Abstract
Medulloblastomas are the most common malignant tumors of the central nervous system during childhood. Radiation-induced medulloblastoma tumor recurrences are aggressive and metastatic in nature. In the present study, we demonstrate that Gadd45a expression can sensitize medulloblastoma cells to radiotherapy. We have elucidated the role of Gadd45a in ionizing radiation (IR)–induced G2-M arrest and invasion and metastatic potential of the medulloblastoma cancer cell lines DAOY and D283. We demonstrate that Gadd45a is induced by IR and results in p53 phosphorylation. The role of IR-induced Gadd45a in G2-M arrest is demonstrated by fluorescence-activated cell sorting analysis in the cells treated with siRNA Gadd45a and Ov-exp Gadd45a. We show that Ov-exp Gadd45a aggravates G2-M blockage and also increases binding of Gadd45a to Cdc2 by immunocytochemistry analysis. Furthermore, we show the anti-tumorigenic role of Gadd45a to be mediated by the negative regulation of IR-induced cancer cell invasion and migration-associated proteins, such as matrix metallopeptidase (MMP)–9 and β-catenin. When compared with IR treatment alone, Ov-exp Gadd45a plus IR treatment resulted in decreased nuclear localization and increased membrane localization of β-catenin, and this was further confirmed by membrane distribution. We also show that Ov-exp Gadd45a resulted in downregulation of MMP-9 and suppression of epithelial-mesenchymal transition (EMT). Alternatively, inhibition of MMP-9 (pM) resulted in upregulation of Gadd45a and suppression of EMT. The anti-tumor effect of pM was correlated with increased expression of Gadd45a protein in nude mice intracranial tumors. Taken together, our studies demonstrate that upregulation of Gadd45a or suppression of MMP-9 (pM) with IR retards medulloblastoma tumor metastatic potential.
Keywords: Epithelial-mesenchymal transition (EMT), ionizing radiation (IR), matrix metallopeptidase 9 (MMP-9), overexpression of Gadd45a (Ov-exp Gadd45a), β-catenin
Medulloblastoma is the most common infratentorial malignant tumor, with an incidence of 19%–20% among children aged <15 years.1 Surgical resection followed by craniospinal irradiation has been the mainstay of medulloblastoma therapy for many years.2 Prognosis is worse for the patients who have metastatic disease, who have unresectable tumors, or who are very young.3 Treatment of medulloblastoma has improved but is associated with significant cognitive morbidity.4 High-energy rays in radiotherapy cause cellular DNA damage and lead to decreased tumor development5 but, in turn, activate cancer cell invasion proteins such as matrix metallopeptidase (MMP)-9,6 leading to sporadic late recurrences. A significant number of retrospective studies have reported cancer recurrences cases with radiotherapy.7,8 Therefore, a greater understanding of the oncogenic pathways leading to medulloblastoma recurrence and its invasion is crucial to identify specific molecular targets with significant therapeutic implications.
In the present study, we attempted to elucidate the role of Gadd45a in ionizing radiation (IR)–induced G2-M arrest, invasion, and metastasis of medulloblastoma cancer cell lines, DAOY and D283. Gadd45a, a DNA-damage-inducible, stress sensor protein, has been implicated in maintenance of genomic fidelity and DNA repair and suppression of cell growth9 in response to a number of DNA-damaging agents and genotoxic stresses, including ultraviolet (UV) radiation, receipt of DNA alkylating agents, and ionizing radiation.10,11 Gadd45a null mice generated by gene targeting exhibit severe genomic instabilities and show susceptibility to DNA damage-induced tumors, including carcinogenesis induced by ionizing radiation and UV radiation.9,12 Interestingly, Gadd45a is the only member of the Gadd gene group that is frequently inducible by ionizing radiation in human cells with wild-type p53.13,14
Wang et al.15 were the first to show that microinjection of the Gadd45a expression into normal human fibroblasts could block cells at the G2-M phase, indicating that Gadd45a may cause cell cycle arrest at the G2-M phase. Additional studies elucidated its role in the G2-M cell cycle checkpoint, and findings were attributed to the capacity of Gadd45a to bind Cdc2 and inhibit Cdc2 kinase activity.16,17 The Cdc2-binding domain of Gadd45a is located at the central region of this protein (amino acids 65–84), and deletion of this central region in the Gadd45a protein halts Gadd45a-mediated G2-M cell cycle arrest and Gadd45a-induced growth suppression, indicating that G2-M arrest is one of the major mechanisms by which Gadd45a suppresses cell growth.18
Currently, the most compelling finding on the biological function of Gadd45a is that the defect of Gadd45a is not only closely associated with genomic instability, but also tumorigenesis. Gadd45a has been identified as a key factor at protecting the epidermis against UV-induced skin tumors.12 High-frequency mutations of the Gadd45a gene in pancreatic cancer and abnormal methylation of the Gadd45a promoter in breast cancer have been identified, which further strengthen its link to cancer.19,20 All of these studies suggested that Gadd45a might play an important role in the negative regulation of cell malignancy. However, the underlying mechanisms by which Gadd45a regulates malignancy are still poorly understood.
Most recently, Gadd45a has been attributed to regulating MMPs, which promote cell migration, invasion, and metastasis.21 According to Hildesheim et al.,22 Gadd45a induced by UV radiation regulates MMP expression by suppression of β-catenin levels in the cytoplasm, via the activation of adenomatous polyposis coli (APC) destruction complex. Free cytoplasmic levels of β-catenin result in the accumulation of β-catenin in the nucleus, where it functions as a bipartite transcription factor complex with the T cell factor/lymphocyte enhancer binding factor (Tcf/lef). Targets of Tcf/lef include invasion and metastasis promoting endopeptidases and MMPs.23 Ji et al.24 demonstrated that Gadd45a is involved in the control of cell contact inhibition (CCI) and cell-cell adhesion (CCA) by inducing β-catenin translocation to the cell membrane. β-catenin plays an important role in maintaining CCA and CCI by linking cadherins to the actin cytoskeleton through its interaction with the intracellular tail of cadherins.25 The loss of cadherins/β-catenin complex function leads to disruption of CCI and CCA, which is important for cell malignancy and cell invasion.26,27 A previous study demonstrated that nerve growth factor-induced Gadd45a expression is independent of JNK and p38 MAPK in medulloblastoma (D283-TrkA) cells.28 However, to our knowledge, no detailed studies have been done that show the role of Gadd45a in medulloblastoma. As a continuation of our previous studies, in which we showed that downregulation of MMP-9 (pM) induces G2-M arrest,29 we demonstrate in this study that Gadd45a expression can sensitize medulloblastoma cells to radiotherapy and that induction of Gadd45a expression by pM in combination with radiotherapy has therapeutic implications.
Materials and Methods
Cell Culture, Chemical Reagents, Transfection Conditions, and Radiation
Human medulloblastoma cell lines DAOY and D283 were purchased from ATCC and maintained in serum containing advanced minimum essential medium (MEM) or improved MEM, respectively, in a 37°C incubator with 5% CO2 humidified atmosphere. The following antibodies were purchased from Santa Cruz Biotechnology: anti-p53, p-p53 (Ser15), Cdc2, p-Cdc2 (Thr14/Tyr15), Gadd45a, β catenin, MMP-9, N-cadherin, E-cadherin, lamin B, flotillin-2, LEF-1, GAPDH and species-specific secondary antibodies conjugated to HRP, and Alexa Fluor 488 and 595; anti-p-β catenin (Ser33/37/Thr41) was purchased from Cell Signaling. Cells were transfected either with shRNA against MMP-9 (pM)30 or with Gadd45a siRNA (h) (sc-35440, Santa Cruz Biotechnology) for knockdown of MMP-9 and Gadd45a, respectively. The human cDNA clone of GFP-tagged Gadd45a, pCMV6-AC-GFP (RG204005, OriGene Technologies), was used for overexpression of Gadd45a (Ov-exp Gadd45a), and purified human MMP-9 (rMMP-9) was obtained from Chemicon International. All transfection experiments were performed using FuGENE HD transfection reagent (Roche Diagnostics) in accordance with the manufacturer's instructions. Control cells were processed in the same way as treated cells and were incubated with either equal volumes of FuGENE HD or transfected with pSV (Scrambled Vector). Radiation treatment was given using the RS 2000 Biological Irradiator X-ray unit (Rad Source Technologies). For the combination treatment (transfection and radiation), cells were irradiated at 8 Gy after 48 h of transfection and incubated for another 16–24 h. The proteins for immunoblot analysis were processed using a protease inhibitor cocktail (Sigma Aldrich).
Cell Proliferation and Viability and TUNEL Assays
Cell growth was assessed using a MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] assay (Sigma Aldrich), as described previously by Rao et al.31 To determine the cell number and the effect of radiation on cell viability, we performed the trypan blue exclusion assay. The cells were suspended in equal volume of trypan blue stain (0.4% w/v), incubated for 5 min, and counted using the Countess Automated Cell Counter (Invitrogen). The TUNEL assay was done using in situ cell death detection kit (Roche) in accordance with the manufacturer's protocol. In brief, treated DAOY and D283 cells for 72 h in 8-well chambered slides were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Next, cells were incubated with a TUNEL reaction mixture for 60 min at 37°C in a humidified incubator. The slides were washed 3 times with phosphate-buffered saline (PBS), and the incorporated biotin-dUTP was detected under a fluorescence microscope.
Cell Cycle Analysis by Fluorescence-Activated Cell Sorting (FACS)
The treated cells were harvested by trypsinization and stained with propidium iodide (PI) (2 mg/mL; Biosure). Suspensions of 1 × 106 cells were analyzed by FACS Caliber System (Becton Dickinson Bioscience) with laser excitation at 488 nm and emission at 639 nm. The percentages of cells in the various phases of the cell cycle (G0/G1, S, and G2/M) were assessed using Cell Quest software (Becton Dickinson Bioscience). The immunofluorometric analysis of Gadd45a protein was performed by incubating the treated cells with primary anti-Gadd45a antibody (1:250) followed by species-specific green Alexa Fluor 488 conjugated secondary antibody, in accordance with standard protocol.
Matrigel Invasion and Wound-Healing Assays
Matrigel invasion assay was performed by following a previously established protocol.31 In brief, 1 × 105 and 2 × 106 control, IR-treated or transfected DAOY and D283 cells, respectively, were plated onto Matrigel-coated transwell inserts that were placed in a 12-well plate containing complete medium. Twenty-fourh after incubation, lower invaded cells were fixed and stained with HEMA-3. The noninvaded cells in the upper chamber were washed using a cotton swab to enable a clear view of the invaded cells. Images of the invaded cells were taken under a light microscope (Olympus IX-71). For the wound healing migration assay, the control and treated cells were grown to full confluence in a 6-well plate to form a monolayer, and a straight scratch was made manually in individual wells using a 200-μL pipette tip, as described previously.30 This point was considered the “0 h” and the width of the wound was immediately photographed under the light microscope. The cells were further incubated for about 12–16 h.
Clonogenic Assay
Clonogenic assay or colony formation assay was performed according to the Franken et al.32 protocol, with slight modification. In brief, single cell suspension of DAOY- and D283-treated cells was prepared. Approximately 500 and 1000 DAOY- and D283-treated cells, respectively, were individually plated in 60-mm plates, spread well, and incubated for 10–15 days until cells in control plates had formed sufficiently large colonies. Next, only DAOY cells were stained with HEMA-3 for 10 min, and colonies were counted and graphically represented. Images were taken directly for D283 colonies without staining, using the standard 200-μm scale bar.
Extraction of Nuclear Proteins and Phase Partitioning Using Triton X-114
Nuclear extracts were prepared using a nuclear extraction kit from Panomics in accordance with the manufacturer's instructions. Phase partitioning for separation of membrane and cytoplasmic proteins was done using the nonionic detergent Triton X-114, as described by Brusca and Radolf,33 with some modifications. The total number of cells in a 100-mm dish with 80%–85% confluence were suspended in 1 mL of ice cold 1X PBS with 1 mM PMSF/10 mM EDTA and disrupted with a glass-glass Potter homogenizer. The cell homogenate was centrifuged at 6000 g for 15 min at 4°C to remove insoluble debris, and Triton X-114 was added to a final concentration of 2%. The solution was vigorously mixed, kept on ice for 20 min, and incubated at 37°C for 15 min. Phase separation was accelerated by centrifuging the sample at 300 rpm for 5 min at room temperature. Finally, proteins from the aqueous and detergent phases were precipitated with 10 volumes of acetone at −20°C for 1 h and pelleted at 14, 000 g for 20 min. The protein concentration was estimated using the bicinchoninic acid procedure (Pierce).
Immunoblotting, Immunoprecipitation Assay and Gelatin Zymograph
Western blot analysis was done using equal amounts of protein separated by SDS-PAGE, transferred onto nitrocellulose membrane (Biorad), incubated with 1:500 dilution of primary antibodies, and subsequently incubated with 1:1000 dilution of species-specific, horseradish peroxidase (HRP)-conjugated secondary antibody according to the standard protocol. Immunoprecipitation assays were performed by incubating ∼400 µg of membrane extracts with the required specific primary antibody (2 µg) overnight at 4°C on a rotating shaker. Approximately 40 μL of protein A/G agarose beads (Miltenyi Biotec) were added to the aforementioned complex and incubated in ice for 30 min. These beads were passed through µM columns, and the bound immunoprecipitates were eluted according to the manufacturer's instructions. The immunoprecipitates were then immunoblotted using specific primary antibodies. Equal volumes of conditioned medium collected from the treated cells were subjected to electrophoresis on 10% acrylamide gels containing 0.5 mg/mL gelatin to detect MMP-9 activity, as described previously.30 Conditioned media of D283 cells were processed using Amicon ultra-4 centrifugal filter units (Millipore) of 30 kDa cut-off size. Approximately 100 µg of protein were used for gelatin zymography of D283 cells.
p53 Transcription Factor Assay
p53 activation in nuclear extracts of DAOY and D283 cells was monitored using the ELISA-based TransAM p53 kit (Active Motif) according to the manufacturer's protocol. Fiveμg of total nuclear protein were loaded onto a 96-well plate coated with an immobilized oligonucleotide containing a p53 consensus binding site. Nuclear extract of H2O2-treated MCF7 cells (provided by the manufacturer) served as a positive control. Anti-p53 and anti-rabbit HRP were used to quantify the amount of bound p53 protein. The HRP signal was developed by a substrate provided by the manufacturer, and samples were analyzed with an ELISA reader at 450 nm with a reference wavelength of 655 nm. The optical density value for negative control was deducted from all the test samples, including the positive control.
Reverse-Transcription Polymerase Chain Reaction (PCR)
Total RNA was isolated from control and treated cells using TRIZOL reagent (Invitrogen) according to the standard protocol. With 1 μg of RNA used as a template, first-strand cDNA was synthesized using Superscript III reverse transcriptase (Roche Applied Science). PCR analysis using 100 ng of first-strand cDNA was completed using specific primers (Table 1). GAPDH was used as an internal control. The PCR reaction was performed using standard conditions in an AB Applied Biosystems Thermocycler (model 9700). PCR-amplified products were electrophoresed on 1.5% agarose gel.
Table 1.
Primer sequences
| Gene name | Forward primer | Reverse primer |
|---|---|---|
| Gadd45a | 5′ ggaggaagtgctcagcaaag 3′ | 5′gcaggatccttccattgaga 3′ |
| β-catenin | 5′ ggtggaccccaagctttagt 3′ | 5′ agtgggatggtgggtgtaag 3′ |
| MMP-9 | 5′ catttcgacgatgacgagttgt 3′ | 5′ cgggtgtagagtctctcgc 3′ |
| N-cadherin | 5′ cctcagtatgtgggaaagctc 3′ | 5′ tggatgagtacttgatattgttctttg 3′ |
| E-cadherin | 5′ accacgtacaagggtcaggt 3′ | 5′ ccgatatgttattttctgttcca 3′ |
| GAPDH | 5′ tgttgccatcaatgacccctt3′ | 5′ ctccacgacgtactcagcg 3′ |
Immunofluorescence
For immunocytochemical analysis, ∼3000 cells were seeded onto 2-well chamber slides. Cells were transfected and/or treated with radiation and then incubated for at least 48–72 h. After the incubation period, cells were washed, fixed, permeabilized with ice-cold methanol, and blocked for 1 h using 3% bovine serum albumin in PBS. Cells were incubated with primary antibodies overnight at 4°C, washed with PBS and incubated with fluorescent-labeled, species-specific secondary antibodies (Alexa Fluor) at 1:500 dilution for 1 h at room temperature. Before mounting, the slides were washed with PBS and incubated for 5 min with a 1:100 dilution of 4′-6-diamidino-2-phenylindole (DAPI) for nuclear staining and analyzed using confocal microscopy (Olympus BX61 Fluoview) at 40× magnification. For immunohistochemical analysis, paraffin-embedded brain sections (5 µm thick) from control and treated animal groups were deparaffinized in xylene and rehydrated through graded ethanol. Antigen retrieval was performed by treatment of the sections with 10 mM of citrate buffer (pH 6) at boiling temperature for 1 h. Sections were blocked and followed by treatment with primary and species-specific, Alexa Fluor secondary antibodies, as described above in the section on immunocytochemical analysis.
In Vivo Studies
DAOY cells (1 × 105) were injected intracerebrally into nude mice under isofluorane anesthesia with the aid of a stereotactic frame, as described earlier.31 After allowing the tumors to establish for 8–10 days, the animals were separated into different treatment groups (a minimum of 5 in each group). pM (shRNA for MMP-9) and pSV (scrambled vector) were delivered into the brains of nude mice using Alzet osmotic mini pumps (model 2001, Alzet Osmotic Pumps). One week after plasmid delivery, animals were treated with radiation. On the basis of their performance and behavior, the animals were euthanized and sacrificed by intracardiac perfusion (first with PBS and then with 4% paraformaldehyde in normal saline). The brains were excised and fixed in 10% buffered formaldehyde, processed, embedded in paraffin, and sectioned (5 μm thick) using a microtome. Paraffin-embedded tissue sections were subjected to hematoxylin and eosin (H&E) staining for visualization of tumor cells, as described elsewhere.31 Tumor volume was calculated from the formula 1/6 II (Rmax) × (Rmin)2, where Rmax and Rmin are the maximum and minimum tumor radii, respectively.
Statistical Analysis
All the Western blot, reverse-transcription PCR, and zymography data were quantified by measuring the band/signal intensity by densitometry analysis using ImageJ software (National Institutes of Health). Results were analyzed using the one-way analysis of variance followed either by Bonferroni's post hoc test (multiple comparison tests, using GraphPad Prism software, version 3.02) or t test to assess statistical significance. The difference for either test was considered statistically significant with a P value <.05.
Results
Antiproliferative Role of Gadd45a
IR treatment induces G2-M arrest accompanied by an increase in Gadd45a expression
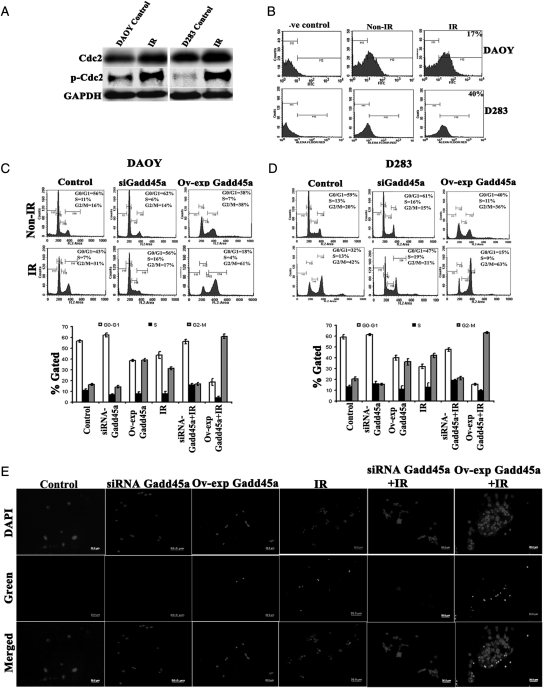
FACS analysis of PI-stained DAOY and D283 cells treated with IR showed G2-M arrest. Additional analysis of cell cycle associated molecules such as Cdc2, pCdc2, and Gadd45a protein showed increased expression after IR treatment, when compared to the non-IR control cells (Fig. 1A and B). The immuno flow cytometry analysis of IR-treated DAOY and D283 cells showed a 17% and 40% increase in Gadd45a expression, respectively (Fig. 1B). Furthermore, to determine the role of Gadd45a associated with IR, FACS analysis was performed in the cells with knockdown (siRNA Gadd45a) and overexpression of Gadd45a (Ov-exp Gadd45a) alone or in combination with IR treatment. With IR treatment, 31% and 42% of DAOY and D283 cells, respectively, were in the G2-M phase (M4). Knockdown of Gadd45a (siRNA Gadd45a) with IR treatment showed 17% of DAOY and 21% of D283 cells in the G2-M phase. In contrast, overexpression of Gadd45a (Ov-exp Gadd45a) with IR treatment showed 61% of DAOY and 63% of D283 cells in the G2-M phase, which indicates increased G2-M arrest (Fig. 1C and D). A TUNEL assay was performed using DAOY and D283 cells treated with siRNA Gadd45a and Ov-exp Gadd45a, alone and in combination with IR treatment. DAOY cells revealed no apoptosis with these treatments (Supplementary material, Fig. S1C), whereas D283 cells showed very little increase in the apoptotic cells and that further increased with Ov-exp Gadd45a with IR treatment (Fig. 1E).
Fig. 1.
Analysis of cell cycle proteins involved in G2-M arrest after ionizing radiation (IR) treatment. (A) Western blot analysis for Cdc-2 and pCdc-2 protein using 40 µg of total cell lysates from non–IR- and IR-treated DAOY and D283 cells. Equal loading of these proteins was confirmed by glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (B) Immuno-flow cytometry analysis of Alexa Fluor-labeled Gadd45a in DAOY and D283 cells. Approximately 1 × 106 non–IR- and IR-treated cells were trypsinized, washed in phosphate-buffered saline (PBS), incubated with anti-Gadd45a antibody overnight at 4°C, and labeled with green Alexa Fluor 488, a fluorescent-labeled, species-specific secondary antibody (Invitrogen) for 1 h at room temperature. Normal immunoglobulin (Ig) G was used as a negative control to set the cut-off point for green fluorescence intensity. (C and D) Fluorescence-activated cell sorting (FACS) analysis of cell cycle progression using knockdown and overexpression of Gadd45a with IR treatment was done as described in Materials and Methods. The y axis denotes cell count and the x axis represents DNA content. The percentages of cells in the G1 (M2), S (M3) and G2/M (M4) phases of the cell cycle were calculated using CellQuest Pro software. Graphical representation of FACS data from 3 independent experiments is shown as the mean ± SD (P< .05). (E) TUNEL nuclear staining on D283 cells. Control cells with knockdown (siRNA Gadd45a) and overexpression of Gadd45a (Ov-exp Gadd45a) in combination with IR treatment on 8-well chambered slides were subjected to TUNEL nuclear staining (Roche Applied Science) and viewed by fluorescence microscopy. Green fluorescence represents apoptotic cells (For the color figure, please refer to supplementary material, Fig. S1).
Gadd45a inhibits Cdc2 kinase activity with IR treatment
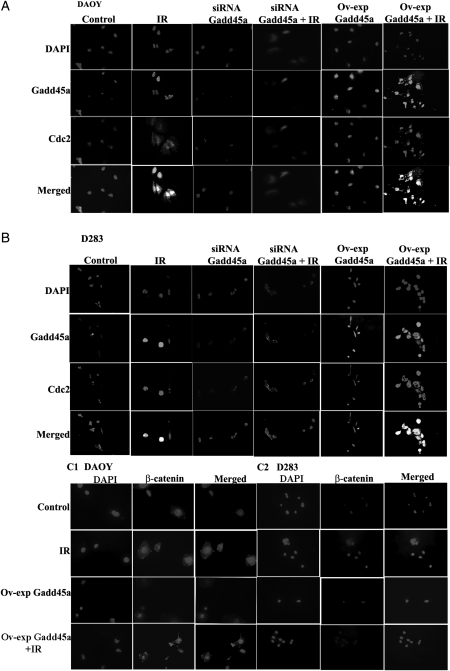
Immunocytochemical and immunoprecipitation analyses using anti-Gadd45a and anti-Cdc2 antibodies in DAOY and D283 cell lines demonstrated that Gadd45a binds to Cdc2 with IR treatment (Fig. 2A and B and Supplementary material, Fig. S1D). We observed that overexpression of Gadd45a accompanied with IR treatment increased its binding to Cdc2. The co-localization of Gadd45a with Cdc2 after IR treatment was more prominent in the nucleus, compared with non–IR-treated cells.
Fig. 2.
Co-localization of Gadd45a with Cdc2 and nuclear translocation of β-catenin with ionizing radiation (IR) treatment. (A and B) Immunocytochemistry analysis was carried out on DAOY (A) and D283 (B) transfected (siRNA GAdd45a and Ov-exp Gadd45a) and IR-treated cells. Microscopic images depict expression of Gadd45a (green fluorescence) and Cdc2 (red fluorescence) proteins in the cells with knockdown (siRNA Gadd45a) and overexpression of Gadd45a (Ov-exp Gadd45a) in combination with IR treatment. (C1-C2) Nuclear translocation of β-catenin (red fluorescence) into the nucleus (blue fluorescence) of DAOY and D283 cells with Ov-exp-Gadd45a in combination with IR treatment. Immunostaining was done according to the protocol described in Materials and Methods. Pictures were taken using confocal microscopy (Olympus BX61 Fluoview) at a 40× magnification. (For the color figure, please refer to supplementary material, Fig. S4)
Antitumorigenic Role of Gadd45a
Gadd45a regulates cellular β-catenin distribution
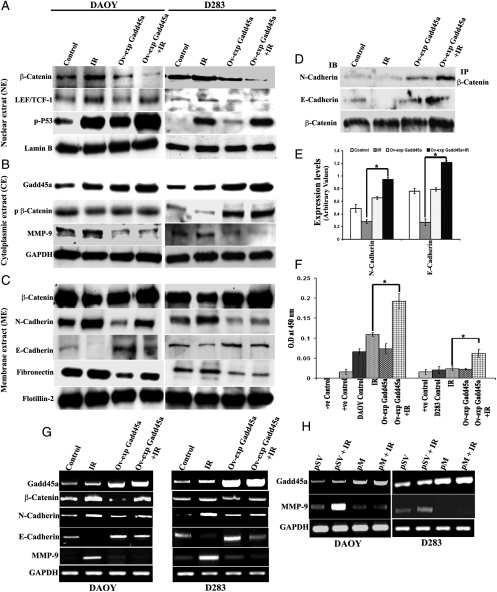
In the aforementioned studies, we demonstrated that Gadd45a expression increased with IR treatment. Furthermore, Gadd45a is known to play a role in cellular distribution of β-catenin. Thus, to determine the role of Gadd45a associated with IR, β-catenin levels were analyzed in the nucleus, cell membrane, and cytoplasm of both DAOY and D283 control cells with overexpression of Gadd45a, alone or in combination with IR treatment. Compared with the control cells, the IR-treated DAOY and D283 cells showed 26% and 24% increased nuclear levels and 26% and 24% decreased membrane levels of β-catenin, respectively (Fig. 3A–C), whereas overexpression of Gadd45a with IR treatment versus IR-treated DAOY and D283 cells showed 41% and 49% increases in membrane localization and 57% and 47% decreases in nuclear localization of β-catenin, respectively. Furthermore, the role of Gadd45a in distribution of β-catenin associated with membrane N- and E-cadherins was also determined by immunoprecipitation (IP) studies. On the basis of the IP studies of membrane proteins using anti-β-catenin antibody, we observed a 39% and 56% increase in binding of β-catenin to N- and, E-cadherins, respectively, with IR treatment accompanied with Gadd45a-overexpressed cells (Fig. 3D and E). The binding of β-catenin to membrane cadherins was negligible in IR-treated cells. IR treatment alone was associated with a 23% increase in N-cadherin levels and a 16% increase in fibronectin levels in both DAOY and D283 cells. Furthermore, IR treatment with overexpression of Gadd45a was associated with a decrease in membrane N-cadherin levels by 26% in DAOY and 34% in D283 cells, whereas fibronectin levels were associated with a 23% and 30% decrease in DAOY and D283 cells, respectively. The levels of E-cadherin with IR treatment were decreased to 35% and 30% in DAOY and D283 cells, respectively. The overexpression of Gadd45a with IR treatment showed a significant increase in membrane E-cadherin levels, compared with the normal IR-treated DAOY and D283 cells (Fig. 3C).
Fig. 3.
Gadd45a regulates distribution of cellular β-catenin. (A) Western blot analysis of nuclear extracts (NEs) to demonstrate β-catenin, LEF-1, and p-p53 expression in DAOY and D283 cells. Nuclear extracts were prepared using nuclear extraction kit (Panomics). Forty µg of total nuclear protein was used for analysis, and Lamin B was used as a nuclear protein marker to confirm equal loads of proteins. (B) Western blot analysis of cytoplasmic extracts (CEs) to demonstrate the levels of Gadd45a, p-β-catenin (Ser33/37/Thr41) and intracellular levels of MMP-9. GAPDH was used to confirm equal loading of proteins. (C) Western blot analysis of membrane extracts (MEs) to demonstrate accumulation of β-catenin on membranes of DAOY and D283 cells. Membrane proteins were isolated using the Triton X-114 phase separation method. Flotillin-2 was used as a membrane marker to confirm equal loading of proteins. (D) Immunoprecipitation (IP) using β-catenin antibody to show its interaction with N- and E-cadherins of treated DAOY cells. Approximately 400 µg of ME protein was immunoprecipitated with 2 µg of β-catenin primary antibody and immunoblotted using anti-N-cadherin, anti-E-cadherin, and anti-β-catenin antibodies. (E) The protein band intensity of IP studies was measured using densitometry and quantified data from 3 different experiments are represented graphically (mean ± SD, P* < .01). (F) Monitoring p53 activation as assessed by p53 DNA binding using TransAM assay (TransAM, p53 Transcription Factor Assay Kit; Active Motif) as described in Materials and Methods. The experiment was performed at least twice in duplicate and results are graphically represented as bar diagrams. Error bars represent mean ± SD (P* < .01). (G) Reverse-transcription polymerase chain reaction (RT-PCR) analysis to determine the β-catenin, N-cadherin, E-cadherin, and matrix metallopeptidase (MMP)–9 transcript levels in DAOY and D283 control, Ov-exp Gadd45a alone and in combination with IR-treated cells. (H) RT-PCR analysis of DAOY and D283 cells transfected with MMP-9 plasmid (pM) and irradiated after 48 h of transfection. PCR analysis was performed using primers specific for Gadd45a, MMP-9, and GAPDH. Total RNA was extracted from treated cells, and cDNA was prepared according to the standard protocols.
In the aforementioned studies, we demonstrated that overexpression of Gadd45a inhibits nuclear translocation of β-catenin. Thus, to determine the role of Gadd45a in degradation of cytoplasmic β-catenin, we performed Western blot analysis of cytoplasmic extracts and showed that, after IR treatment, levels of p-β-catenin decreased by 13% and 32% in DAOY and D283 cells, respectively, whereas overexpression of Gadd45a with IR treatment was associated with a significant increase in the levels of p-β-catenin (Fig. 3B).
The results above were confirmed by reverse-transcription PCR analysis using primers specific for β-catenin, N-cadherin, and E-cadherin in non–IR-treated and IR-treated control and Gadd45a overexpressed DAOY and D283 cells. Reverse-transcription PCR results demonstrated that IR treatment with overexpression of Gadd45a showed a decrease in β-catenin and N-cadherin mRNA transcript levels by 21% and 13% in DAOY cells and 15% and 39% in D283 cells, respectively. Furthermore, the overexpression of Gadd45a with IR treatment was associated with a significant increase in membrane E-cadherin levels, compared with the normal IR-treated DAOY and D283 cells (Fig. 3G).
Overexpression of Gadd45a causes increased phosphorylation of p53
p53, an upstream molecule to Gadd45a, is known to be stabilized by phosphorylation at Ser15. Western blot analysis of nuclear extracts revealed increased levels of p-p53 with IR treatment in both DAOY and D283 cells. Furthermore, nuclear levels of p-p53 increased to 27% and 6% in DAOY and D283 cells, respectively, in Gadd45a-overexpressed cells with IR treatment (Fig. 3A). In addition, we used the TransAM p53 kit to determine the levels of activated p53 in nuclear extracts of DAOY and D283 cells. On the basis of the TransAM p53 kit results, we observed that, with IR treatment, p53 protein binds to an immobilized oligonucleotide probe containing the p53 consensus sequence, and its binding efficiency to this probe increased with overexpression of Gadd45a with IR treatment (Fig. 3F). These results demonstrate a positive feedback loop between Gadd45a and p53 activation.
Overexpression of Gadd45a retards IR-induced MMP-9, cancer cell invasion, and migration by sensitizing the cells to IR
β-Catenin level in the nucleus is known to be proportional to the expression of cell proliferation and invasion proteins. We have observed that overexpression of Gadd45a affects expression of MMP-9, which is a molecule involved in invasion. Intracellular levels of MMP-9 were analyzed by Western blot in both DAOY and D283 cell lines, and our studies demonstrated that, with radiation treatment, MMP-9 levels increased, whereas the overexpression of Gadd45a and radiation treatment resulted in a decrease in MMP-9 levels (Fig. 3B). These results were further confirmed by reverse-transcription PCR analysis in which a decrease in expression levels of MMP-9 mRNA was observed in Gadd45a-overexpressed and IR-treated cells when compared to IR-treated cells (Fig. 3G). Reverse-transcription PCR analysis also revealed that cells with knockdown of MMP-9 showed increased Gadd45a mRNA levels (Fig. 3H).
Immunocytochemical analysis revealed enhanced nuclear translocation of β-catenin in DAOY and D283 cells with IR treatment, compared with control cells, whereas overexpression of Gadd45a with IR treatment in both cell lines demonstrated a clear inhibition of nuclear translocation of β-catenin (Fig. 2C1–C2).
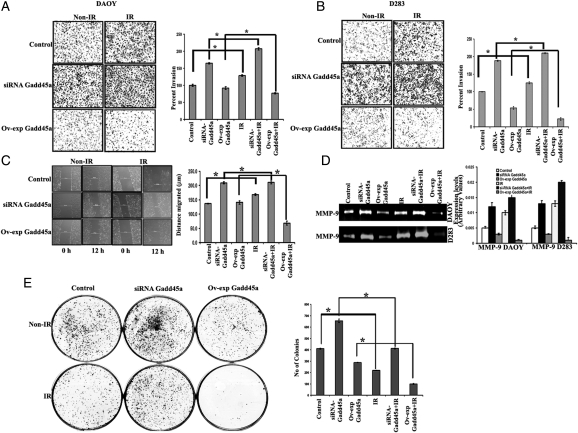
Results of the Matrigel invasion assay and gelatin zymography demonstrated the potential role of Gadd45a in inhibition of IR-induced cell invasion of the cancer cell lines DAOY and D283 (Fig. 4A, B and D). In vitro cancer cell invasion assay using Matrigel-coated transwell inserts showed that the number of cells that invaded through Matrigel increased with IR treatment. Overexpression of Gadd45a in combination with IR treatment demonstrated a marked decrease in the invasive potential, whereas knockdown of Gadd45a in combination with IR treatment was associated with an increase in the invasive potential of DAOY and D283 cells. The extracellular levels of MMP-9 protein in these cells were analyzed using gelatin zymography (Fig. 4D). Zymography results demonstrated that with IR treatment, MMP-9 expression levels increased by 30% and 58% in DAOY and D283 cells, respectively, whereas overexpression of Gadd45a with IR treatment was associated with a 60% and 80% decrease in MMP-9 levels of DAOY and D283 cells, respectively. Furthermore, knockdown of Gadd45a using specific siRNA in combination with IR treatment was associated with upregulation of MMP-9 expression by 30% and 45% in DAOY and D283 cells, respectively, compared with IR-treated cells.
Fig. 4.
Gadd45a-mediated decrease in the invasive, migratory, and proliferative potential of medulloblastoma cell lines. Approximately 1 × 105 DAOY (A) and 2 × 106 D283 (B) cells treated with ionizing radiation (IR), knockdown (siRNA Gadd45a) or overexpression of Gadd45a (Ov-exp Gadd45a) were suspended in serum-free media and plated onto Matrigel-coated transwell inserts, as described in Materials and Methods. After a 24-hr incubation period, lower invaded cells were stained with HEMA-3. Images of invaded cells were taken under a light microscope (Olympus IX-71). The invasive potential of treated cells was quantified, and the percentage of cells invading from 3 independent experiments are graphically represented as bar diagrams. Error bars represent mean ± SD (*P< .05). (C) Wound healing assay, which is indicative of migration potential of cancer cells, was performed using 80%–85% confluent-treated DAOY cells, as described in Materials and Methods. Photographs were taken using standard 200-μm scale bar. Percent wound repair was calculated from the mean of the average width of the wound obtained from 3 independent experiments and graphically represented as bar diagrams. Error bars represent mean ± SD (P*< .05). (D) Gelatin zymography was performed to determine matrix metallopeptidase (MMP)–9 activity in the conditioned media of the aforementioned treated cells. The intensity of clear halo band of MMP-9 in zymography gels was measured using densitometry and graphically represented, and the error bars represent mean ± SD (P < .05, with IR; P < .01 with control). (E) Clonogenic assay for DAOY cells. The control cells treated with siRNA-Gadd45a, Ov-exp Gadd45a, and in combination with IR treatment were trypsinized to produce single-cell suspension. Approximately 500 cells from each treatment were plated individually in 60-mm plates containing complete media. The plates were incubated for 12 days until they formed sufficiently large colonies. Next, the cells were fixed and stained using HEMA-3 stain. The number of colonies were quantified from 3 independent experiments and are graphically represented as the measure of clonogenecity. Error bars represent mean ± SD (P*< .05).
The wound healing assay was used to determine the migration potential of DAOY cells. In our studies, we demonstrated that the migration capacity of cancer cells during the wound healing process increased with IR treatment (Fig. 4C). Furthermore, overexpression of Gadd45a with IR treatment demonstrated a prominent decrease (63%) in the migration rate of DAOY cells. In contrast, knockdown of Gadd45a and IR treatment resulted in a 40% increase in the wound healing capacity of cells, compared with IR-treated control cells. Knockdown of Gadd45a further increased the IR-induced migration rate and resulted in a faster wound closing. Because D283 cells are semi-adherent, the wound healing assay was not appropriate for this cell line.
Furthermore, the clonogenic assay was performed on DAOY (Fig. 4E) and D283 (Supplementary material, Fig. S2) cells with knockdown and overexpression of Gadd45a alone and in combination with IR treatment. Compared with the control DAOY cells, the IR-treated cells revealed a 30% decrease in the colony-forming efficiency, which was further decreased by 70% in cells with Ov-exp Gadd45a with IR treatment. We also observed that the number of clonogenic cells was increased after siRNA treatment targeting Gadd45a.
Gadd45a induces expression of E-cadherin
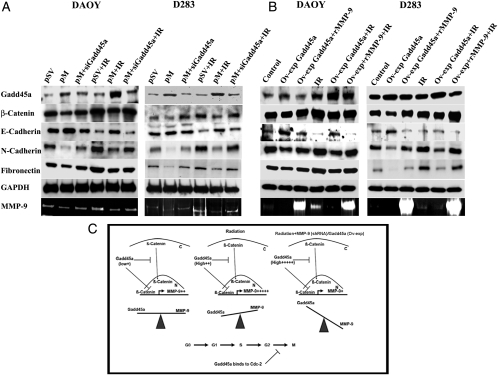
Western blot analysis demonstrated that the knockdown of MMP-9 (pM) with IR treatment causes an increase in the expression levels of E-cadherin by 25% in DAOY and by 21% in D283 cells, compared with IR-treated cells. The levels of N-cadherin decreased by 50% and 30% and fibronectin decreased by 19% and 23% in DAOY and D283 cells, respectively. Furthermore, Western blot analysis demonstrated that the treatment of cells with pM in combination with IR treatment increased the expression levels of Gadd45a in the DAOY and D283 cells by 55% and 40%, respectively. The simultaneous treatment of these cell lines with pM and siRNA for Gadd45a was associated with a decrease in E-cadherin and increases in N-cadherin and fibronectin levels. The N-cadherin levels were increased by 29% and E-cadherin levels were decreased by 31% in DAOY and D283 cells, respectively, compared with the pM with IR-treated cells (Fig. 5A).
Fig. 5.
Role of Gadd45a in suppression of matrix metallopeptidase (MMP)–9 and epithelial-mesenchymal transition (EMT). The DAOY and D283 cells were treated with pM, with pM + siRNA Gadd45a alone, and with IR treatment (A) and were separately treated with Ov-exp Gadd45a, Ov-exp Gadd45a + rMMP-9 alone and in combination with IR treatment (B). The control cells were treated with scrambled vector (pSV) alone and with IR treatment. Western analysis was done using 40 µg of total lysates from the treated cells to show the levels of Gadd45a, β-catenin, E-cadherin, N-cadherin, and fibronectin. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as loading control. Gelatin zymography was performed to determine MMP-9 activity using conditioned media from treated samples. (C) Diagramatic representation showing the role of Gadd45a in the negative regulation of MMP-9 induction with IR treatment; the overexpression of Gadd45a accompanied by IR inhibits MMP-9 induction and nuclear translocation of β-catenin. Compared to the control cells and IR treated cells, the downregulation of MMP-9 (pM) or upregulation of Gadd45a (Ov-exp Gadd45a) accompanied by IR shifts the balance towards Gadd45a and thus has significant therapeutic implications.
Alternatively, overexpression of Gadd45a (Ov-exp Gadd45a) in these cell lines caused the downregulation of MMP-9, as observed by gelatin zymography (Fig. 5B). Furthermore, addition of recombinant MMP-9 (100 ng) to the cells overexpressing Gadd45a with IR treatment showed decrease in the E-cadherin levels by 42% and 40%, an increase in the N-cadherin levels by 14% and 20%, and an increase in fibronectin levels by 19% and 30% in DAOY and D283 cells, respectively.
Suppression of MMP-9 with IR treatment retards medulloblastoma tumor development
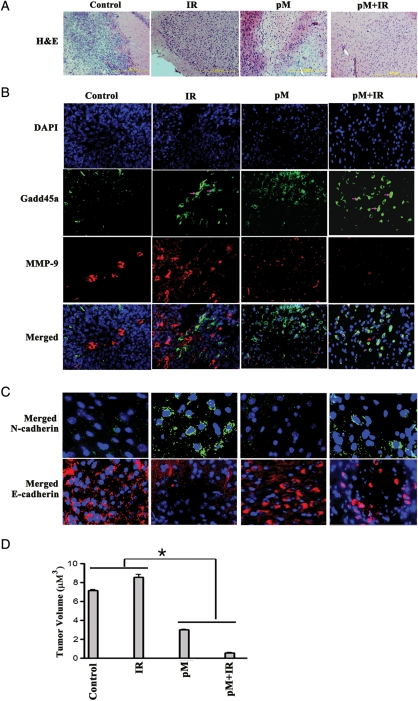
Tumors were raised in the cerebellum region of nude mice and confirmed by H&E staining (Fig. 6A). Immunohistochemical studies were performed to demonstrate MMP-9 (red) and Gadd45a (green) expression in control, IR-, pM-, and pM- plus IR-treated tumors (Fig. 6B). The results demonstrated that, in the control cells, MMP-9 expression was localized into a particular tumor region, compared with IR-treated tumors, whereas expression of MMP-9 was seen over the entire tumor region. Gadd45a expression in control cells was lower than in IR-treated tumors. With IR treatment, nuclear localization of Gadd45a was observed in tumors. The expression of Gadd45a in IR-treated tumors was found to be more in the regions where MMP-9 expression levels were negligible. Tumors treated with pM alone demonstrated an increase in Gadd45a expression, but tumor invasion was not completely halted. Furthermore, pM + IR treatment showed significant increase in Gadd45a expression and showed reduced tumor volume (Fig. 6D). Furthermore, the tumors treated with pM alone and in combination with radiation were associated with low levels of N-cadherin (green) and high levels of E-cadherin (red) proteins (Fig. 6C).
Fig. 6.
Downregulation of MMP-9 (pM) in in vivo tumors increased radioresponse by inducing Gadd45a expression and suppression of epithelial-mesenchymal transition (EMT). Intracerebral tumors were established in nude mice by injecting 1 × 105 DAOY cells. A group of 5 animals was used for each treatment condition (control, IR, pM, and pM + IR). Alzet osmotic pumps (model 2001; Alzet Osmotic Pumps) were implanted into the animals for pM delivery (6–8 mg/kg body weight) followed by IR treatment after 1 week. (A) Brain tissue sections were subjected to hematoxylin and eoisin staining. (B) Immunohistochemical comparison of treated tumor sections using green Alexa Fluor 488 and red Alexa Fluor 594 secondary antibodies for Gadd45a and matrix metallopeptidase (MMP)–9, respectively. (C) Immunohistochemical analysis of the tumor sections to show the levels of EMT markers, N-cadherin (green) and E-cadherin (red). The merged figures are represented here and the individual stained figures are shown in the Supplementary material, Fig. S2. (D) Semiquantification of tumor volume in control, IR, pM, and pM + IR vector-treated groups was done as described in Materials and Methods. Data shown are the mean ± SD values from 3 animals from each group (*P< .005).
Discussion
Genomic instability plays a key role in tumor pathogenesis and progression.34 Regulation of genomic integrity is very crucial for cells and is maintained by regulating cellular processes, such as DNA repair, cell cycle checkpoints, and apoptosis. Deregulation of one or more of these processes may contribute to tumorigenesis. The tumor-suppressor molecule p53 and its downstream molecules have been demonstrated to participate in these biological events.14,35 As the first well-defined p53 downstream gene, Gadd45a has been implicated in the maintenance of genomic fidelity,9 probably via its roles in the control of cell cycle at the G2-M checkpoint, induction of cell death, and DNA repair induced by genotoxic stress, including IR and UV radiation. Furthermore, Santucci et al.36 correlated the radiation-induced Gadd45a expression in patients who have cervical carcinoma with their clinical response to radiotherapy and suggested that induction of Gadd45 proteins may be useful for predicting radioresponse in patients with cancer.
In the present study, we show the induction of Gadd45a protein in medulloblastoma cell lines with IR treatment. We also demonstrate that Gadd45a sensitizes medulloblastoma cells to radiation and has a negative role as a regulator of cancer cell proliferation, invasion, and metastatic potential. Previous studies reported that Gadd45a, an 18.4-kDa protein, can oligomerize into dimeric, trimeric, and tetrameric forms and can also interact with different cell cycle and DNA repair proteins.37 In our studies, Western blot analysis using Gadd45a monoclonal antibody consistently showed reactivity with a protein band of molecular size ∼50 kDa (as shown in Western blots). Therefore, we performed alternative immunoflow cytometry analysis to confirm the induction of Gadd45a protein with IR treatment. Furthermore, our results showed that both DAOY and D283 cell lines undergo increased G2-M arrest after radiation treatment in a IR dose-dependent manner (2–10 Gy; data not shown). Mechanisms that control the G2-M checkpoint currently remain poorly understood; however, many of the G2-M regulators appear to ultimately target Cdc2, a protein kinase required for mitotic entry in mammalian cells.38 According to Zhan et al.,17 Gadd45a induced by UV radiation is shown to interact with Cdc2 as shown by immunoprecipitation studies, and the addition of Gadd45a to immunoprecipitated Cdc2/cyclin B1 complex led to dissociation of this complex, indicating a possible interaction of Gadd45a and Cdc2. Furthermore, mutation of a unique region on Gadd45 containing DEDDDR residues was shown to inhibit Cdc2 binding and Gadd45a-induced G2-M arrest.39 With use of immunocytochemistry and immunoprecipitation analysis, we show—to our knowledge, for the first time—in medulloblastoma cells that Gadd45a protein, induced by IR, physically interacts with Cdc2. We observed that overexpression of Gadd45a along with IR treatment increases the levels of Cdc2. Overexpression of Gadd45a with IR treatment resulted in prominent Gadd45a-Cdc2 co-localization in the nucleus. The presence of Gadd45a and Cdc2 in the nucleus is closely associated with its role in cell-cycle G2-M arrest.40 Furthermore, with use of the MTT assay (Supplementary material, Fig. S1A and B), we demonstrate that Gadd45a suppresses the growth of medulloblastoma cells by the induction of G2-M arrest, indicating the anti-proliferative role of Gadd45a. However, our data indicated that overexpression of Gadd45a led only to cell-cycle arrest at the G2-M phase but was not sufficient to cause apoptosis in DAOY cells (Supplementary material, Fig. S1C). D283 cells showed scant apoptosis with Ov-exp Gadd45a in combination with a sublethal dose of IR (8 Gy). The number of apoptotic cells was negligible when compared with the total number of cells, as observed in TUNEL assay (Fig. 1E).
Expression of Gadd45a is shown to be both dependent41 and independent of p53. Its p53 independence is known to be induced by BRCA1.42 In the absence of the wild-type p53 gene, GADD45 promoter is strongly activated after expression of the wild-type breast cancer susceptibility gene, BRCA1.43 After DNA damage, mediated by IR, p53 protein is known to stabilize transiently and accumulate in the nucleus, where it functions as a transcription factor and upregulates multiple downstream-targeted genes, including p21, Gadd45a, and Bax.14,44,45 However, regulation of p53 stabilization is complex and involves post-translational modification of p53, such as phosphorylation at the Ser-15 position. Jin et al.,46 demonstrated that Gadd45a, a conventional downstream gene of p53, may play a role as an upstream effector in p53 stabilization after DNA damage. In the present study, we showed that an increase in nuclear levels of p-p53 is associated with overexpression of Gadd45a, which is further enhanced with IR treatment. This indicates that p53 and Gadd45a may be connected via a positive feedback mechanism, activating each other and mediating cell cycle arrest. Therefore, our studies indicate that stabilization of p53 is required in medulloblastoma cells for Gadd45a activity and vice versa.
Gadd45 proteins represent a novel class of targets for therapeutic interventions in cancers, because Gadd45a has been suggested to have important roles in tumorigenesis and tumor progression.47 Tront et al.8 showed by using a novel mouse model that loss of Gadd45a significantly accelerates the onset of breast tumorigenesis. A later study demonstrated the role of UV radiation–induced Gadd45a in tumor progression.22 This study demonstrates that Gadd45a negatively regulates expression of cell migration and invasion mediated by MMPs and further by the suppression of the β-catenin signaling via stress-activated p38 MAPK. Ji et al.24 demonstrated that Gadd45a is involved in control of cell-cell contact inhibition and cell-cell adhesion. Their study demonstrated that the Gadd45a protein can serve as an adapter to enhance interaction between β-catenin and caveolin-1 and, in turn, induce β-catenin translocation to the cell membrane for maintenance of cell-cell adhesion/contact inhibition. Cadherins are also implicated in this process and are known to be located at intercellular adhesion junctions.49
To understand the role of Gadd45a in cell invasion and tumorigenesis, we overexpressed Gadd45a protein in medulloblastoma cell lines and have shown that overexpression of Gadd45a with IR treatment causes an increase in membrane localization and a decrease in nuclear localization of β-catenin. Overexpression of Gadd45a seems to mediate increased distribution of β-catenin on the cell membrane and decreased nuclear translocation after IR treatment, whereas control cells, after IR treatment, demonstrate decreased membrane levels of β-catenin and increased nuclear levels of β-catenin. Binding of β-catenin to membrane adhesion glycoproteins such as cadherins indicates that overexpression of Gadd45a mediates cell adhesion dynamics. β-catenin in the nucleus is known to act as a transcription factor for cell proliferation molecules, such as cyclin D1, c-Myc and cell invasion molecules like MMPs.23,50,51 Our results from Matrigel, wound healing, and zymography assays confirm that Gadd45a induction with IR treatment may play an important role in the negative regulation of cell invasion and migration-associated molecules, such as MMP-9 and β-catenin,52 in DAOY and D283 cells.
Membrane levels of β-catenin play an important role in maintaining cell contact inhibition and cell-cell adhesion, linking cadherins to the actin cytoskeleton.25 Loss of cadherin/β-catenin complex function leads to disruption of cell contact inhibition and cell-cell adhesion, which is known to be important for malignancy.26,27 Loss of epithelial E-cadherin and gain of mesenchymal N-cadherin and fibronectin expression are major hallmarks of epithelial-mesenchymal transition (EMT), which we show to be induced in medulloblastoma cell lines after IR treatment. We also show that overexpression of Gadd45a in these cell lines retards IR-induced EMT by increasing the expression of E-cadherins and regulating distribution of membrane β-catenin bound to cadherins.
The role of MMP-9 at mediating EMT in cancers is already known.52 Our studies show that Gadd45a may play an important role in the negative regulation of IR-induced MMP-9 and that MMP-9 mediates EMT. This was confirmed by overexpression of Gadd45a, which resulted in downregulation of MMP-9 and suppression of EMT in medulloblastoma cells, as demonstrated by Western blot analysis. Alternatively, the inhibition of MMP-9 (pM) resulted in upregulation of Gadd45a and suppresseion of EMT as demonstrated by Western blot analysis and in vivo tumors. Thus, we conclude from our studies that the overexpression of Gadd45a accompanied by IR inhibits MMP-9 induction and retards the nuclear translocation of β-catenin (Fig. 5C).
Most recently, Bru et al.53 demonstrated the position-dependent expression of Gadd45a in rat brain tumors and showed that Gadd45a was expressed at much lower levels in the peripheral zone (invasive front) versus the center tumor core. Our in vivo studies demonstrated that, compared with control tumors, the IR-treated tumors demonstrated increased levels of MMP-9, with a corresponding increase in Gadd45a levels, which was localized in MMP-9–poor regions. The control tumor region with high MMP-9 levels had very low or negligible levels of Gadd45a. In pM-treated tumors, a significant increase in Gadd45a protein levels was observed that was further enhanced with IR treatment, suggesting the existence of a negative feedback loop between Gadd45a and MMP-9. Nuclear translocation of Gadd45a was observed with IR treatment and was further enhanced with pM + IR treatment, indicating its role in G2-M arrest. Because our in vitro studies showed that overexpression of Gadd45a downregulated MMP-9 and alternatively downregulation of MMP-9 (pM) resulted in upregulation of Gadd45a, we therefore used pM treatment for the in vivo studies as it is therapeutically feasible. Taken together, our results demonstrate that Gadd45a expression suppresses invasion, migration, and metastatic potential of medulloblastoma cells. Furthermore, this suppression is mediated by downregulation of MMP-9 and the stabilization of the β-catenin/E-cadherin complex. In conclusion, our study demonstrated that the downregulation of MMP-9 or upregulation of Gadd45a accompanied by IR has significant therapeutic implications.
Supplementary Material
Conflict of interest statement. None declared.
Funding
This research was supported by National Cancer Institute Grant CA138409 (to J.S.R.). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
Acknowledgments
We thank Peggy Mankin and Noorjehan Ali for their technical assistance. We also thank Shellee Abraham for manuscript preparation and Diana Meister and Sushma Jasti for manuscript review.
References
- 1.Rutkowski S, Cohen B, Finlay J, et al. Medulloblastoma in young children. Pediatr Blood Cancer. 2010;54:635–637. doi: 10.1002/pbc.22372. doi:10.1002/pbc.22372. [DOI] [PubMed] [Google Scholar]
- 2.Reddick WE, Russell JM, Glass JO, et al. Subtle white matter volume differences in children treated for medulloblastoma with conventional or reduced dose craniospinal irradiation. Magn Reson Imaging. 2000;18:787–793. doi: 10.1016/s0730-725x(00)00182-x. doi:10.1016/S0730-725X(00)00182-X. [DOI] [PubMed] [Google Scholar]
- 3.Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352:978–986. doi: 10.1056/NEJMoa042176. doi:10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 4.Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children's Cancer Group study. J Clin Oncol. 2001;19:3470–3476. doi: 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- 5.Fu KK, Phillips TL. Biologic rationale of combined radiotherapy and chemotherapy. Hematol Oncol Clin North Am. 1991;5:737–751. [PubMed] [Google Scholar]
- 6.Cheng JC, Chou CH, Kuo ML, Hsieh CY. Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction pathway. Oncogene. 2006;25:7009–7018. doi: 10.1038/sj.onc.1209706. doi:10.1038/sj.onc.1209706. [DOI] [PubMed] [Google Scholar]
- 7.Mascarin M, Dall'Oglio S, Palazzi M, et al. A case of relapsed medulloblastoma treated with intensity-modulated radiotherapy and temozolomide. Tumori. 2010;96:327–331. doi: 10.1177/030089161009600223. [DOI] [PubMed] [Google Scholar]
- 8.Stevens B, Razzaqi F, Yu L, Craver R. Late recurrence of medulloblastoma. J La State Med Soc. 2008;160:138–141. [PubMed] [Google Scholar]
- 9.Hollander MC, Sheikh MS, Bulavin DV, et al. Genomic instability in Gadd45a-deficient mice. Nat Genet. 1999;23:176–184. doi: 10.1038/13802. doi:10.1038/13802. [DOI] [PubMed] [Google Scholar]
- 10.Daino K, Ichimura S, Nenoi M. Both the basal transcriptional activity of the GADD45A gene and its enhancement after ionizing irradiation are mediated by AP-1 element. Biochim Biophys Acta. 2006;1759:458–469. doi: 10.1016/j.bbaexp.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Fayolle C, Pourchet J, de Fromentel CC, Puisieux A, Dore JF, Voeltzel T. Gadd45a activation protects melanoma cells from ultraviolet B-induced apoptosis. J Invest Dermatol. 2008;128:196–202. doi: 10.1038/sj.jid.5700963. doi:10.1038/sj.jid.5700963. [DOI] [PubMed] [Google Scholar]
- 12.Hildesheim J, Bulavin DV, Anver MR, et al. Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res. 2002;62:7305–7315. [PubMed] [Google Scholar]
- 13.Kastan MB, Zhan Q, el-Deiry WS, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. doi:10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhan Q, Bae I, Kastan MB, Fornace AJ., Jr The p53-dependent gamma-ray response of GADD45. Cancer Res. 1994;54:2755–2760. [PubMed] [Google Scholar]
- 15.Wang XW, Zhan Q, Coursen JD, et al. GADD45 induction of a G2/M cell cycle checkpoint. Proc Natl Acad Sci USA. 1999;96:3706–3711. doi: 10.1073/pnas.96.7.3706. doi:10.1073/pnas.96.7.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin S, Tong T, Fan W, et al. GADD45-induced cell cycle G2-M arrest associates with altered subcellular distribution of cyclin B1 and is independent of p38 kinase activity. Oncogene. 2002;21:8696–8704. doi: 10.1038/sj.onc.1206034. doi:10.1038/sj.onc.1206034. [DOI] [PubMed] [Google Scholar]
- 17.Zhan Q, Antinore MJ, Wang XW, et al. Association with Cdc2 and inhibition of Cdc2/Cyclin B1 kinase activity by the p53-regulated protein Gadd45. Oncogene. 1999;18:2892–2900. doi: 10.1038/sj.onc.1202667. doi:10.1038/sj.onc.1202667. [DOI] [PubMed] [Google Scholar]
- 18.Jin S, Antinore MJ, Lung FD, et al. The GADD45 inhibition of Cdc2 kinase correlates with GADD45-mediated growth suppression. J Biol Chem. 2000;275:16602–16608. doi: 10.1074/jbc.M000284200. doi:10.1074/jbc.M000284200. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Huper G, Guo Y, Murphy SK, Olson JA, Jr, Marks JR. Analysis of methylation-sensitive transcriptome identifies GADD45a as a frequently methylated gene in breast cancer. Oncogene. 2005;24:2705–2714. doi: 10.1038/sj.onc.1208464. doi:10.1038/sj.onc.1208464. [DOI] [PubMed] [Google Scholar]
- 20.Yamasawa K, Nio Y, Dong M, Yamaguchi K, Itakura M. Clinicopathological significance of abnormalities in Gadd45 expression and its relationship to p53 in human pancreatic cancer. Clin Cancer Res. 2002;8:2563–2569. [PubMed] [Google Scholar]
- 21.Mendes O, Kim HT, Stoica G. Expression of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat model. Clin Exp Metastasis. 2005;22:237–246. doi: 10.1007/s10585-005-8115-6. doi:10.1007/s10585-005-8115-6. [DOI] [PubMed] [Google Scholar]
- 22.Hildesheim J, Belova GI, Tyner SD, Zhou X, Vardanian L, Fornace AJ., Jr Gadd45a regulates matrix metalloproteinases by suppressing DeltaNp63alpha and beta-catenin via p38 MAP kinase and APC complex activation. Oncogene. 2004;23:1829–1837. doi: 10.1038/sj.onc.1207301. doi:10.1038/sj.onc.1207301. [DOI] [PubMed] [Google Scholar]
- 23.Behrens J. Control of beta-catenin signaling in tumor development. Ann NY Acad Sci. 2000;910:21–33. doi: 10.1111/j.1749-6632.2000.tb06698.x. doi:10.1111/j.1749-6632.2000.tb06698.x. [DOI] [PubMed] [Google Scholar]
- 24.Ji J, Liu R, Tong T, et al. Gadd45a regulates beta-catenin distribution and maintains cell-cell adhesion/contact. Oncogene. 2007;26:6396–6405. doi: 10.1038/sj.onc.1210469. doi:10.1038/sj.onc.1210469. [DOI] [PubMed] [Google Scholar]
- 25.Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. doi:10.1016/0168-9525(93)90250-L. [DOI] [PubMed] [Google Scholar]
- 26.Kudo Y, Kitajima S, Ogawa I, et al. Invasion and metastasis of oral cancer cells require methylation of E-cadherin and/or degradation of membranous beta-catenin. Clin Cancer Res. 2004;10:5455–5463. doi: 10.1158/1078-0432.CCR-04-0372. doi:10.1158/1078-0432.CCR-04-0372. [DOI] [PubMed] [Google Scholar]
- 27.Salon C, Moro D, Lantuejoul S, et al. E-cadherin-beta-catenin adhesion complex in neuroendocrine tumors of the lung: a suggested role upon local invasion and metastasis. Hum Pathol. 2004;35:1148–1155. doi: 10.1016/j.humpath.2004.04.015. doi:10.1016/j.humpath.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Chou TT, Trojanowski JQ, Lee VM. p38 mitogen-activated protein kinase-independent induction of gadd45 expression in nerve growth factor-induced apoptosis in medulloblastomas. J Biol Chem. 2001;276:41120–41127. doi: 10.1074/jbc.M102832200. doi:10.1074/jbc.M102832200. [DOI] [PubMed] [Google Scholar]
- 29.Ganji PC, Nalla AK, Gupta R, et al. siRNA-mediated downregulation of MMP-9 and uPAR in combination with radiation induces G2/M cell-cycle arrest in medulloblastoma. Mol Cancer Res. 2011;9:51–66. doi: 10.1158/1541-7786.MCR-10-0399. doi:10.1158/1541-7786.MCR-10-0399. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Lakka SS, Gondi CS, Dinh DH, et al. Specific interference of uPAR and MMP-9 gene expression induced by double-stranded RNA results in decreased invasion, tumor growth and angiogenesis in gliomas. J Biol Chem. 2005;280:21882–21892. doi: 10.1074/jbc.M408520200. doi:10.1074/jbc.M408520200. [DOI] [PubMed] [Google Scholar]
- 31.Rao JS, Bhoopathi P, Chetty C, Gujrati M, Lakka SS. Matrix metalloproteinase-9 short interfering RNA induced senescence resulting in inhibition of medulloblastoma growth via p16INK4 and mitogen-activated protein kinase pathway. Cancer Res. 2007;67:4956–4964. doi: 10.1158/0008-5472.CAN-07-0380. doi:10.1158/0008-5472.CAN-07-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. doi:10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 33.Brusca JS, Radolf JD. Isolation of integral membrane proteins by phase partitioning with Triton X-114. Methods Enzymol. 1994;228:182–193. doi: 10.1016/0076-6879(94)28019-3. doi:10.1016/0076-6879(94)28019-3. [DOI] [PubMed] [Google Scholar]
- 34.Kwei KA, Kung Y, Salari K, Holcomb IN, Pollack JR. Genomic instability in breast cancer: pathogenesis and clinical implications. Mol Oncol. 2010;4:255–266. doi: 10.1016/j.molonc.2010.04.001. doi:10.1016/j.molonc.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 36.Santucci MA, Barbieri E, Frezza G, et al. Radiation-induced gadd45 expression correlates with clinical response to radiotherapy of cervical carcinoma. Int J Radiat Oncol Biol Phys. 2000;46:411–416. doi: 10.1016/s0360-3016(99)00459-9. doi:10.1016/S0360-3016(99)00459-9. [DOI] [PubMed] [Google Scholar]
- 37.Kovalsky O, Lung FD, Roller PP, Fornace AJ., Jr Oligomerization of human Gadd45a protein. J Biol Chem. 2001;276:39330–39339. doi: 10.1074/jbc.M105115200. doi:10.1074/jbc.M105115200. [DOI] [PubMed] [Google Scholar]
- 38.O'Connor PM. Mammalian G1 and G2 phase checkpoints. Cancer Surv. 1997;29:151–182. [PubMed] [Google Scholar]
- 39.Yang Q, Manicone A, Coursen JD, et al. Identification of a functional domain in a GADD45-mediated G2/M checkpoint. J Biol Chem. 2000;275:36892–36898. doi: 10.1074/jbc.M005319200. doi:10.1074/jbc.M005319200. [DOI] [PubMed] [Google Scholar]
- 40.Gao H, Jin S, Song Y, et al. B23 regulates GADD45a nuclear translocation and contributes to GADD45a-induced cell cycle G2-M arrest. J Biol Chem. 2005;280:10988–10996. doi: 10.1074/jbc.M412720200. doi:10.1074/jbc.M412720200. [DOI] [PubMed] [Google Scholar]
- 41.Carrier F, Georgel PT, Pourquier P, et al. Gadd45, a p53-responsive stress protein, modifies DNA accessibility on damaged chromatin. Mol Cell Biol. 1999;19:1673–1685. doi: 10.1128/mcb.19.3.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harkin DP, Bean JM, Miklos D, et al. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell. 1999;97:575–586. doi: 10.1016/s0092-8674(00)80769-2. doi:10.1016/S0092-8674(00)80769-2. [DOI] [PubMed] [Google Scholar]
- 43.Jin S, Zhao H, Fan F, et al. BRCA1 activation of the GADD45 promoter. Oncogene. 2000;19:4050–4057. doi: 10.1038/sj.onc.1203759. doi:10.1038/sj.onc.1203759. [DOI] [PubMed] [Google Scholar]
- 44.el-Deiry WS, Tokino T, Velculescu VE, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. doi:10.1016/0092-8674(93)90500-P. [DOI] [PubMed] [Google Scholar]
- 45.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. doi:10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 46.Jin S, Mazzacurati L, Zhu X, et al. Gadd45a contributes to p53 stabilization in response to DNA damage. Oncogene. 2003;22:8536–8540. doi: 10.1038/sj.onc.1206907. doi:10.1038/sj.onc.1206907. [DOI] [PubMed] [Google Scholar]
- 47.Cretu A, Sha X, Tront J, Hoffman B, Liebermann DA. Stress sensor Gadd45 genes as therapeutic targets in cancer. Cancer Ther. 2009;7:268–276. [PMC free article] [PubMed] [Google Scholar]
- 48.Tront JS, Hoffman B, Liebermann DA. Gadd45a suppresses Ras-driven mammary tumorigenesis by activation of c-Jun NH2-terminal kinase and p38 stress signaling resulting in apoptosis and senescence. Cancer Res. 2006;66:8448–8454. doi: 10.1158/0008-5472.CAN-06-2013. doi:10.1158/0008-5472.CAN-06-2013. [DOI] [PubMed] [Google Scholar]
- 49.Gottardi CJ, Gumbiner BM. Adhesion signaling: how beta-catenin interacts with its partners. Curr Biol. 2001;11:R792–R794. doi: 10.1016/s0960-9822(01)00473-0. doi:10.1016/S0960-9822(01)00473-0. [DOI] [PubMed] [Google Scholar]
- 50.Shtutman M, Zhurinsky J, Simcha I, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. doi:10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi M, Tsunoda T, Seiki M, Nakamura Y, Furukawa Y. Identification of membrane-type matrix metalloproteinase-1 as a target of the beta-catenin/Tcf4 complex in human colorectal cancers. Oncogene. 2002;21:5861–5867. doi: 10.1038/sj.onc.1205755. doi:10.1038/sj.onc.1205755. [DOI] [PubMed] [Google Scholar]
- 52.Bozzuto G, Ruggieri P, Molinari A. Molecular aspects of tumor cell migration and invasion. Ann Ist Super Sanita. 2010;46:66–80. doi: 10.4415/ANN_10_01_09. [DOI] [PubMed] [Google Scholar]
- 53.Bru A, del Fresno C, Soares-Schanoski A, et al. Position-dependent expression of GADD45alpha in rat brain tumours. Med Oncol. 2007;24:436–444. doi: 10.1007/s12032-007-0025-9. doi:10.1007/s12032-007-0025-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.