Abstract
Background and Aims
Interspecific Diphasiastrum hybrids have been assumed to be homoploid and to produce well-formed spores serving sexual reproduction. If this were the case, forms intermediate between hybrids and parents or hybrid swarms should be expected. The purpose of this study was: (1) to check whether homoploidy consistently applies to the three hybrids throughout their Central European range; (2) to examine whether their genome sizes confirm their parentage as assumed by morphology; and (3) to perform a screening for detection of ploidy levels other than diploid and variation in DNA content due to backcrossing.
Methods
Flow cytometry was used first to measure the relative DNA values [with 4′,6-diamidino-2-phenylindole (DAPI) staining] and ploidy level as a general screening, and secondly to determine the absolute DNA 2C values [with propidium iodide (PI) staining] in a number of selected samples with the main focus on the hybrids.
Key Results
A considerable variation of DNA 2C values (5·26–7·52 pg) was detected between the three European Diphasiastrum species. The values of the diploid hybrids are highly constant without significant variation between regions. They are also intermediate between their assumed parents and agree closely with those calculated from their putative parents. This confirms their hybrid origin, assumed parentage and homoploid status. Considerably higher DNA amounts (9·48–10·30 pg) were obtained for three populations, suggesting that these represent triploid hybrids, an interpretation that is strongly supported by their morphology.
Conclusions
Diploid hybrids have retained their genetic and morphological identites throughout their Central European range, and thus no indications for diploid backcrossing were found. The triploid hybrids have probably originated from backcrossing between a diploid gametophyte of a hybrid (derived from a diplospore) and a haploid gametophyte of a diploid parental species. By repeated crossing events, reticulate evolution patterns arise that are similar to those known for a number of ferns.
Keywords: Diphasiastrum, Lycopodiaceae, flow cytometry, nuclear DNA content, homoploidy, triploidy, hybridization, reticulate evolution
INTRODUCTION
The lycophytes are the oldest extant group of spore-producing vascular plants, and their earliest fossil remains are from the Devonian or possibly (the genus Baragwanathia) even from the late Silurian period (Hueber, 1983; Garratt et al., 1984; Rickards, 2000; Gensel and Berry, 2001; Hao and Gensel, 2001; Wikström, 2001; Kotyk et al., 2002).
While their extant representatives comprise a number of rather small and inconspicuous herbaceous plants, in the Carboniferous, lycophytes (Lepidodendron and Sigillaria) grew as tall trees (Stewart and Rothwell, 1993; Berry and Fairon-Demaret, 2001; Pigg, 2001; Taylor et al., 2009) and, in association with the arborescent giant horsetails (Calamitaceae and Archaeocalamitaceae), formed swampland forests for 40 million years and were important contributors to coal formation.
Reconstructions of relationships for the main lineages of vascular plants that diverged since the Devonian period have revealed a basal dichotomy, separating the lycophytes from the so-called euphyllophytes (Euphyllophytina, all other vascular plants) approx. 400 million years ago (Raubeson and Jansen, 1992; Kenrick and Crane, 1997; Duff and Nickrent, 1999; Pryer et al., 2001, 2004a, b; Tsuji et al., 2007). The latter comprise two major clades: the spermatophytes (seed plants, Soltis and Soltis, 2004), and the monilophytes (ferns, sensu Pryer et al., 2004b), including horsetails, whisk ferns and all eusporangiate and leptosporangiate ferns.
The extant lycophytes include members of the homosporous Lycopodiaceae, as well as the heterosporous Selaginellaceae and Isoëtaceae. Although being extremely diverse and of virtually cosmopolitan distribution, the family of Lycopodiaceae is considered to be monophyletic (Wikström and Kenrick, 1997; Yatsentyuk et al., 2001). While in the past all species of the Lycopodiaceae were placed generally in the single genus Lycopodium, in the last several decades the family has been variously divided into 2–16 genera (Øllgaard, 1987; Wagner and Beitel, 1993; Haines, 2003). In Europe, currently four genera are recognized: Diphasiastrum, Huperzia, Lycopodium and Lycopodiella (Valentine and Moore, 1993).
The genus Diphasiastrum comprises a relatively small group of lycopods that differ morphologically from the other genera by their leaves being usually decussate (or 4–5-ranked), di- or even trimorphic, and the aerial branchlets being quadrate to flattened. It includes about 25 species (and taxa of hybrid origin) of mainly north temperate and sub-arctic distribution (Wagner and Beitel, 1993). Only a few species occur in the tropics or sub-tropics, where they are restricted to mountain areas (e.g. D. multispicatum; see Bennert et al., 2007). The base number is generally accepted to be x = 23 (Wagner, 1992), and diploidy prevails by far (Aagaard et al., 2009c).
In Continental Europe, six diploid taxa are recognized, including three parental species (D. alpinum, D. complanatum and D. tristachyum, called ‘species’ in the following), and three intermediate taxa of putative hybrid origin (D. × issleri, D. × oellgaardii and D. × zeilleri, called ‘hybrids’ in the following).
It generally has been supposed that these diploid hybrids reproduce sexually via spores, giving rise to F2 and subsequent generations, but this never has been proven beyond doubt. Also, it is not clear whether backcrosses between hybrids and parent species occur, as would be expected regarding the high crossability of species. Judging from morphology, the parental species seem to retain their identity and no transitions between parents and hybrids have been found so far, with rare putative triploids as a possible exception (see below). A total of seven homoploid hybrids have been reported from North America, Northern Asia and Europe (Wagner et al., 1985).
Flow cytometry was applied in order to check whether (a) the known Diphasiastrum hybrids in Central Europe are invariably homoploid; (b) their genome sizes would be indicative of their origin; and (c) cytotypes other than diploid exist.
MATERIALS AND METHODS
Plants of Diphasiastrum were identified by their morphological characters. While the species are easy to differentiate, recognition of the hybrids requires advanced experience. Diagnostically useful are the dimensions of the ventral and dorsal leaves and their size in relation to the stem (see Horn, 2006, 2008; Horn and Tribsch, 2009).
For flow cytometric studies, fresh plant material was collected (by K.H., B.Ø., I.J. and R.V.) in Denmark, France, the Czech Republic, the Central German Uplands and northern Italy (Table 1), and kept in the refrigerator prior to analysis for no longer than 2 weeks. Three to five branchlets were collected per colony; discontiguous parts of larger colonies were sampled separately. For all accessions an initial ploidy screening was performed on a Ploidy Analyser (Partec GmbH, Münster, Germany) using 4′,6-diamidino-2-phenylindole (DAPI)-stained samples (1·6 µg mL−1) isolated in 2·1 % (w/v) citric acid monohydrate, 0·5 % (w/v) Tween-20, together with Agave sisalana (5x, 2n = 150, 2C = 20·2 pg; Zonneveld et al., 2005) as an internal reference standard. Absolute 2C DNA contents were determined for selected accessions (of 2–6 phytogeographic regions with one to several localities each) using propidium iodide (PI) staining. Diphasiastrum samples and leaf sections of Pisum sativum ‘Viktoria, Kifejtö Borsó’ (Genebank Gatersleben accession number: PIS 630; 2C = 9·09 pg; Doležel et al., 2007) as an internal reference standard were simultaneously chopped and stained using the ‘CyStain PI absolute P’ nuclei extraction and staining kit (Partec GmbH, Münster) according to the manufacturer's instruction. Samples were analysed using either a Partec CyFlow SL flow cytometer (Münster, Germany) or a FACStarPLUS (BD Biosciences, San José, CA, USA). Usually 10 000 nuclei per sample were analysed in at least five replicates per accession. The absolute DNA contents of Diphasiastrum species were calculated based on the ratios of the G1 peak means of sample and reference standard.
Table 1.
Diphasiastrum samples used for flow cytometric analyses
| Taxon | Accession number | Date of collection | Locality | Region | Type of study |
|---|---|---|---|---|---|
| D. alpinum | H 08/01 | 20/8/2008 | Gr. Sonnen-Berg N St. Andreasberg, NI, D | Har | b |
| D. alpinum | H 09/01, H 09/73 | 11/9/2009, 18/11/2009 | NNW Finsterau, BY, D | BaFo | a, b |
| D. alpinum | H 09/03, H 09/75 | 11/9/2009, 18/11/2009 | W Finsterau, BY, D | BaFo | a, b |
| D. alpinum | H 09/09 | 11/9/2009 | SW Altschönau, BY, D | BaFo | a |
| D. alpinum | H 09/11 | 11/9/2009 | SW Guglöd, BY, D | BaFo | a |
| D. alpinum | H 09/27 | 11/9/2009 | NE Frauenau, eastern locality, BY, D | BaFo | a |
| D. alpinum | H 09/30 | 11/9/2009 | NE Frauenau, western locality, BY, D | BaFo | a |
| D. alpinum | H 09/36 | 12/9/2009 | Kiesruck NE Buchenau, BY, D | BaFo | a |
| D. alpinum | H 09/65 | 17/11/2009 | Hirschenstein N Böbrach, BY, D | BaFo | a, b |
| D. alpinum | H 09/68 | 17/11/2009 | Zlíbský vrch N Strázny, C, CZ | BoFo | a, b |
| D. alpinum | H 09/87b | 18/11/2009 | Voithenberg, western locality, BY, D | ObWa | a |
| D. alpinum | H 09/57 | 1/10/2009 | Hundseck SE Bühlertal, BW, D | BlFo | a |
| D. alpinum | H 09/63 | 3/10/2009 | Kandel N St. Peter, BW, D | BlFo | a |
| D. alpinum | V 12664, V 12736 | 29/10/2009 | Vexaincourt W Schirmeck, Vosges, F | Vos | a |
| D. alpinum | V 12647 | 1/10/2009 | SE St. Quirin, Moselle, F | Vos | a |
| D. alpinum | V 12669–71, V 12681, V 12685 | 1/10/2009 | Champ du Feu W le Hohwald, Bas-Rhin, F | Vos | a |
| D. alpinum | V 12713 | 2/10/2009 | Col du Schlucht, Haut-Rhin, F | Vos | a |
| D. alpinum | V 12740 | 4/11/2009 | Forét d'Abreschviller, Moselle, F | Vos | a |
| D. alpinum | Ho 09/12 | 19/11/2009 | Rosskopf SW Engenthal, Moselle, F | Vos | a |
| D. alpinum | V ST/01–09 | 4/8/2010 | Martalm, Ridnauntal, South Tyrol, I | ST | a |
| D. complanatum | BØ 09/01, BØ 09/03 | 27/10/2009 | Nørlund Plantage S Ikast, Midtjylland, DK | Jut | a |
| D. complanatum | H 08/02 | 20/8/2008 | Gr. Sonnen-Berg N St. Andreasberg, NI, D | Har | b |
| D. complanatum | H 09/04, H 09/76 | 11/9/2009, 18/11/2009 | W Finsterau, BY, D | BaFo | a, b |
| D. complanatum | H 09/12 | 11/9/2009 | SW Guglöd, BY, D | BaFo | a |
| D. complanatum | H 09/17 | 11/9/2009 | E Spiegelau, BY, D | BaFo | a, |
| D. complanatum | H 09/31 | 11/9/2009 | NE Frauenau, western locality, BY, D | BaFo | a |
| D. complanatum | H 09/37 | 12/9/2009 | Kiesruck NE Buchenau, BY, D | BaFo | a |
| D. complanatum | H 09/43, H 09/88 | 12/9/2009, 18/11/2009 | Voithenberg, western locality, BY, D | ObWa | a, b |
| D. complanatum | H 09/69 | 17/11/2009 | Zlíbský vrch N Strázny, C, CZ | BoFo | a, b |
| D. complanatum | V ST/10–12 | 4/8/2010 | Martalm, Ridnauntal, South Tyrol, I | ST | a |
| D. × issleri | H 08/03 | 20/8/2008 | Gr. Sonnen-Berg N St. Andreasberg, NI, D | Har | b |
| D. × issleri | H 09/02, H 09/74 | 11/9/2009, 18/11/2009 | NNW Finsterau, BY, D | BaFo | a, b |
| D. × issleri | H 09/05, H 09/77 | 11/9/2009, 18/11/2009 | W Finsterau, BY, D | BaFo | a, b |
| D. × issleri | H 09/13, H 09/14 | 11/9/2009 | SW Guglöd, BY, D | BaFo | a |
| D. × issleri | H 09/18 | 11/9/2009 | E Spiegelau, BY, D | BaFo | a |
| D. × issleri | H 09/24 | 11/9/2009 | Spiegelau, BY, D | BaFo | a |
| D. × issleri | H 09/32, H 09/33 | 11/9/2009 | NE Frauenau, western locality, BY, D | BaFo | a |
| D. × issleri | H 06/07, H 09/38 | 12/10/2006, 12/9/2009 | Kiesruck NE Buchenau, BY, D | BaFo | a |
| D. × issleri | H 09/44, H 09/45, H 09/89 | 12/9/2009, 18/11/2009 | Voithenberg, western locality, BY, D | ObWa | a, b |
| D. × issleri | H 09/66 | 17/11/2009 | Hirschenstein N Böbrach, BY, D | BaFo | a, b |
| D. × issleri | H 09/67 | 17/11/2009 | S Haidmühle, BY, D | BaFo | a, b |
| D. × issleri | H 09/81 | 18/11/2009 | Rindel-Berg E Altschönau, BY, D | BaFo | a, b |
| D. × issleri | H 09/070 | 17/11/2009 | Zlíbský vrch N Strázny, C, CZ | BoFo | a, b |
| D. × issleri | H 09/59 | 1/10/2009 | Lochrütte, Feldberg region, BW, D | BlFo | a, b |
| D. × issleri | H 09/60, V 12698 | 2/10/2009 | Le Tanet, type locality, Haut-Rhin, F | Vos | a, b |
| D. × issleri | H 09/61 | 2/10/2009 | NE Le Tanet, Haut-Rhin, F | Vos | a, b |
| D. × issleri | V ST/13–16 | 4/8/2010 | Martalm, Ridnauntal, South Tyrol, I | ST | a |
| D. × oellgaardii | BØ 09/06 | 27/10/2009 | Præstbjerg Plantage SE Vind, Midtjylland, DK | Jut | a |
| D. × oellgaardii | H 09/06, H 09/78 | 11/9/2009, 18/11/2009 | W Finsterau, BY, D | BaFo | a, b |
| D. × oellgaardii | H 09/15 | 11/9/2009 | SW Guglöd, BY, D | BaFo | a |
| D. × oellgaardii | H 09/20, H 09/83 | 11/9/2009, 18/11/2009 | E Spiegelau, BY, D | BaFo | a, b |
| D. × oellgaardii | H 09/25 | 11/9/2009 | Spiegelau, BY, D | BaFo | a |
| D. × oellgaardii | H 09/34 | 11/9/2009 | NE Frauenau, western locality, BY, D | BaFo | a |
| D. × oellgaardii | H 09/39 | 12/9/2009 | Kiesruck NE Buchenau, BY, D | BaFo | a |
| D. × oellgaardii | H 09/71 | 17/11/2009 | Zlíbský vrch N Strázny, C, CZ | BoFo | a, b |
| D. × oellgaardii | H 06/19 | 16/11/2006 | Dreiwappen ENE Althütte, BY, D | ObWa | a |
| D. × oellgaardii | H 09/46, H 09/47, H 09/90 | 12/9/2009, 18/11/2009 | Voithenberg, western locality, BY, D | ObWa | a, b |
| D. × oellgaardii | H 09/54 | 1/10/2009 | Beerfelden, HE, D | HeOd | a |
| D. × oellgaardii | H 09/58 | 1/10/2009 | Hundseck SE Bühlertal, BW, D | BlFo | a |
| D. × oellgaardii | H 09/62 | 3/10/2009 | Weiherkopf S Münsterhalden, BW, D | BlFo | a |
| D. × oellgaardii | V 12672, V 12673, V 12676, V 12678, V 12679, V 12686 | 1/10/2009 | Champ du Feu W le Hohwald, Bas-Rhin, F | Vos | a |
| D. × oellgaardii | V ST/17 | 4/8/2010 | Martalm, Ridnauntal, South Tyrol, I | ST | a |
| D. tristachyum | BØ 09/02, BØ 09/05 | 27/10/2009 | Nørlund Plantage S Ikast, Midtjylland, DK | Jut | a |
| D. tristachyum | H 09/07, H 09/79 | 11/9/2009, 18/11/2009 | W Finsterau, BY, D | BaFo | a, b |
| D. tristachyum | H 09/10 | 11/9/2009 | SW Altschönau, BY, D | BaFo | a |
| D. tristachyum | H 09/21 | 11/9/2009 | E Spiegelau, BY, D | BaFo | a |
| D. tristachyum | H 09/23, H 09/85 | 11/9/2009, 18/11/2009 | Filz-Wald E Spiegelau, BY, D | BaFo | a, b |
| D. tristachyum | H 09/26 | 11/9/2009 | Spiegelau, BY, D | BaFo | a |
| D. tristachyum | H 09/28 | 11/9/2009 | NE Frauenau, eastern locality, BY, D | BaFo | a |
| D. tristachyum | H 09/40 | 12/9/2009 | Kiesruck NE Buchenau, BY, D | BaFo | a |
| D. tristachyum | H 09/48, H 09/91 | 12/9/2009, 18/11/2009 | Voithenberg, western locality, BY, D | ObWa | a, b |
| D. tristachyum | H 09/55 | 1/10/2009 | Beerfelden, HE, D | HeOd | a |
| D. tristachyum | H 09/56 | 1/10/2009 | Olfen, HE, D | HeOd | a |
| D. tristachyum | H 09/64 | 3/10/2009 | Kandel N St. Peter, BW, D | BlFo | a |
| D. tristachyum | V 12639, V 12649 | 1/10/2009 | Altdorfkopf WSW Dabo, Moselle, F | Vos | a |
| D. tristachyum | V 12640, V 12645 | 1/10/2009 | Le Canceley SE Abreschviller, Moselle, F | Vos | a |
| D. tristachyum | V 12665–67, V 12668, V 12674, V 12677, V 12684, V 12687 | 1/10/2009 | Champ du Feu W le Hohwald, Bas-Rhin, F | Vos | a |
| D. tristachyum | V 12690 | 1/10/2009 | ENE Moussey, Moselle, F | Vos | a |
| D. tristachyum | V 1273 | 12/10/2009 | St. Quirin (Tête du Calice), Moselle, F | Vos | a |
| D. tristachyum | V 12738 | 27/10/2009 | Singristerkopf near Reinhardsmunster, Bas-Rhin, F | Vos | a |
| D. tristachyum | V 12735 | 29/10/2009 | Vallée de la Sarre Blanche, Moselle, F | Vos | a |
| D. tristachyum | V 12737 | 29/10/2009 | Kappelbronn N Lutzelhouse, Bas-Rhin, F | Vos | a |
| D. tristachyum | V 12739 | 4/11/2009 | Forét domaniale de Walscheid, Moselle, F | Vos | a |
| D. × zeilleri | BØ 09/07 | 27/10/2009 | Tihøje ved Røddinglund Plantage WNW Vildbjerg, Midtjylland, DK | Jut | a |
| D. × zeilleri | H 08/04 | 20/8/2008 | Gr. Sonnen-Berg N St. Andreasberg, NI, D | Har | b |
| D. × zeilleri | H 09/08, H 09/80 | 11/9/2009, 18/11/2009 | W Finsterau, BY, D | BaFo | a, b |
| D. × zeilleri | H 09/16 | 11/9/2009 | SW Guglöd, BY, D | BaFo | a |
| D. × zeilleri | H 09/22, H 09/84 | 11/9/2009, 18/11/2009 | E Spiegelau, BY, D | BaFo | a, b |
| D. × zeilleri | H 09/29 | 11/9/2009 | NE Frauenau, eastern locality, BY, D | BaFo | a |
| D. × zeilleri | H 09/35 | 11/9/2009 | NE Frauenau, western locality, BY, D | BaFo | a |
| D. × zeilleri | H 09/41 | 12/9/2009 | Kiesruck NE Buchenau, BY, D | BaFo | a |
| D. × zeilleri | H 09/49, H 09/50, H 09/92 | 12/9/2009, 18/11/2009 | Voithenberg, western locality, BY, D | ObWa | a, b |
| D. × zeilleri | H 09/72 | 17/11/2009 | Zlíbský vrch N Strázny, C, CZ | BoFo | a |
| D. × zeilleri | H 09/52, H 09/53 | 1/10/2009 | Beerfelden, HE, D | HeOd | a, b |
| D. × zeilleri | V 12679, V 12680, V 12682 | 1/10/2009 | Champ du Feu W le Hohwald, Bas-Rhin, F | Vos | a |
| D. × zeilleri | V 12728 | 9/10/2009 | Baerenthal (Lindenkopf), Moselle, F | Vos | a |
| D. × zeilleri | V 12729 | 9/10/2009 | Wingen (Col du Litschhof, Wingen, Bas-Rhin, F | Vos | a |
| D. × zeilleri | V 12732 | 9/10/2009 | Jungenwald, Wingen, Bas-Rhin, F | Vos | a |
| D. × zeilleri | V 12733 | 9/10/2009 | Diebhalt, Climbach, Bas-Rhin, F | Vos | a |
| D. hybrid, triploid | H 09/19, H 09/82 | 11/9/2009, 18/11/2009 | E Spiegelau, BY, D | BaFo | a, b |
| D. hybrid, triploid | H 09/51, H 09/86 | 12/9/2009, 18/11/2009 | Voithenberg, eastern locality, BY, D | ObWa | a, b |
| D. hybrid, triploid | H 09/42, H 09/87a | 12/9/2009, 18/11/2009 | Voithenberg, western locality, BY, D | ObWa | a, b |
Abbreviations used for accession numbers: H, K. Horn; V, R. Viane; Ho, P. Holveck; BØ, I.S. Bisgaard Jakobsen and B. Øllgaard. For localities: D, Germany; BW, Baden-Württemberg; BY, Bavaria; HE, Hesse; NI, Lower Saxony; ST, Sachsen-Anhalt; CZ, Czech Republic; C, Budejovicky Kraj; DK, Denmark; F. France; I, Italy. For regions: BaFo, Bavarian Forest; BlFo, Black Forest; BoFo, Bohemian Forest; Har, Harz Mountains; HeOd, Hessischer Odenwald; Jut, Jutland; ObWa, Oberpfälzer Wald; ST, South Tyrol; V, Vosges Mountains. For types of study: a, flow cytometry by DAPI; b, flow cytometry by PI.
Using the 2C DNA contents, a Student's test was performed to test (a) for differences between the populations within one taxon and (b) for differences between taxa based on all populations studied.
RESULTS AND DISCUSSION
Relative DNA values (DAPI measurements)
Values for relative fluorescence intensity show a 1·38-fold variation between the parent species, with D. alpinum possessing the largest and D. tristachyum the smallest genome (Table 2). The genome sizes of the three known hybrids are not only in the same range as those of the diploid species, but also intermediate between those of the assumed parents, confirming their diploid status as well as their correct identification. Within the taxa the variation between regions is small and does not exceed the inaccuracy threshold.
Table 2.
Relative fluorescence intensity (DAPI measurements) for the Diphasiastrum taxa studied, summarized by the phytogeographic regions
| Taxon | Genome composition | Ploidy level | Region | No. of sampled colonies | Relative fluorescence intensity (mean ± s.d.) | Overall mean (± s.d.) |
|---|---|---|---|---|---|---|
| D. alpinum | AA | 2x | BaFo | 7 | 0·321 ± 0·006 | |
| BlFo | 2 | 0·319 ± 0·006 | ||||
| BoFo | 1 | 0·313 | ||||
| Vos | 6 | 0·317 ± 0·006 | ||||
| ST | 9 | 0·319 ± 0·006 | 0·317 ± 0·006 | |||
| D. complanatum | CC | 2x | BaFo | 5 | 0·255 ± 0·009 | |
| BoFo | 1 | 0·243 | ||||
| Jut | 2 | 0·253 ± 0·009 | ||||
| ObWa | 1 | 0·260 | ||||
| ST | 3 | 0·248 ± 0·002 | 0·249 ± 0·011 | |||
| D. tristachyum | TT | 2x | BaFo | 7 | 0·235 ± 0·012 | |
| BlFo | 1 | 0·226 | ||||
| HeOd | 2 | 0·226 ± 0·001 | ||||
| Jut | 2 | 0·241 ± 0·001 | ||||
| ObWa | 2 | 0·231 ± 0·003 | ||||
| Vos | 9 | 0·225 ± 0·008 | 0·229 ± 0·010 | |||
| D. × issleri | AC | 2x | BaFo | 7 | 0·287 ± 0·012 | |
| BlFo | 1 | 0·286 | ||||
| BoFo | 1 | 0·281 | ||||
| ObWa | 3 | 0·285 ± 0·007 | ||||
| Vos | 2 | 0·284 ± 0·005 | ||||
| ST | 4 | 0·280 ± 0·005 | 0·284 ± 0·009 | |||
| D. × oellgaardii | AT | 2x | BaFo | 6 | 0·279 ± 0·009 | |
| BlFo | 2 | 0·268 ± 0·010 | ||||
| HeOd | 1 | 0·263 | ||||
| Jut | 1 | 0·283 | ||||
| ObWa | 3 | 0·281 ± 0·007 | ||||
| Vos | 6 | 0·267 ± 0·005 | ||||
| ST | 1 | 0·278 | 0·273 ± 0·009 | |||
| D. × zeilleri | CT | 2x | BaFo | 6 | 0·246 ± 0·007 | |
| BoFo | 1 | 0·230 | ||||
| HeOd | 2 | 0·234 ± 0·001 | ||||
| Jut | 1 | 0·243 | ||||
| ObWa | 3 | 0·250 ± 0·002 | ||||
| Vos | 5 | 0·231 ± 0·006 | 0·238 ± 0·009 | |||
| D. hybrid | AAC | 3x | ObWa | 1 | 0·429 ± 0·003 | 0·429 ± 0·003 |
| D. hybrid | AAT | 3x | ObWa | 1 | 0·416 ± 0·004 | 0·416 ± 0·004 |
| D. hybrid | ACC | 3x | BaFo | 1 | 0·402 ± 0·003 | 0·402 ± 0·003 |
For abbreviations used, see Table 1; letters used for genome composition: A, D. alpinum; C, D. complanatum; T, D. tristachyum.
Considerably higher values were found in only three accessions, one from the Bavarian Forest and two from the Oberpfälzer Wald. These exceed those of the species by a factor of 1·30–1·80 (average 1·59). Therefore, these three colonies represent plants with a ploidy level other than diploid; they are very probably triploids (see below).
Nuclear DNA C values of the species (PI measurements)
Analogous to the results of the DAPI measurements, a considerable variation (1·42-fold) in nuclear DNA C amounts was found (Table 3), with D. alpinum having the largest 2C content (7·49 pg), D. tristachyum the smallest (5·26 pg) and D. complanatum being intermediate (5·72 pg), but closer to D. tristachyum. The intraspecific differences between localities and regions are small (usually <0·1 pg) and statistically not significant (P < 0·01).
Table 3.
DNA 2C values (pg) for the diploid Diphasiastrum taxa studied
| Taxon | Accession number | Region | Locality | 2C ± s.d. mean for locality | 2C ± s.d. mean for region | 2C ± s.d. overall mean |
|---|---|---|---|---|---|---|
| D. alpinum | H 09/75 | BaFo | W Finsterau | 7·50 ± 0·04 | ||
| H 09/65 | BaFo | Hirschenstein | 7·49 ± 0·07 | |||
| H 09/73 | BaFo | NNW Finsterau | 7·45 ± 0·03 | 7·49 ± 0·05 | ||
| H 08/01 | Har | Gr. Sonnenberg | 7·55 ± 0·05 | 7·55 ± 0·05 | 7·52 ± 0·06 | |
| D. complanatum | H 09/76 | BaFo | W Finsterau | 5·73 ± 0·06 | 5·73 ± 0·06 | |
| H 09/69 | BoFo | Strázny | 5·74 ± 0·01 | 5·74 ± 0·01 | ||
| H 09/43 and H 09/88 | ObWa | Voithenberg | 5·73 ± 0·07 | 5·73 ± 0·07 | ||
| H 08/02 | Har | Gr. Sonnenberg | 5·78 ± 0·03 | 5·73 ± 0·07 | 5·76 ± 0·05 | |
| D. tristachyum | H 09/79 | BaFo | W Finsterau | 5·26 ± 0·05 | ||
| H 09/85 | BaFo | E Spiegelau | 5·23 ± 0·07 | 5·25 ± 0·06 | ||
| H 09/48 and H 09/91 | ObWa | Voithenberg | 5·29 ± 0·06 | 5·29 ± 0·06 | 5·26 ± 0·06 | |
| D. × issleri | H 09/77 | BaFo | W Finsterau | 6·68 ± 0·05 | ||
| H 09/66 | BaFo | Hirschenstein | 6·59 ± 0·07 | |||
| H 09/67 | BaFo | Haidmühle | 6·59 ± 0·05 | |||
| H 09/74 | BaFo | NNW Finsterau | 6·67 ± 0·09 | |||
| H 09/81 | BaFo | Rindel-Berg | 6·52 ± 0·06 | 6·61 ± 0·07 | ||
| H 09/70 | BoFo | Strázny | 6·61 ± 0·06 | 6·61 ± 0·06 | ||
| H 09/45 and H 09/89 | ObWa | Voithenberg | 6·61 ± 0·04 | 6·61 ± 0·04 | ||
| H 09/59 | BlFo | Feldberg | 6·73 ± 0·08 | 6·73 ± 0·08 | ||
| H 09/60 | Vos | Tanneck, type locality | 6·62 ± 0·01 | |||
| H 09/61 | Vos | Tanneck | 6·62 ± 0·02 | 6·62 ± 0·02 | ||
| H 08/03 | Har | Gr. Sonnenberg | 6·65 ± 0·05 | 6·65 ± 0·05 | 6·64 ± 0·07 | |
| D. × oellgaardii | H 09/78 | BaFo | W Finsterau | 6·45 ± 0·06 | ||
| H 09/83 | BaFo | E Spiegelau | 6·36 ± 0·10 | 6·40 ± 0·09 | ||
| H 09/71 | BoFo | Strázny | 6·34 ± 0·08 | 6·34 ± 0·08 | ||
| H 09/46 and H 09/90 | ObWa | Voithenberg | 6·40 ± 0·06 | 6·40 ± 0·06 | 6·39 ± 0·08 | |
| D. × zeilleri | H 09/80 | BaFo | W Finsterau | 5·51 ± 0·03 | ||
| H 09/84 | BaFo | E Spiegelau | 5·46 ± 0·04 | 5·50 ± 0·05 | ||
| H 09/49 and H 09/92 | ObWa | Voithenberg | 5·52 ± 0·07 | 5·52 ± 0·07 | ||
| H 09/52 | HeOd | Beerfelden 1 | 5·55 ± 0·04 | |||
| H 09/53 | HeOd | Beerfelden 2 | 5·56 ± 0·02 | 5·55 ± 0·03 | ||
| H 08/04 | Har | Gr. Sonnenberg | 5·56 ± 0·04 | 5·56 ± 0·04 | 5·53 ± 0·05 |
For abbreviations used, see Table 1.
To our knowledge, there are only very few reports on 2C DNA amounts in Diphasiastrum (Table 4). Our estimates exceed both the values published by Aagaard et al. (2009c) and those that were extracted from appendix 2 in Aagaard et al. (2009b) by about 1 pg. The use of different methods, Feulgen densitometry (Aagaard et al., 2009b, c) compared with flow cytometry (present study), cannot be responsible for these differences (see Doležel et al., 1998; Vilhar et al., 2001). However, the use of frozen or dried samples (as done by Aagaard et al. 2009b, c) has to be considered with caution as, due to an altered accessibility of the stain to the DNA, an apparent nucleic acid loss cannot be excluded, especially after long-term storage (Suda and Trávníček, 2006; Aagaard et al., 2009b, c).
Table 4.
DNA 2C values (pg) for several north temperate Diphasiastrum species and hybrids
| Taxon | Wang et al. (2005) | Aagaard et al. (2009c) | Aagaard et al. (2009b) | This study |
|---|---|---|---|---|
| D. alpinum | 6·40 | 6·45 (6·78) [6·97] | 7·52 | |
| D. complanatum | 4·82 | 4·81 (5·12) [5·37] | 5·76 | |
| D. digitatum | 5·45 | |||
| D. madeirense | 5·63 | |||
| D. tristachyum | 4·45 | 4·28 (4·70) | 5·26 | |
| D. × issleri | 5·61 | 6·64 | ||
| D. × oellgaardii | 5·21 | 6·39 | ||
| D. × zeilleri | 4·53 | 5·53 |
Data are from Wang et al. (2005), Aagaard et al. (2009b, c) and from this study; values in parentheses are mentioned by Aagaard et al. (2009b) under results and were obtained using Feulgen densitometry and those in square brackets were obtained by flow cytometric measurements using fresh material.
Aagaard et al. (2009b, appendix 2) presented a comprehensive list of Feulgen DNA image densitometry data on numerous Diphasiastrum accessions without, however, naming the taxa. The only information given is that of the origin of the chloroplast DNA (‘maternal lineage’). As a certain maternal chloroplast haplotype occurs in the corresponding species, but may also be present in the two hybrids derived from it, each accession could belong to three different taxa. For example, the chloroplast haplotype of D. alpinum is contained in D. alpinum itself, but also occurs in D. × issleri and D. × oellgaardii, provided D. alpinum was the female parent. We have attempted to identify the taxa of the accessions analysed by Aagaard et al. (2009b, appendix 2) by first selecting the three possible candidates on the basis of the maternal lineage and then comparing the reported 2C content with the values given by Aagaard et al. (2009c) for the three species. Taxa with intermediate values were interpreted as the corresponding hybrids. Some data sets could not be assigned due to incompatibility of data.
Data on genome size in other Lycopodiaceae are scarce. Omitting those of Bouchard (1976) because of large discrepancies compared with more recent studies (see Bennett and Leitch, 2001), there seem to be only two reports on members of the Lycopodiaceae. Lycopodium clavatum was determined to have a 2C DNA content of 5·72 pg (Hanson and Leitch, 2002), which is in the same range as our results for Diphasiastrum. In Huperzia lucidula a higher 2C DNA content of 11·40 pg was found (Wang et al., 2005), probably reflecting the high base number (x = 67 or 68) in this genus (Wagner and Beitel, 1993).
Nuclear DNA C values in the diploid hybrids (PI measurements)
The three diploid Diphasiastrum hybrids yielded 2C DNA values which are similar to those of the (diploid) non-hybrid species, ranging from 5·52 pg in D. × zeilleri to 6·63 pg in D. × issleri (Table 3). The values are exactly intermediate between those of their assumed parents and agree closely with those calculated from their putative parents by adding the corresponding 1C values for a single genome (Table 5). The concordance between the measured and expected values convincingly supports their hybrid origin, the assumed parentage (as inferred from morphological characters) as well as their homoploid status. Similarly to the species, the geographical variation (within and between regions) is small and statistically not significant (P < 0·01). Homoploid hybrids as confirmed by flow cytometry occur in all nine geographical regions studied (Fig. 1). In the Vosges Mountains, the Bavarian Forest, the Oberpfälzer Wald and the Bohemian Forest the occurrence of all three hybrids was confirmed, while in the remaining five areas the existence of two hybrids was established. Diphasiastrum × zeilleri appears to be the most common hybrid taxon and was shown to occur in seven out of nine regions.
Table 5.
DNA 2C values for the species and hybrids of Diphasiastrum studied
| Taxon and genome formula | Genome formula | Values measured (pg ± s.d.) | Values predicted (pg) | Difference measured – predicted (pg) |
|---|---|---|---|---|
| Species | ||||
| D. alpinum | AA | 7·52 ± 0·06 | ||
| D. complanatum | CC | 5·76 ± 0·05 | ||
| D. tristachyum | TT | 5·26 ± 0·06 | ||
| Diploid hybrids | ||||
| D. × issleri | AC | 6·64 ± 0·07 | 6·64 | 0·00 |
| D. × oellgaardii | AT | 6·39 ± 0·08 | 6·39 | 0·00 |
| D. × zeilleri | CT | 5·53 ± 0·05 | 5·51 | 0·02 |
| Triploid hybrids | ||||
| D. alpinum × D. × issleri | AAC | 10·30 ± 0·06 | 10·40 | –0·10 |
| D. alpinum × D. × oellgaardii | AAT | 9·97 ± 0·07 | 10·15 | –0·18 |
| D. complanatum × D. × issleri | ACC | 9·48 ± 0·12 | 9·52 | –0·04 |
For the hybrids, a comparison is made between the values measured and those predicted on the basis of the parental genome sizes; the calculation for the triploids was made in accordance with the assumed origin (e.g. by summing the size of an unreduced genome of a hybrid and one half of the parental species' genome; see text); the assumed hybrid origin is indicated by genome formulae.
Fig. 1.
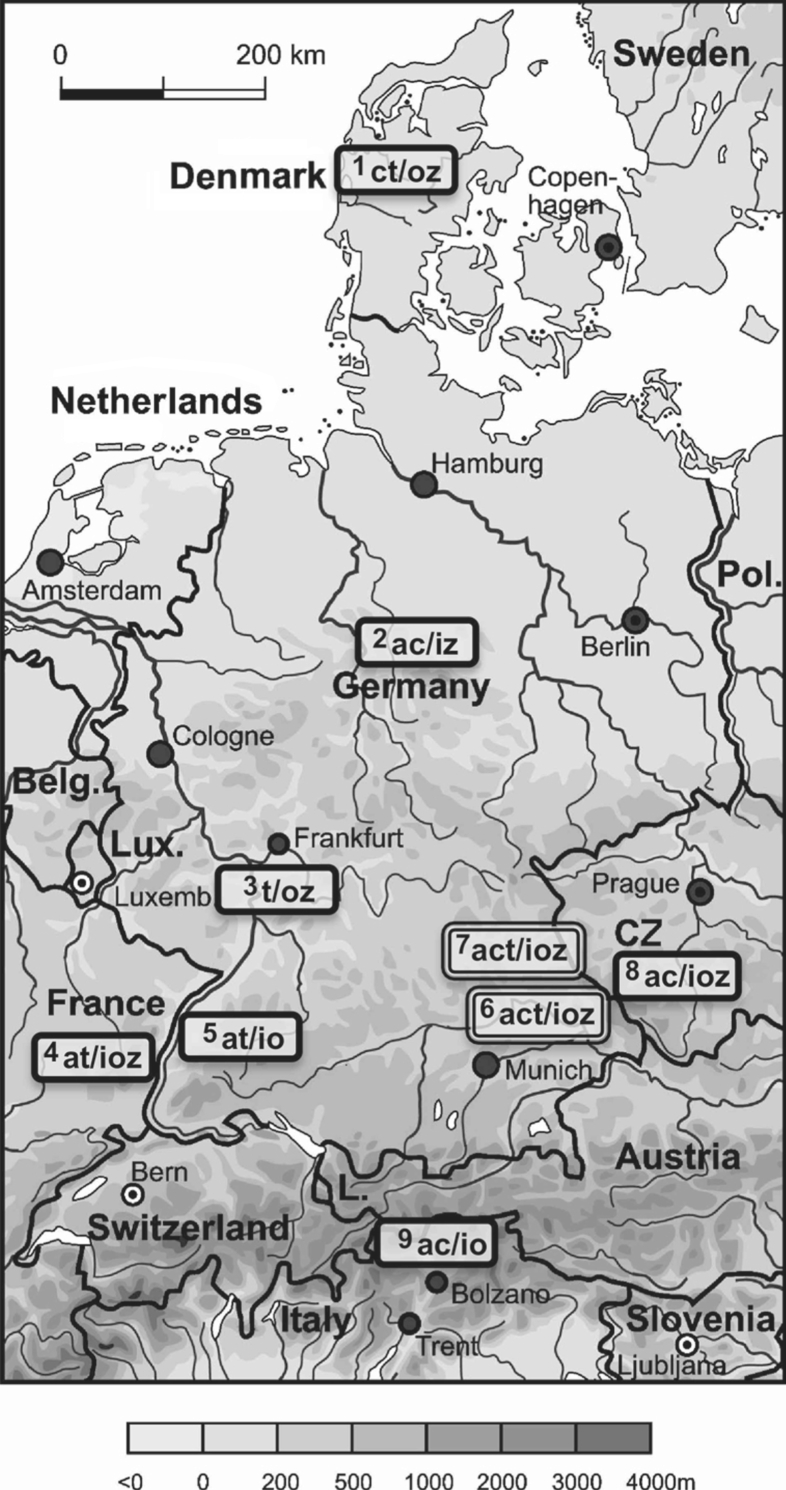
Map of Central Europe showing the distribution of Diphasiastrum taxa identified; a, D. alpinum; c, D. complanatum; i, D. × issleri; o, D. × oellgaardii; t, D. tristachyum; z, D. × zeilleri. Code numbers for the regions: 1Denmark, 2Harz Mountains, 3Hessischer Odenwald, 4Vosges Mountains, 5Black Forest, 6Bavarian Forest, 7Oberpfälzer Wald, 8Bohemian Forest, 9South Tyrol.
Just like in the species, our estimates exceed the values extracted from appendix 2 in Aagaard et al. (2009b) by about 1 pg (Table 4).
Diploid backcrossing
It has been proposed that in Diphasiastrum, backcrosses between hybrids and their parent species occur at the diploid level (Aagaard et al., 2009a). Exemplified for D. × issleri, backcrossing would require this primary hybrid to produce germinable haploid meiospores (with 23 chromosomes) containing chromosomes from both parents, D. alpinum and D. complanatum. If we assume the assortment of homologues in meiosis to be random (which is likely) and to follow a Gaussian distribution, 99 % of the spores produced contain 6–17 chromosomes of each parent. During fertilization, such a mixed chromosome set would be added to a single D. alpinum genome (23 chromosomes), resulting in a sporophyte with the majority (63–87 %) of its chromosomes being derived from D. alpinum and only a minor fraction (13–37 %) from D. complanatum.
Backcrosses exhibiting such more or less intermediate genotypes should not only be morphologically striking but also detectable by flow cytometry due to their intermediate DNA contents. Our 2C DNA values, however, are virtually invariable within each species and hybrid (P < 0·01), and the differences in genome sizes between all taxa are highly significant (P < 0·0001).
A rough estimate for Central Europe, based on data from field observations, revision of herbarium specimens and regional literature, reveals that the 15–17 populations of each hybrid included in this study (see Table 1) represent 7 % of the approx. 230 known populations in D. × zeilleri, 15 % in D. × issleri with about 110 colonies, and 71 % in D. × oellgaardii with 21 records. Considering the high crossability of species and the fact that agglomerations with 4–6 taxa co-occurring are not rare, backcrosses would be expected to be not uncommon and should be detectable in our sampling.
Aagaard et al. (2009b) presented evidence that reciprocal crosses occur repeatedly in D. × issleri and × zeilleri. In such reciprocal crosses no DNA content differences would be expected.
Genetic variability was observed in neighbouring or intermixed clones in two of the diploid hybrids (D. × issleri and × zeilleri) by means of isoenzyme gel electrophoresis (see Horn, 1997), which also suggests repeated hybridization events.
Molecular studies are in preparation by one of the authors (M. Schnittler) to (a) prove the status of the hybrid taxa by using species-specific uniparental inherited markers and (b) develop markers that allow genotyping of individuals to test the occurrence of diploid backcrossing.
Nuclear DNA C values and possible parentage of three putative triploid hybrids
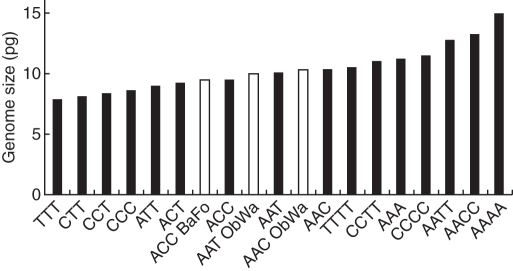
Considerably higher 2C DNA amounts than in the diploids were found in three accessions, with values ranging from 9·48 to 10·30 pg (Table 5). These exceed those of D. alpinum (which has the largest genome size of the diploids) by 2 to almost 3 pg, providing strong evidence that these plants are polyploids. Figure 2 shows the various genome sizes to be expected for the triploid and tetraploid genotype combinations based on the three diploid species. They range from 7·89 pg (in an autotriploid D. tristachyum, TTT) to 15·04 pg (in an autotetraploid D. alpinum, AAAA). As expected, the tetraploid cytotypes occupy the upper range, starting with 10·52 pg for an autotetraploid D. tristachyum (TTTT). This value is 0·22 pg higher than the largest 2C DNA amount measured for the new hybrids, allowing the conclusion that they represent triploids and not tetraploids. The closest concordances between measured and expected values were found assuming the following genome compositions: ACC for the accession 09/82 from the Bavarian Forest, and AAC for the accession 09/86 and AAT for the accession 09/87a, both from the Oberpfälzer Wald (Table 5). For unknown reasons, the matches are not as close as in the case of the diploid hybrids, a phenomenon observed in diploid and triploid Equisetum hybrids as well (Bennert et al., 2005). In all three Diphasiastrum hybrids the predicted values are slightly higher than those measured. Genome downsizing may be involved (see Leitch and Bennett, 2004), but, to our knowledge, has not been reported for lycophytes or ferns so far.
Fig. 2.
Bar chart displaying genome sizes (DNA values in pg) to be expected for the ten triploid (seven allotriploid and three autotriploid) and six tetraploid (three allotetraploid and three autotetraploid) genotype combinations (black columns) as calculated from the values measured for the six diploid taxa. The DNA contents of the three triploids found in nature are shown by white columns; for abbreviations used for regions, see Table 1; for letters used for genome composition, see Table 2.
The morphology of the triploid hybrids corroborates our interpretation (Fig. 3). The plant of accession 09/82 is generally intermediate between D. complanatum and D. × issleri, but displays a strong influence of D. complanatum, such as the distinctly flattened upright axes and the spreading lateral leaves, which narrow abruptly toward the slender apices and reduced lanceolate ventral leaves. In accession 09/86, considered to be an intermediate between D. alpinum and D. × issleri (AAC), the D. complanatum-like characters are less distinct, and the influence of D. alpinum is more pronounced as becomes manifest by longer ventral and dorsal leaves (Fig. 3). Accession 09/87 (intermediate between D. alpinum and D. × oellgaardii, AAT) is not reminiscent of D. complanatum, but shares characters with D. × oellgaardii, such as the length and shape of the ventral leaves; the shoots, however, are terete to triangular, approaching those of D. alpinum. Additional morphological details (growth form and habit of plants, number and insertion of strobili), giving further evidence for the assumed origin of the three triploids, will be published separately.
Fig. 3.
Photographs of shoots of the three putative triploid Diphasiastrum hybrids: (A, B) dorsal and ventral view of the hybrid interpreted as D. complanatum × D. × issleri (ACC), (C, D) dorsal and ventral view of the hybrid interpreted as D. alpinum × D. × issleri (AAC), (E, F) dorsal and ventral view of the hybrid interpreted as D. alpinum × D. × oellgaardii (AAT). Scale bar = 2·5 mm.
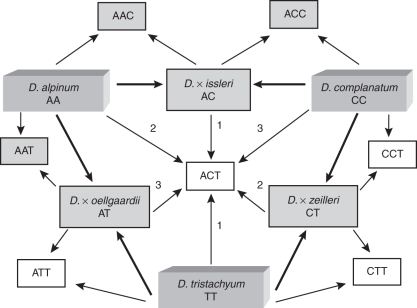
In total, seven allotriploid genome combinations are possible (Figs 2 and 4): six with a genome pair derived from the same parental species combined with a third chromosome set from another species (like AAC), and a unique combination composed of one genome each from all three diploid species (ACT). As shown in Fig. 4, there are three different routes by which this hybrid could be achieved. While in Diphasiastrum this three-parent hybrid is unknown, such a hybrid was detected in the genus Equisetum (Bennert et al., 2005; Lubienski et al., 2010).
Fig. 4.
Hybridization scheme showing the origin of the three known diploid and all seven possible triploid hybrids within Diphasiastrum in Central Europe; diploid species and hybrids as well as the three new triploids are shown in the grey boxes; for the triploid genotype ACT, all three possible origins are indicated.
Out of the seven possible triploids, three apparently exist in nature and, together with the diploid hybrids, deliver an impressive example of reticulate evolution in Diphasiastrum.
How could triploids have formed in nature?
When studying spore morphology, in all three diploid hybrids, besides well-formed spores and aborted spores, diplospores were discovered, which are recognizable by their larger size and more globose shape; details of this study will be published separately. The occurrence of diplospores was reported earlier for D. × oellgaardii by Øllgaard and Tind (1993). These unreduced diplospores, sometimes called ‘giant’ or ‘basketball’ spores (Wagner et al., 1986; Øllgaard and Tind, 1993), result from incomplete meiotic divisions and are believed to be capable of germinating and producing diploid gametophytes.
Tetraploids are unknown in European Diphasiastrum species, and our screening also failed to prove the existence of tetraploids in the regions studied. Thus, the common route of yielding triploids, i.e. from a cross between a tetraploid and a diploid species or cytotype, obviously is unlikely. We propose that the triploids arose instead as crosses between diploid gametophytes (stemming from a hybrid's diplospore) and a haploid gametophyte (derived from a normal meiospore of the second parent).
For the formation of the three putative triploid hybrids (ACC, AAC and AAT), only two diplospore-producing diploid hybrids would be required, namely D. × issleri (AC) and D. × oellgaardii (AT). For establishment of the genotypes AAC and ACC, a diploid D. × issleri gametophyte would backcross with either parent (D. alpinum or D. complanatum), while to obtain AAT a diploid gametophyte of D. × oellgaardii is required, which would backcross with D. alpinum. At the locality in the Bavarian Forest where the triploid ACC is growing, all six diploid taxa are present, and the same applies for the stand of the triploid AAT in the Oberpfälzer Wald, while the colony of AAC is separated by about 750 m from the main Diphasiastrum assemblage also containing the maximum number of six diploid taxa. So far, the range of the triploid hybrids is confined to southeastern Germany (Fig. 1). This is one centre of distribution of the diploid hybrids (D. × issleri and D. × oellgaardii) that are considered as parents (Bennert, 1999).
The significance of diplospores for the formation of triploids has been shown, e.g. for Athyrium (Schneller and Rasbach, 1984; Rasbach et al., 1991) and Equisetum, where several triploid taxa exist (Bennert et al., 2005), and, as in European Diphasiastrum, no tetraploids are known.
ACKNOWLEDGEMENTS
We thank Mrs Ilse Wessel, Bochum, Germany, and Mr Guy Van der Kinderen, Ghent, Belgium, for careful help with part of the lab work, Mr Pascal Holveck, Rauhwiller, France, for collecting a number of samples in the Vosges Mountains, and Mr Ralf Wieland, Bochum, Germany, for providing the geographical map of Central Europe.
LITERATURE CITED
- Aagaard SMD, Gyllenstrand N, Wikström N. Homoploid hybridization in Central European Diphasiastrum (Lycopodiaceae) 2009a In: Aagaard SMD. Reticulate evolution in Diphasiastrum (Lycopodiaceae). PhD thesis, Uppsala University, Sweden. [Google Scholar]
- Aagaard SMD, Greilhuber J, Vogel JC, Wikström N. Reticulate phylogenetic patterns in diploid European Diphasiastrum (Lycopodiaceae) 2009b In: Aagaard SMD. Reticulate evolution in Diphasiastrum (Lycopodiaceae). PhD thesis, Uppsala University, Sweden. [Google Scholar]
- Aagaard SMD, Greilhuber J, Zhang X-C, Wikström N. Occurrence and evolutionary origins of polyploids in the clubmoss genus Diphasiastrum (Lycopodiaceae) Molecular Phylogenetics and Evolution. 2009c;52:746–754. doi: 10.1016/j.ympev.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Bennert HW. Die seltenen und gefährdeten Farnpflanzen Deutschlands – Biologie, Verbreitung, Schutz. Münster-Hiltrup: Landwirtschaftsverlag; 1999. [Google Scholar]
- Bennert HW, Suksathan P, Horn K. Diphasiastrum multispicatum (J.H. Wilce) Holub (Lycopodiaceae) in Thailand. American Fern Journal. 2007;97:155–165. [Google Scholar]
- Bennert W, Lubienski M, Körner S, Steinberg M. Triploidy in Equisetum subgenus Hippochaete (Equisetaceae, Pteridophyta) Annals of Botany. 2005;95:807–815. doi: 10.1093/aob/mci084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MD, Leitch IJ. Nuclear DNA amounts in pteridophytes. Annals of Botany. 2001;87:335–345. [Google Scholar]
- Berry C, Fairon-Demaret MM. The Middle Devonian flora revisited. In: Gensel PG, Edwards D, editors. Plants invade the land: evolutionary and environmental perspectives. New York: Columbia University Press; 2001. pp. 120–139. [Google Scholar]
- Bouchard RA. DNA amount and organisation in some lower vascular plants. 1976 PhD Thesis, University of Chicago, USA. [Google Scholar]
- Doležel J, Greilhuber J, Suda J. Estimation of nuclear DNA content in plants using flow cytometry. Nature Protocols. 2007;2:2233–2244. doi: 10.1038/nprot.2007.310. [DOI] [PubMed] [Google Scholar]
- Doležel J, Greilhuber J, Lucretti S, et al. Plant genome size estimation by flow cytometry: inter-laboratory comparison. Annals of Botany. 1998;82(Suppl. A):17–26. [Google Scholar]
- Duff RJ, Nickrent DL. Phylogenetic relationships of land plants using mitochondrial small-subunit rDNA sequences. American Journal of Botany. 1999;86:372–386. [PubMed] [Google Scholar]
- Garratt MJ, Tims JD, Rickards RB, Chambers TC, Douglas JG. The appearance of Baragwanathia (Lycophytina) in the Silurian. Botanical Journal of the Linnean Society. 1984;89:355–358. [Google Scholar]
- Gensel PG, Berry CM. Early lycophyte evolution. American Fern Journal. 2001;91:74–98. [Google Scholar]
- Haines A. The families Huperziaceae and Lycopodiaceae of New England – a taxonomic and ecological reference. Bowdoin: VF Thomas Co; 2003. [Google Scholar]
- Hanson L, Leitch IJ. DNA amounts for five pteridophyte species fill phylogenetic gaps in C-value data. Botanical Journal of the Linnean Society. 2002;140:169–173. [Google Scholar]
- Hao SG, Gensel PG. The Posongchang floral assemblages of Southeastern Yunnan, China – diversity and disparity in early Devonian plant assemblages. In: Gensel PG, Edwards D, editors. Plants invade the land. Evolutionary and environmental perspectives. New York: Columbia University Press; 2001. pp. 103–119. [Google Scholar]
- Horn K. Verbreitung, Ökologie und Gefährdung der Flachbärlappe (Diphasiastrum spp., Lycopodiaceae, Pteridophyta) in Niedersachsen und Bremen. Naturschutz und Landschaftspflege in Niedersachsen. 1997;38:1–83. [Google Scholar]
- Horn K. Diphasiastrum Holub. Flachbärlapp. In: Zündorf HJ, Günther K-F, Korsch H, Westhus W, editors. Flora von Thüringen. Die wildwachsenden Farn- und Blütenpflanzen Thüringens. Jena: Weissdorn-Verlag; 2006. pp. 34–37. [Google Scholar]
- Horn K, editor. Steckbriefe zu den Gefäßpflanzen Bayerns. Diphasiastrum Holub – Flachbärlapp. 2008 http://www.bayernflora.de/de/info_pflanzen.php?taxnr=65151 , accessed 22 February 2011. [Google Scholar]
- Horn K, Tribsch A, editors. Diphasiastrum – Flachbärlapp. Online-Flora von Österreich. 2009 http://188.118.193.31/flora/Diphasiastrum , accessed 22 February 2011. [Google Scholar]
- Hueber FM. A new species of Baragwanathia from the Sextant Formation (Emsian) Northern Ontario, Canada. Botanical Journal of the Linnean Society. 1983;86:57–79. [Google Scholar]
- Kenrick P, Crane PR. The origin and early evolution of plants on land. Nature. 1997;389:33–39. [Google Scholar]
- Kotyk ME, Basinger JF, Gensel PG, De Freitas TA. Morphologically complex plant macrofossils from the late Silurian of arctic Canada. American Journal of Botany. 2002;89:1004–1013. doi: 10.3732/ajb.89.6.1004. [DOI] [PubMed] [Google Scholar]
- Leitch IJ, Bennett MD. Genome downsizing in polyploid plants. Biological Journal of the Linnean Society. 2004;82:651–663. [Google Scholar]
- Lubienski M, Bennert HW, Körner S. Two new triploid hybrids in Equisetum subgenus Hippochaete for Central Europe and notes on the taxonomic value of ‘Equisetum trachyodon forma Fuchsii’ (Equisetaceae, Pteridophyta) Nova Hedwigia. 2010;90:321–341. [Google Scholar]
- Øllgaard B. A revised classification of the Lycopodiaceae s. lat. Opera Botanica. 1987;92:153–178. [Google Scholar]
- Øllgaard B, Tind K. Scandinavian ferns. Copenhagen: Rhodos; 1993. [Google Scholar]
- Pigg KB. Isoetalean lycopsid evolution: from the Devonian to the present. American Fern Journal. 2001;91:99–114. [Google Scholar]
- Pryer KM, Schneider H, Magallón S. The radiation of vascular plants. In: Cracraft J, Donoghue MJ, editors. Assembling the Tree of Life. London: Oxford University Press; 2004a. pp. 138–153. [Google Scholar]
- Pryer KM, Schuettpelz E, Wolf PG, Schneider H, Smith AR, Cranfill R. Phylogeny and evolution of ferns (monilophytes) with a focus on the early leptosporangiate divergences. American Journal of Botany. 2004b;91:1582–1598. doi: 10.3732/ajb.91.10.1582. [DOI] [PubMed] [Google Scholar]
- Pryer KM, Schneider H, Smith AR, et al. Horsetails and ferns are a monophyletic group and the closest living relatives to seed plants. Nature. 2001;409:618–622. doi: 10.1038/35054555. [DOI] [PubMed] [Google Scholar]
- Rasbach H, Reichstein T, Schneller JJ. Hybrids and polyploidy in the genus Athyrium (Pteridophyta) in Europe. 2. Botanica Helvetica. 1991;101:209–225. Origin and description of two triploid hybrids and synthesis of allotetraploids. [Google Scholar]
- Raubeson LA, Jansen RK. Chloroplast DNA evidence on the ancient evolutionary split in vascular land plants. Science. 1992;255:1697–1699. doi: 10.1126/science.255.5052.1697. [DOI] [PubMed] [Google Scholar]
- Rickards RB. The age of the earliest club mosses: the Silurian Baragwanathia flora in Victoria, Australia. Geological Magazine. 2000;137:207–209. [Google Scholar]
- Schneller JJ, Rasbach H. Hybrids and polyploidy in the genus Athyrium (Pteridophyta) in Europe. Botanica Helvetica. 1984;94:81–99. [Google Scholar]
- Soltis PS, Soltis DE. The origin and diversification of angiosperms. American Journal of Botany. 2004;91:1614–1626. doi: 10.3732/ajb.91.10.1614. [DOI] [PubMed] [Google Scholar]
- Stewart WN, Rothwell GW. Paleobotany and the evolution of plants. 2nd edn. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Suda J, Trávnicek P. Reliable DNA ploidy determination in dehydrated tissues of vascular plants by DAPI flow cytometry – new prospects for plant research. Cytometry Part A. 2006;69:273–280. doi: 10.1002/cyto.a.20253. [DOI] [PubMed] [Google Scholar]
- Taylor E, Taylor TN, Krings M. Paleobotany: the biology and evolution of fossil plants. 2nd edn. Burlington, MA: Academic Press; 2009. [Google Scholar]
- Tsuji S, Ueda K, Nishiyama T, et al. The chloroplast genome from a lycophyte (microphyllophyte), Selaginella uncinata, has a unique inversion, transpositions and many gene losses. Journal of Plant Research. 2007;120:281–90. doi: 10.1007/s10265-006-0055-y. [DOI] [PubMed] [Google Scholar]
- Valentine DH, Moore DM, editors; Tutin T, Burges G, Chater NA, et al., editors. Lycopodiaceae. 2nd edn. Cambridge: Cambridge University Press; 1993. pp. 3–5. Flora Europaea. Vol. 1: Psilotaceae to Platanaceae. [Google Scholar]
- Vilhar B, Greilhuber J, Koce JD, Temsch EM, Dermastia M. Plant genome size measurement with DNA image cytometry. Annals of Botany. 2001;87:719–728. [Google Scholar]
- Wagner FS. Cytological problems in Lycopodium sens. lat. Annals of the Missouri Botanical Garden. 1992;79:718–729. [Google Scholar]
- Wagner WH, Beitel MJ Flora of North Americaial Committee. Flora of North America North of Mexico, vol. 2: Pteridophytes and gymnosperms. New York: Oxford University Press; 1993. Lycopodiaceae Mirbel – clubmoss family; pp. 18–37. [Google Scholar]
- Wagner WH, Wagner FS, Beitel JM. Evidence for interspecific hybridisation in pteridophytes with subterranean mycoparasitic gametophytes. Proceedings of the Royal Society of Edinburgh. 1985;86B:273–281. [Google Scholar]
- Wagner WH, Wagner FS, Taylor WC. Detecting abortive spores in herbarium specimens of sterile hybrids. American Fern Journal. 1986;76:129–140. [Google Scholar]
- Wang W, Tanurdzic M, Luo M, et al. Construction of a bacterial artificial chromosome library from the spikemoss Selaginella moellendorffii: a new resource for plant comparative genomics. BMC Plant Biology. 2005;5:10. doi: 10.1186/1471-2229-5-10. doi:10.1186/1471-2229-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström N. Diversification and relationships of extant homosporous lycopods. American Fern Journal. 2001;91:150–165. [Google Scholar]
- Wikström N, Kenrick P. Phylogeny of Lycopodiaceae (Lycopsida) and the relationship of Phylloglossum drumondii Kunze based on rbcL sequence data. International Journal of Plant Sciences. 1997;158:862–871. [Google Scholar]
- Yatsentyuk SP, Valiejo-Roman KM, Samigullin TH, Wilkström N, Troitsky AV. Evolution of Lycopodiaceae inferred from spacer sequencing of chloroplast rRNA genes. Russian Journal of Genetics. 2001;37:1068–1073. [Google Scholar]
- Zonneveld BJ, Leitch IJ, Bennett MD. First nuclear DNA amounts in more than 300 angiosperms. Annals of Botany. 2005;96:229–44. doi: 10.1093/aob/mci170. [DOI] [PMC free article] [PubMed] [Google Scholar]