaire to measure primary and secondary effects of neurofibromatosis type 2. J Laryngol Otol. 2010;124(7):720–728. doi: 10.1017/S0022215110000460. doi:10.1017/S0022215110000460. [DOI] [PubMed] [Google Scholar]
- 25.Mathieu D, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for vestibular schwannomas in patients with neurofibromatosis type 2: an analysis of tumor control, complications, and hearing preservation rates. Neurosurgery. 2007;60(3):460–468. doi: 10.1227/01.NEU.0000255340.26027.53. discussion 468–470. [DOI] [PubMed] [Google Scholar]
- 26.Phi JH, Kim DG, Chung HT, Lee J, Paek SH, Jung HW. Radiosurgical treatment of vestibular schwannomas in patients with neurofibromatosis type 2: tumor control and hearing preservation. Cancer. 2009;115(2):390–398. doi: 10.1002/cncr.24036. doi:10.1002/cncr.24036. [DOI] [PubMed] [Google Scholar]
- 27.Rowe JG, Radatz MW, Walton L, Soanes T, Rodgers J, Kemeny AA. Clinical experience with gamma knife stereotactic radiosurgery in the management of vestibular schwannomas secondary to type 2 neurofibromatosis. J Neurol Neurosurg Psychiatry. 2003;74(9):1288–1293. doi: 10.1136/jnnp.74.9.1288. doi:10.1136/jnnp.74.9.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.