Review on the cellular and molecular sources of IFN-I during virus infections with emphasis on plasmacytoid dendritic cells (pDC) and dsRNA sensors.
Keywords: interferon, plasmacytoid dendritic cell, Toll-like receptor 3, melanoma differentiation-associated gene 5, autoimmunity
Abstract
IFN-I are pleiotropic cytokines that impact innate and adaptive immune responses. In this article, we discuss TLR7/9 versus TLR3/MDA5 signaling in antiviral responses and diabetes. pDCs are thought to have a critical role in antiviral defense because of their ability to rapidly secrete large amounts of IFN-I through TLR7/9 signaling. A recent study demonstrates that although pDCs are a source of IFN-I in vivo, their overall contribution to viral containment is limited and time-dependent, such that additional cellular sources of IFN-I are required to fully control viral infections. dsRNA sensors, such as TLR3 and MDA5, provide another important trigger for antiviral IFN-I responses, which can be exploited to enhance immune responses to vaccines. In the absence of infection, IFN-I production by pDCs or from signaling through dsRNA sensors has been implicated in the pathogenesis of autoimmune diseases such as diabetes. However, recent data demonstrate that IFN-I production via TLR3 and MDA5 is critical to counter diabetes caused by a virus with preferential tropism for pancreatic β-cells. This highlights the complexity of the host antiviral response and how multiple cellular and molecular components balance protective versus pathological responses.
Introduction
The majority of cells in the body is equipped with the machinery to produce and respond to IFN-I (i.e., IFN-α, IFN-β). IFN-I were first described over 50 years ago as factors that interfere with virus replication [1–3]. Since then, it has become clear that in addition to having potent antiviral properties, IFN-I possess immunomodulatory activities [4]. IFN-I promote T cell responses, including CD8+ T cell effector functions, survival and memory [5–8], and Th1 polarization of CD4+ T cells [9]. IFN-I enhance NK cell activation [10] and support the differentiation and maturation of DCs, allowing them to prime naïve T cells effectively [11–13]. The differentiation of B cells into antibody-secreting plasma cells [14–16] and the proliferation of dormant hematopoietic stem cells [17] are also induced by IFN-I. Thus, IFN-I confer resistance to viruses and promote multiple immune functions. This review focuses on cellular and molecular sources for IFN-I during virus infections and diabetes with emphasis on pDCs and the dsRNA sensors, TLR3 and MDA5.
pDCs SPECIALIZE IN THE SECRETION OF IFN-I
pDCs are bone marrow-derived cells that specialize in the secretion of IFN-I. They were first described over 30 years ago as natural IPCs that activate NK cells upon exposure to viruses [18, 19]. The murine equivalent to human IPC was described in 2001 [20–22]. pDCs detect RNA and DNA viruses through two endosomal sensors, TLR7 and TLR9, respectively, which induce secretion of IFN-I through the MyD88-IRF7 signaling pathway [23, 24]. pDCs can produce IFN-I independently of IFNAR feedback signaling [25]; however, they do respond to IFN-I, generating an autocrine circuit through IFNAR, which augments IFN-I secretion and induces activation and migration [26, 27].
Upon engagement with nucleic acid ligands, TLR7 and TLR9 signal through the cytosolic adaptor MyD88. To induce IFN-I, MyD88 associates with IRAK1/4, TRAF3, IKKα, and osteopontin, leading to the phosphorylation and activation of IRF7 [23, 24, 28]. Phosphorylated IRF7 translocates to the nucleus and induces the transcriptional activation of IFN-I genes [29, 30]. IRF7 is positively regulated by the PI3K-mammalian target of rapamycin-p70S6K pathway [31] and repressed at the translational level by eukaryotic initiation factor 4E-binding proteins [32]. To induce inflammatory cytokines, MyD88 associates with IRAK1/4, triggering the TRAF6-TGF-β-activated kinase 1 pathway, which activates NF-κB and MAPK. MyD88 also recruits IRF5, which cooperates with NF-κB to induce inflammatory cytokines and chemokines [33].
The endosomal compartmentalization of TLR7/9 and their ligands determines whether IFN-I or inflammatory cytokines are produced. Condensed or multimeric RNA/DNA aggregates in early endosomes, eliciting the production of IFN-I, whereas monomeric forms reach late endosomes, resulting in cytokine secretion and pDC maturation [34, 35]. To induce IFN-I, it is necessary for TLR7 and TLR9 to translocate from the ER to a specialized lysosome-related organelle. This process requires adaptor protein 3, as well as Slc15a4, BLOC-1, and BLOC-2 [36, 37]. TLR9 activation also requires proteolytic cleavage in the endosome as well as Ly49Q and granulin [38–42].
Many viruses can promote pDC activation and secretion of IFN-I via the TLR7/9-MyD88-IRF7 pathway, such as MCMV, LCMV, HSV, VSV, HIV, HTLV-1, MHV, HCV, NDV, influenza, SeV, CVB, pneumovirus, and EBV [43–62]. Although TLR7/9 signaling promotes pDC activation and IFN-I secretion, TLR7 and TLR9 are also expressed in other immune cells, including monocytes, classical DCs, and B cell subsets [63, 64]. Thus, it is important to clarify to what extent pDCs contribute to innate and adaptive antiviral responses elicited by viruses sensed by the TLR7/9 pathway. TLR8 also uses the MyD88 signaling pathway and was initially shown to sense ssRNA in human monocytes, whereas the murine homologue was thought to be nonfunctional [65]. However, it was reported recently that viral DNA from vaccinia virus promotes pDC activation in a TLR8-dependent manner, suggesting the involvement of TLR8 in detecting ssRNA in mice [66].
IN VIVO ABLATION OF pDCs
To determine the contribution of pDCs in host-pathogen interactions, pDCs have been depleted in animal models with mAb. In early studies, pDCs were eliminated with a mAb specific for the antigen Gr-1 [20, 67], which is shared by Ly6C and Ly6G. Ly6C is expressed on pDCs but also on plasma cells [68], memory T cells [69], and inflammatory monocytes [70], whereas Ly6G is expressed on granulocytes. Thus, anti-Gr-1 mAb can deplete multiple cell types in addition to pDCs.
Several other pDC-depleting mAbs have been developed, including 120G8 [71], mouse pDC antigen 1 [47], and 927 [72]. These mAbs recognize BST2, which is expressed selectively on mouse pDCs and plasma cells in naïve mice but is up-regulated on most cell types following stimulation with IFN-I and IFN-γ [72]. Thus, the activation-induced, nonspecific expression of BST2 has important implications for using anti-BST2 mAb to deplete pDCs, as they may also deplete additional cell types during an immune response.
Given the limitations of pDC-depleting mAbs, mice lacking pDCs would be extremely valuable. Mice carrying a hypomorphic mutation in the Ikaros gene (IkL/L) lack pDCs [73]; however, Ikaros is a lymphoid cell-specific transcription factor that influences the development of other immune cell types as well [74–77]. Within the past few years, mice have been generated that more specifically lack pDCs, either constitutively or by inducible depletion [78, 79]. pDCs are absent in mice lacking the basic helix–loop–helix transcription factor E2-2/Tcf4, which is essential for pDC development [78]. In BDCA2-DTR Tg mice, which selectively express the DTR on pDCs, administration of DT results in the transient but specific depletion of pDCs [79]. Thus, E2-2-deficient mice and BDCA2-DTR Tg mice will be useful for assessing the contribution of pDCs to innate and adaptive immune responses to pathogens.
pDCs ARE AN EARLY BUT LIMITED SOURCE OF IFN-I DURING VIRUS INFECTIONS
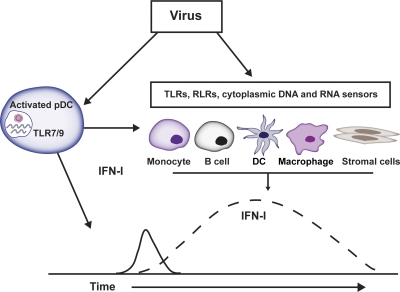
In a recent study, BDCA2-DTR Tg mice were challenged with representative RNA and DNA viruses, VSV and MCMV, respectively [79]. It was shown that pDCs provide an early but limited source of IFN-I during VSV and MCMV infections (Fig. 1). In the case of VSV infection, IFN-I produced by pDCs limits viral replication and perpetuates the survival and accumulation of antigen-specific CD8+ T cells. During MCMV infection, pDC-derived IFN-I can partially control viral burden, which reduces the expansion of MCMV-specific Ly49H+ NK cells. The ability of pDCs to contain MCMV replication is highly dependent on the initial viral inoculum. At low viral doses, pDCs are effective at restraining viral replication; however, at higher doses, their contribution to antiviral responses appears to be negligible. The restricted capacity of pDCs to augment antiviral responses when higher doses of virus are administered may be a result of their limited numbers at sites of viral replication. Although pDCs are only capable of controlling viral replication at low-to-intermediate doses, many natural infections occur in vivo by transmission of low numbers of virions. Therefore, in physiological settings, pDCs might be sufficient for antiviral responses. Furthermore, pDC-derived IFN-I might augment the expression of IFN-induced genes, including viral sensors, which promote an antiviral state [1–3], and further amplify IFN-I responses.
Figure 1. Cellular sources of IFN-I during viral infections.
pDCs detect RNA and DNA viruses through endosomal TLR7 and TLR9, respectively, leading to the secretion of IFN-I. IFN-I production by pDCs is early and transient, suggesting a need for additional cellular sources that provide a more broad, extended production of IFN-I to resolve viral infections. Other cellular sources that are critical in the production or responsive to IFN-I during virus infections include monocytes, B cells, DCs, macrophages, and stromal cells, which can produce IFN-I via TLRs (TLR2, TLR3, TLR7, TLR9) and RLRs (RIG-I, MDA5, and LGP2), and cytoplasmic DNA sensors, which are not discussed in this review (DNA-dependent activator of IRFs; IFN-γ-inducible protein IFI16 or p204; stimulator of IFN genes; HMGB1 and HMGB2; DHX36) [79–86]. Cytoplasmic helicases (DDX1-DDX21-DHX36 complex) have been identified, which sense dsRNA and activate IFN-I responses in the cytosol of myeloid DCs [87]. Macrophage subsets include subcapsular sinus macrophages, splenic marginal zone macrophages, metallophilic macrophages, microglia, and tissue macrophages, such as those found in the liver and lung (i.e., Kupffer and alveolar).
DIVERSITY AND SPECIFICITY OF dsRNA SENSORS
TLR7 and TLR9 are only two of multiple pathogen recognition sensors that detect viral nucleic acids. Viral dsRNA is sensed by TLR3 and the RLRs: RIG-I, MDA5, and LGP2 [23, 89–93]. TLR3 is mainly expressed in hematopoietic cells, particularly DCs and macrophages, but also in some stromal cells. TLR3 detects dsRNA, which gains access to the endosomal compartment by phagocytosis of virus-infected cells or apoptotic cell debris, internalization of antibodies bound to viruses, or autophagy [48, 58, 94]. TLR3 transmits signals through the TRIF pathway, which results in the phosphorylation and nuclear translocation of IRF3 and the transcription and secretion of IFN-β [95, 96]. TLR3-TRIF signaling also activates NF-κB and the transcription of inflammatory cytokine genes. The specificity of TLR3 for dsRNA allows recognition of RNA viruses such as EMCV, influenza A virus, CVB, WNV, DV, reovirus, and rhinovirus [97–106]. Moreover, TLR3 detects the DNA viruses MCMV and HSV-1, most likely through recognition of RNA intermediates, which may be generated during viral replication [107–111].
RIG-I and MDA5 are two cytosolic helicases induced by IFN-I in most cell types. RIG-I and MDA5 detect dsRNA intermediates that accumulate in the cytosol during viral replication [23, 91–93] and interact with the adaptor molecule IPS-1 (also known as mitochondrial antiviral-signaling protein, virus-induced signaling adapter, or Cardif) [112–115]. IPS-1 is localized on the mitochondria and recruits TRAF3, which activates TRAF family member-associated NF-κB-binding kinase 1 and IKKε, leading to the phosphorylation and nuclear translocation of IRF3 and IRF7 and production of IFN-β and IFN-α [116–118]. Additionally, IPS-1 associates with FADD protein and receptor-interacting protein-1, which activate caspase-8 and caspase-10, resulting in NF-κB activation and production of inflammatory cytokines [112, 119, 120]. IPS-1 is also located on peroxisomes and facilitates rapid antiviral responses through IRF1 [121].
RIG-I and MDA5 detect distinct dsRNA forms that differ in structure, length, and 5′ cap structures [122–128]. The distinct ligand preferences of the MDA5 and RIG-I receptors confer specific recognition of disparate viruses. RIG-I has been shown to detect paramyxoviruses (SeV, NDV, respiratory syncytial virus, and Measles virus); orthomyxovirus (influenza A and B viruses); rhabdovirus (VSV and Rabies virus); flavivirus (Japanese encephalitis virus, HCV, WNV, and DV); filovirus (Ebola virus); reovirus; and metapneumovirus [106, 123, 128–144]. Recent studies have shown that RIG-I also recognizes DNA viruses by detecting RNA intermediates generated through the RNA polymerase III-mediated transcription of dsDNA [145, 146]. MDA5 detects picornaviruses such as EMCV, CVB, Mengo virus, and Theiler virus, as well as murine norovirus 1 [132, 146–149]. MDA5 is also involved in the recognition of WNV, DV, reovirus, SeV, MHV, Measles virus, and LCMV [106, 134, 138, 139, 150–153].
LGP2 is another RLR that detects dsRNA [154–156]. LGP2 does not contain any signaling domains and was initially thought to negatively regulate MDA5 and RIG-I [156]. Accordingly, LGP2-deficient mice have more robust IFN-I responses following poly(I:C) stimulation and VSV infection compared with WT mice [155]. However, recent data have demonstrated that LGP2 may positively influence antiviral responses, as RLR-mediated IFN-I responses were impaired in mice lacking LGP2 or the LGP2 ATP-binding site [154].
DISTINCT ROLES OF TLR3 AND MDA5 IN poly(I:C)-STIMULATED IMMUNE RESPONSES
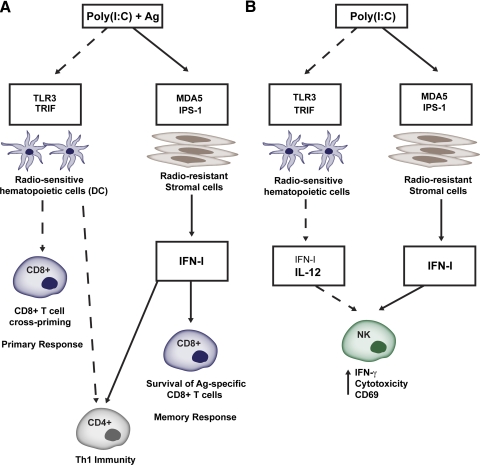
Recent studies demonstrate that IFN-I responses via dsRNA sensors can be exploited in vaccination to enhance adaptive immune responses to antigens. Poly(I:C) is a synthetic analog of viral dsRNA, which is mainly detected by TLR3 and MDA5 [98, 132, 147, 157, 158], although RIG-I can also detect short forms of poly(I:C) [126]. By stimulating hematopoietic and stromal cells through TLR3 and MDA5, poly(I:C) induces a rise in systemic IFN-I, which activates DCs, boosting their capacity to elicit antigen-specific CD4+ T cells [159–162]. Poly(I:C) also stimulates antigen-specific CD8+ T cell immunity. Initial studies showed that poly(I:C) triggers TLR3 in CD8α+ DCs, which then acquire the capacity to cross-prime CD8+ T cells [94]. Then, it was found that poly(I:C) requires TRIF and IPS-1 signaling pathways to boost antigen-specific CD8+ T cell and antigen-specific B cell responses [163, 164], suggesting that TLR3 and MDA5 impact T and B cell responses. Accordingly, recent bone marrow chimera experiments demonstrated that poly(I:C) stimulates TLR3 in hematopoietic cells, most likely DCs, promoting cross-priming of antigen-specific CD8+ T cells, i.e., primary responses [165]. Additionally, poly(I:C) stimulates MDA5 in stromal cells, inducing a rise in systemic IFN-I, which supports the survival of antigen-primed CD8+ T cells and the establishment of CD8+ T cell memory [165] (Fig. 2).
Figure 2. Poly(I:C) promotes T cell responses and NK cell activation through TLR3 and MDA5 signaling.
(A) TLR3 and MDA5 impact CD8+ and CD4+ T cell responses. Poly(I:C)-mediated stimulation of TLR3 is required in hematopoietic cells to promote cross-priming of antigen-specific CD8+ T cells, i.e., primary responses, whereas poly(I:C)-mediated stimulation of MDA5 is required in stromal cells to induce a systemic rise in IFN-I secretion that promotes the survival of antigen-primed CD8+ T cells and the establishment of CD8+ T cell memory. IFN-I, from hematopoietic and stromal cells, is necessary for the adjuvant function of poly(I:C) to induce effective CD4+ Th1 immunity. (B) Roles of TLR3 and MDA5 in poly(I:C)-mediated NK cell activation. TLR3 contributes to NK cell activation by promoting IFN-I and IL-12 release by hematopoietic cells, whereas MDA5 activates NK cells indirectly, by promoting stromal cell release of IFN-I.
Poly(I:C)-mediated triggering of TLR3 and MDA5 can also be used to promote NK cell activity against cancer cells. Studies in humans showed that poly(I:C) promotes NK activities by triggering TLR3 expressed in cultured NK cells [166–169]. In contrast, murine NK cells do not express TLR3, and poly(I:C)-mediated activation of NK cells is indirect. Initially, it was shown that poly(I:C) activates DCs through TLR3 and that DCs in turn activate NK cells [170]. Subsequently, it was demonstrated that poly(I:C) triggers the TRIF and IPS-1 pathways in CD8α+ DCs, which then acquire the ability to activate NK cells [171], implicating TLR3 and MDA5 in poly(I:C)-mediated NK cell activation. A recent study showed that NK cell activation is mostly dependent on MDA5, whereas TLR3 has a modest impact, only evident in the absence of MDA5 [172] (Fig. 2). TLR3 contributed to NK cell activation by promoting IFN-I and IL-12 release by hematopoietic cells. MDA5 acted indirectly by promoting stromal cell release of IFN-I, which activated NK cells. Thus, in mice, poly(I:C) stimulates NK cell responses by inducing the production of various cytokines in a manner dependent on the cell type and dsRNA sensor.
pDCs AND dsRNA SENSORS IN DIABETES
A major outcome of TLR7/9 and TLR3/MDA5 signaling is IFN-I production. Although IFN-I is critical for inducing an antiviral state and promoting protective immune responses, it has been proposed that excessive IFN-I production can result in autoimmunity [4, 173–176]. Exaggerated IFN-I has been associated with T1D, an autoimmune disease caused by T cell destruction of pancreatic β-cells [177–179]. One report demonstrated that Tg mice expressing IFN-I in insulin-producing β-cells develop islet inflammation and hypoinsulinemic diabetes, which can be prevented by neutralizing IFN-I [180]. Subsequently, it was shown that IFN-I accelerates T1D in NOD mice and breaks tolerance to β-cells in nondiabetes-prone mice [181]. A more recent study revealed that NOD mice have increased IFN-I levels and IFN-I-producing pDCs in pancreatic LNs prior to the onset of diabetes [182], shortly after the wave of β-cell apoptosis, which peaks at postnatal days 14–17 [183]. Blockade of IFNAR in NOD mice at 2–3 weeks of age delayed the onset and incidence of T1D. Moreover, mAb-mediated depletion of pDCs in young NOD mice also delayed the onset and incidence of T1D [184]. Although these studies were conducted in mice, the expansion of IFN-I-producing pDCs has been documented in human patients with T1D around the time of diagnosis [185]. Thus, pDC-derived IFN-I may serve a pathogenic role in the development of T1D.
poly(I:C) can hasten T1D in animal models [186, 187], suggesting that dsRNA sensors, such as TLR3 and RLRs, may also promote T1D in an IFN-I-dependent manner. In support of this hypothesis, genome-wide association studies have demonstrated that human polymorphisms in the RIG-I and MDA5 genes, which reduce IFN-I responses to dsRNA, are linked with resistance to T1D [188–192]. Interestingly, It has been reported that NK cells mediate islet inflammation in humans with T1D, infiltrate the islets of NOD mice, and are required for accelerated T1D driven by IFN-I [193–195]. A recent study found that the activating receptor NKp46, expressed almost exclusively by NK cells, is essential for the development of T1D in NOD mice and in the streptozotocin diabetes model [196]. Thus, IFN-I produced by pDCs (TLR7/9) or other cell types via dsRNA sensors may promote NK cell accumulation and activation in islets, which results in NK cell-mediated destruction of β-cells and T1D.
Paradoxically, other studies have found that treatment with IFN-I can be protective and reduce the incidence of T1D in NOD mice [197–199]. Furthermore, NOD mice have delayed onset or are protected completely from diabetes after exposure to certain viruses [200–202], suggesting that viral detection and IFN-I responses may promote T cell tolerance by enhancing T regulatory cell activity and up-regulation of inhibitory molecules and cytokines [202–205].
Certain viruses have tropism for the pancreas and can induce diabetes by directly destroying β-cells or by causing immune cell-mediated β-cell death [177]. In the context of these viral infections, IFN-I may be protective [177]. Acute LCMV infection in Tg mice expressing the LCMV glycoprotein under control of the RIP-GP causes diabetes [206]. In this model, pDCs counter infection and diabetes through IFN-I secretion [203]. Paradoxically, another study reported that hematopoietic-derived IFN-I was essential for CD8+ T cell-mediated destruction of β-cells and induction of T1D in LCMV-infected RIP-GP mice [207]. CVB and EMCV-D replicate in the pancreas of mice, causing extensive tissue damage and diabetes [208, 209]. Diabetes onset in CVB-infected mice has been attributed to bystander damage of β-cells, leading to the release of sequestered islet antigens, which results in the restimulation of resting, autoreactive T cells [210]. In contrast, diabetes induced by EMCV-D appears to be a consequence of direct β-cell destruction mediated by macrophages rather than T cells [211]. Clinical studies have also linked coxsackievirus and enterovirus infections to human T1D, but whether these viruses induce direct β-cell damage or trigger autoimmunity remains unclear [212, 213]. It has been shown that IFN-I induces an antiviral state in human islet cells and protects them from coxsackievirus replication [214]. Thus, IFN-I may be critical for β-cell survival and protection from virus-induced T1D in humans.
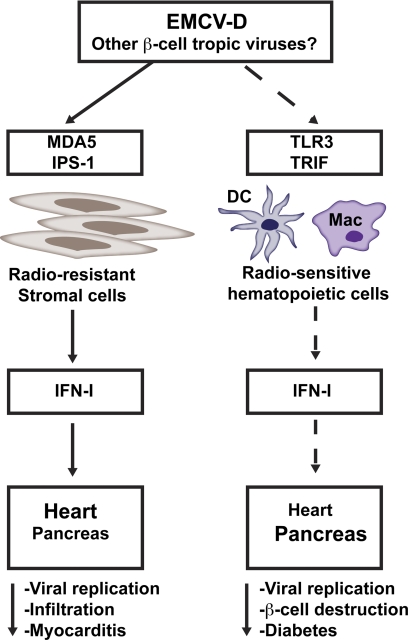
Given that EMCV replication generates dsRNA intermediates that trigger IFN-I responses through MDA5 [132, 147] and TLR3 [97], we recently examined the relative contributions of these sensors in the host response to EMCV-D and development of diabetes [215] (Fig. 3). We found that TLR3-deficient mice develop diabetes as a result of hematopoietic cells failing to mount an early IFN-I response that protects β-cells from virus-induced damage. The role of MDA5 in diabetes could not be assessed precisely, as MDA5 has a dominant role in protecting the heart against EMCV-D infection, and MDA5−/− mice die from severe myocarditis before they can develop diabetes. However, MDA5+/− mice, which survive EMCV-D infection, do develop transient hyperglycemia, suggesting a role for MDA5 in preventing virus-induced diabetes [215]. Altogether, these data indicate that IFN-I responses mediated by TLR3 and MDA5 during EMCV-D infection influence the pathogenesis of T1D. As LGP2 positively regulates RLR-mediated responses to picornaviruses, such as EMCV [154, 155], it will be worthwhile to investigate whether mice deficient in LGP2 are more susceptible to virus-induced diabetes. In summary, IFN-I responses may trigger the activation of autoreactive T cells in autoimmune T1D; however, during infection with a pancreatic-tropic virus, IFN-I responses reduce viral replication, thereby preventing β-cell damage and diabetes (Fig. 3).
Figure 3. Roles of MDA5 and TLR3 in virus-induced diabetes.
MDA5 has a dominant role in protecting the heart against EMCV-D infection. TLR3-deficient mice develop diabetes as a result of hematopoietic cells, failing to mount an early IFN-I response that protects β-cells from virus-induced damage. The role of MDA5 in virus-induced diabetes could not be assessed, as MDA5-deficient mice die from myocarditis within the first 5 days of infection. However, studies in MDA5+/− mice, which survive EMCV-D infection, do develop transient hyperglycemia, suggesting at least a partial for MDA5 in preventing virus-induced diabetes. Thus, IFN-I responses mediated by MDA5 and TLR3 can reduce viral replication, preventing β-cell destruction and diabetes.
CONCLUDING REMARKS
Virtually all cells in the body are capable of producing IFN-I. As pDCs are more specialized than other cell types, it was thought that they might be the predominant source of IFN-I during viral infections. However, increasing evidence suggests that in vivo, pDC contribution to antiviral response is limited in magnitude and time and that multiple cellular sources contribute to protective or in the case of autoimmunity, detrimental responses mediated by IFN-I (Fig. 1). Recently, it was shown that subcapsular sinus macrophages produce IFN-I during peripheral VSV infection and prevent CNS invasion [216]. Splenic marginal zone and metallophilic macrophages are important for IFN-I production during systemic HSV-1 [217] and LCMV [218] infections. Tissue resident macrophages in the liver control viral replication in an IFN-I-dependent matter in LCMV-infected mice, although they were not reported to produce IFN-I themselves [219], whereas alveolar macrophages and microglia are dominant sources of IFN-I during respiratory or brain viral infections, respectively [51, 151]. Inflammatory monocytes secrete IFN-I in a TLR2-dependent manner and are necessary for early restriction of vaccinia virus replication [220]. Splenic DCs produce IFN-I in response to adenoviruses [221] and MCMV [222]. It has also been shown that splenic macrophages [223] and a subset of B cells [224] are major producers of IFN-I during Listeria infection and that splenic DCs secrete IFN-I in response to group B streptococcus [224]. Our studies indicate that stromal cells and DCs are critical sources of IFN-I during infection with EMCV [215]. Altogether, these findings indicate that several cellular sources of IFN-I exist in vivo and probably depend on the pathogen and site of infection (Fig. 1).
A multiplicity of viral sensors seems essential for effective IFN-I responses. Clearly, distinct sensors differ in their specificity for viruses and viral products. Furthermore, distinct sensors may differ in their tissue distribution, such that sensors required for responses to a given virus in one organ may vary in another. During EMCV-D infection, for example, MDA5 is critical in the heart, whereas MDA5 and TLR3 restrict virus-induced damage in the pancreas. To make matters more complex, organs and tissues might express different sensors at different time-points during infections. MDA5 is an IFN-induced protein, whereas TLR3 is constitutively expressed; therefore, TLR3-mediated responses might occur earlier than MDA5-mediated responses [215, 226].
Specificity, tissue distribution, and time-frame of action of viral sensors are all critical factors contributing to protective or detrimental immune responses. Understanding whether viral sensors have complementary and nonredundant or redundant roles in particular organs and cell types, whether they synergize or antagonize each other during host responses, will aid in the design of better vaccines and therapies for infections and autoimmune diseases.
ACKNOWLEDGMENTS
These studies were supported by the Juvenile Diabetes Research Foundation grant 24-2007-420 and NIH grant CA109673 (to M.C.). M.S. was supported by the NRSA training grant 5T32DK007296 from the NIDDK. S.A.M. was supported by NRSA F30HL096354 from the NHLBI and T32DK007296 from NIDDK. Y.W. was supported by Pulmonary and Critical Care training grant 2T32HL007317 from the NHLBI.
Footnotes
- BDCA2
- blood DC antigen 2
- BLOC-1/2
- biogenesis of lysosome-related organelles, complex 1/2
- BST2
- bone marrow stromal cell antigen 2
- CVB
- coxsackievirus B
- DDX1/21
- Asp-Glu-Ala-Asp box polypeptide 1/21
- DHX36
- DexD/H box-containing helicase 36
- DT
- diphtheria toxin
- DTR
- diphtheria toxin receptor
- DV
- Dengue virus
- EMCV
- encephalomyocarditis virus
- HCV
- hepatitis C virus
- HMGB1/2
- high-mobility group box 1/2 proteins
- HSV
- herpes simplex virus
- HTLV-1
- human T-cell leukemia virus 1
- IFN-I
- type I IFN(s)
- IFNAR
- IFN-α/β receptor
- IPC
- IFN-producing cell
- IPS-1
- IFN-β-promoter stimulator 1
- IRAK1/4
- IL-1R-associated kinase 1/4
- IRF
- IFN regulatory factor
- LCMV
- lymphocytic choriomeningitis virus
- LGP2
- laboratory of genetics and physiology-2
- MCMV
- murine CMV
- MDA5
- melanoma differentiation-associated gene 5
- MHV
- murine hepatitis virus
- NDV
- Newcastle disease virus
- NHLBI
- National Heart, Lung, and Blood Institute
- NIDDK
- National Institute of Diabetes and Digestive and Kidney Diseases
- NRSA
- National Research Service Award
- pDC
- plasmacytoid DC
- poly(I:C)
- polyinosinic:polycytidylic acid
- RIG-I
- retinoic acid-inducible gene-I
- RIP-GP
- rat insulin promoter-glycoprotein
- RLR
- retinoic acid-inducible gene-I-like receptor
- SeV
- Sendai virus
- T1D
- type I diabetes
- Tg
- transgenic
- TRAF
- TNF receptor-associated factor
- TRIF
- Toll/IL-1R domain-containing adaptor-inducing IFN-β
- VSV
- vesicular stomatitis virus
- WNV
- West Nile virus
AUTHORSHIP
M.S. contributed data and wrote the manuscript. S.A.M. and Y.W. contributed data and reviewed the manuscript. M.C. reviewed the manuscript.
REFERENCES
- 1. Nagano Y., Kojima Y. (1954) Immunizing property of vaccinia virus inactivated by ultraviolets rays. C. R. Seances Soc. Biol. Fil. 148, 1700–1702 [PubMed] [Google Scholar]
- 2. Lindenmann J., Burke D. C., Isaacs A. (1957) Studies on the production, mode of action and properties of interferon. Br. J. Exp. Pathol. 38, 551–562 [PMC free article] [PubMed] [Google Scholar]
- 3. Pestka S., Krause C. D., Walter M. R. (2004) Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 202, 8–32 [DOI] [PubMed] [Google Scholar]
- 4. Trinchieri G. (2010) Type I interferon: friend or foe? J. Exp. Med. 207, 2053–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marrack P., Kappler J., Mitchell T. (1999) Type I interferons keep activated T cells alive. J. Exp. Med. 189, 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Le Bon A., Tough D. F. (2002) Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14, 432–436 [DOI] [PubMed] [Google Scholar]
- 7. Kolumam G. A., Thomas S., Thompson L. J., Sprent J., Murali-Krishna K. (2005) Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202, 637–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mescher M. F., Curtsinger J. M., Agarwal P., Casey K. A., Gerner M., Hammerbeck C. D., Popescu F., Xiao Z. (2006) Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 211, 81–92 [DOI] [PubMed] [Google Scholar]
- 9. Trinchieri G. (2003) Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3, 133–146 [DOI] [PubMed] [Google Scholar]
- 10. Biron C. A. (2001) Interferons α and β as immune regulators—a new look. Immunity 14, 661–664 [DOI] [PubMed] [Google Scholar]
- 11. Blanco P., Palucka A. K., Gill M., Pascual V., Banchereau J. (2001) Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science 294, 1540–1543 [DOI] [PubMed] [Google Scholar]
- 12. Santini S. M., Di Pucchio T., Lapenta C., Parlato S., Logozzi M., Belardelli F. (2002) The natural alliance between type I interferon and dendritic cells and its role in linking innate and adaptive immunity. J. Interferon Cytokine Res. 22, 1071–1080 [DOI] [PubMed] [Google Scholar]
- 13. Le Bon A., Etchart N., Rossmann C., Ashton M., Hou S., Gewert D., Borrow P., Tough D. F. (2003) Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4, 1009–1015 [DOI] [PubMed] [Google Scholar]
- 14. Le Bon A., Schiavoni G., D'Agostino G., Gresser I., Belardelli F., Tough D. F. (2001) Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14, 461–470 [DOI] [PubMed] [Google Scholar]
- 15. Jego G., Palucka A. K., Blanck J. P., Chalouni C., Pascual V., Banchereau J. (2003) Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 19, 225–234 [DOI] [PubMed] [Google Scholar]
- 16. Poeck H., Wagner M., Battiany J., Rothenfusser S., Wellisch D., Hornung V., Jahrsdorfer B., Giese T., Endres S., Hartmann G. (2004) Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood 103, 3058–3064 [DOI] [PubMed] [Google Scholar]
- 17. Essers M. A., Offner S., Blanco-Bose W. E., Waibler Z., Kalinke U., Duchosal M. A., Trumpp A. (2009) IFNα activates dormant hematopoietic stem cells in vivo. Nature 458, 904–908 [DOI] [PubMed] [Google Scholar]
- 18. Trinchieri G., Santoli D. (1978) Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J. Exp. Med. 147, 1314–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trinchieri G., Santoli D., Dee R. R., Knowles B. B. (1978) Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Identification of the anti-viral activity as interferon and characterization of the human effector lymphocyte subpopulation. J. Exp. Med. 147, 1299–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Asselin-Paturel C., Boonstra A., Dalod M., Durand I., Yessaad N., Dezutter-Dambuyant C., Vicari A., O'Garra A., Biron C., Briere F., Trinchieri G. (2001) Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2, 1144–1150 [DOI] [PubMed] [Google Scholar]
- 21. Nakano H., Yanagita M., Gunn M. D. (2001) CD11c(+)B220(+)Gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 194, 1171–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Björck P. (2001) Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood 98, 3520–3526 [DOI] [PubMed] [Google Scholar]
- 23. Kawai T., Akira S. (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 [DOI] [PubMed] [Google Scholar]
- 24. Blasius A. L., Beutler B. (2010) Intracellular Toll-like receptors. Immunity 32, 305–315 [DOI] [PubMed] [Google Scholar]
- 25. Barchet W., Cella M., Odermatt B., Asselin-Paturel C., Colonna M., Kalinke U. (2002) Virus-induced interferon α production by a dendritic cell subset in the absence of feedback signaling in vivo. J. Exp. Med. 195, 507–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Asselin-Paturel C., Brizard G., Chemin K., Boonstra A., O'Garra A., Vicari A., Trinchieri G. (2005) Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J. Exp. Med. 201, 1157–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumagai Y., Kumar H., Koyama S., Kawai T., Takeuchi O., Akira S. (2009) Cutting edge: TLR-dependent viral recognition along with type I IFN positive feedback signaling masks the requirement of viral replication for IFN-{α} production in plasmacytoid dendritic cells. J. Immunol. 182, 3960–3964 [DOI] [PubMed] [Google Scholar]
- 28. Shinohara M. L., Lu L., Bu J., Werneck M. B., Kobayashi K. S., Glimcher L. H., Cantor H. (2006) Osteopontin expression is essential for interferon-α production by plasmacytoid dendritic cells. Nat. Immunol. 7, 498–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Honda K., Yanai H., Negishi H., Asagiri M., Sato M., Mizutani T., Shimada N., Ohba Y., Takaoka A., Yoshida N., Taniguchi T. (2005) IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434, 772–777 [DOI] [PubMed] [Google Scholar]
- 30. Honda K., Yanai H., Takaoka A., Taniguchi T. (2005) Regulation of the type I IFN induction: a current view. Int. Immunol. 17, 1367–1378 [DOI] [PubMed] [Google Scholar]
- 31. Cao W., Manicassamy S., Tang H., Kasturi S. P., Pirani A., Murthy N., Pulendran B. (2008) Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat. Immunol. 9, 1157–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Colina R., Costa-Mattioli M., Dowling R. J., Jaramillo M., Tai L. H., Breitbach C. J., Martineau Y., Larsson O., Rong L., Svitkin Y. V., Makrigiannis A. P., Bell J. C., Sonenberg N. (2008) Translational control of the innate immune response through IRF-7. Nature 452, 323–328 [DOI] [PubMed] [Google Scholar]
- 33. Takaoka A., Yanai H., Kondo S., Duncan G., Negishi H., Mizutani T., Kano S., Honda K., Ohba Y., Mak T. W., Taniguchi T. (2005) Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature 434, 243–249 [DOI] [PubMed] [Google Scholar]
- 34. Honda K., Ohba Y., Yanai H., Negishi H., Mizutani T., Takaoka A., Taya C., Taniguchi T. (2005) Spatiotemporal regulation of MyD88-IRF-7 signaling for robust type-I interferon induction. Nature 434, 1035–1040 [DOI] [PubMed] [Google Scholar]
- 35. Guiducci C., Ott G., Chan J. H., Damon E., Calacsan C., Matray T., Lee K. D., Coffman R. L., Barrat F. J. (2006) Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J. Exp. Med. 203, 1999–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sasai M., Linehan M. M., Iwasaki A. (2010) Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science 329, 1530–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blasius A. L., Arnold C. N., Georgel P., Rutschmann S., Xia Y., Lin P., Ross C., Li X., Smart N. G., Beutler B. (2010) Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA 107, 19973–19978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park B., Brinkmann M. M., Spooner E., Lee C. C., Kim Y. M., Ploegh H. L. (2008) Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat. Immunol. 9, 1407–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ewald S. E., Lee B. L., Lau L., Wickliffe K. E., Shi G. P., Chapman H. A., Barton G. M. (2008) The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature 456, 658–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ewald S. E., Engel A., Lee J., Wang M., Bogyo M., Barton G. M. (2011) Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J. Exp. Med. 208, 643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yoshizaki M., Tazawa A., Kasumi E., Sasawatari S., Itoh K., Dohi T., Sasazuki T., Inaba K., Makrigiannis A. P., Toyama-Sorimachi N. (2009) Spatiotemporal regulation of intracellular trafficking of Toll-like receptor 9 by an inhibitory receptor, Ly49Q. Blood 114, 1518–1527 [DOI] [PubMed] [Google Scholar]
- 42. Park B., Buti L., Lee S., Matsuwaki T., Spooner E., Brinkmann M. M., Nishihara M., Ploegh H. L. (2011) Granulin is a soluble cofactor for Toll-like receptor 9 signaling. Immunity 34, 505–513 [DOI] [PubMed] [Google Scholar]
- 43. Lund J., Sato A., Akira S., Medzhitov R., Iwasaki A. (2003) Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198, 513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lund J. M., Alexopoulou L., Sato A., Karow M., Adams N. C., Gale N. W., Iwasaki A., Flavell R. A. (2004) Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 101, 5598–5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krug A., Luker G. D., Barchet W., Leib D. A., Akira S., Colonna M. (2004) Herpes simplex virus type 1 activates murine natural interferon-producing cells through Toll-like receptor 9. Blood 103, 1433–1437 [DOI] [PubMed] [Google Scholar]
- 46. Yoneyama H., Matsuno K., Toda E., Nishiwaki T., Matsuo N., Nakano A., Narumi S., Lu B., Gerard C., Ishikawa S., Matsushima K. (2005) Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J. Exp. Med. 202, 425–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Krug A., French A. R., Barchet W., Fischer J. A., Dzionek A., Pingel J. T., Orihuela M. M., Akira S., Yokoyama W. M., Colonna M. (2004) TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21, 107–119 [DOI] [PubMed] [Google Scholar]
- 48. Lee H. K., Lund J. M., Ramanathan B., Mizushima N., Iwasaki A. (2007) Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science 315, 1398–1401 [DOI] [PubMed] [Google Scholar]
- 49. Delale T., Paquin A., Asselin-Paturel C., Dalod M., Brizard G., Bates E. E., Kastner P., Chan S., Akira S., Vicari A., Biron C. A., Trinchieri G., Briere F. (2005) MyD88-dependent and -independent murine cytomegalovirus sensing for IFN-α release and initiation of immune responses in vivo. J. Immunol. 175, 6723–6732 [DOI] [PubMed] [Google Scholar]
- 50. Beignon A. S., McKenna K., Skoberne M., Manches O., DaSilva I., Kavanagh D. G., Larsson M., Gorelick R. J., Lifson J. D., Bhardwaj N. (2005) Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Invest. 115, 3265–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kumagai Y., Takeuchi O., Kato H., Kumar H., Matsui K., Morii E., Aozasa K., Kawai T., Akira S. (2007) Alveolar macrophages are the primary interferon-α producer in pulmonary infection with RNA viruses. Immunity 27, 240–252 [DOI] [PubMed] [Google Scholar]
- 52. Zucchini N., Bessou G., Traub S., Robbins S. H., Uematsu S., Akira S., Alexopoulou L., Dalod M. (2008) Cutting edge: overlapping functions of TLR7 and TLR9 for innate defense against a herpesvirus infection. J. Immunol. 180, 5799–5803 [DOI] [PubMed] [Google Scholar]
- 53. Colisson R., Barblu L., Gras C., Raynaud F., Hadj-Slimane R., Pique C., Hermine O., Lepelletier Y., Herbeuval J. P. (2010) Free HTLV-1 induces TLR7-dependent innate immune response and TRAIL relocalization in killer plasmacytoid dendritic cells. Blood 115, 2177–2185 [DOI] [PubMed] [Google Scholar]
- 54. Steinberg C., Eisenacher K., Gross O., Reindl W., Schmitz F., Ruland J., Krug A. (2009) The IFN regulatory factor 7-dependent type I IFN response is not essential for early resistance against murine cytomegalovirus infection. Eur. J. Immunol. 39, 1007–1018 [DOI] [PubMed] [Google Scholar]
- 55. Jung A., Kato H., Kumagai Y., Kumar H., Kawai T., Takeuchi O., Akira S. (2008) Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces a cytotoxic T-cell response via MyD88. J. Virol. 82, 196–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Diebold S. S., Kaisho T., Hemmi H., Akira S., Reis e Sousa C. (2004) Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 [DOI] [PubMed] [Google Scholar]
- 57. Barchet W., Krug A., Cella M., Newby C., Fischer J. A., Dzionek A., Pekosz A., Colonna M. (2005) Dendritic cells respond to influenza virus through TLR7- and PKR-independent pathways. Eur. J. Immunol. 35, 236–242 [DOI] [PubMed] [Google Scholar]
- 58. Cervantes-Barragan L., Zust R., Weber F., Spiegel M., Lang K. S., Akira S., Thiel V., Ludewig B. (2007) Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood 109, 1131–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang J. P., Asher D. R., Chan M., Kurt-Jones E. A., Finberg R. W. (2007) Cutting edge: antibody-mediated TLR7-dependent recognition of viral RNA. J. Immunol. 178, 3363–3367 [DOI] [PubMed] [Google Scholar]
- 60. Takahashi K., Asabe S., Wieland S., Garaigorta U., Gastaminza P., Isogawa M., Chisari F. V. (2010) Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc. Natl. Acad. Sci. USA 107, 7431–7436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Davidson S., Kaiko G., Loh Z., Lalwani A., Zhang V., Spann K., Foo S. Y., Hansbro N., Uematsu S., Akira S., Matthaei K. I., Rosenberg H. F., Foster P. S., Phipps S. (2011) Plasmacytoid dendritic cells promote host defense against acute pneumovirus infection via the TLR7-MyD88-dependent signaling pathway. J. Immunol. 186, 5938–5948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fiola S., Gosselin D., Takada K., Gosselin J. (2010) TLR9 contributes to the recognition of EBV by primary monocytes and plasmacytoid dendritic cells. J. Immunol. 185, 3620–3631 [DOI] [PubMed] [Google Scholar]
- 63. Iwasaki A., Medzhitov R. (2004) Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 [DOI] [PubMed] [Google Scholar]
- 64. Gururajan M., Jacob J., Pulendran B. (2007) Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS ONE 2, e863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S., Lipford G., Wagner H., Bauer S. (2004) Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303, 1526–1529 [DOI] [PubMed] [Google Scholar]
- 66. Martinez J., Huang X., Yang Y. (2010) Toll-like receptor 8-mediated activation of murine plasmacytoid dendritic cells by vaccinia viral DNA. Proc. Natl. Acad. Sci. USA 107, 6442–6447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dalod M., Salazar-Mather T. P., Malmgaard L., Lewis C., Asselin-Paturel C., Briere F., Trinchieri G., Biron C. A. (2002) Interferon α/β and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J. Exp. Med. 195, 517–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wrammert J., Kallberg E., Agace W. W., Leanderson T. (2002) Ly6C expression differentiates plasma cells from other B cell subsets in mice. Eur. J. Immunol. 32, 97–103 [DOI] [PubMed] [Google Scholar]
- 69. Walunas T. L., Bruce D. S., Dustin L., Loh D. Y., Bluestone J. A. (1995) Ly-6C is a marker of memory CD8+ T cells. J. Immunol. 155, 1873–1883 [PubMed] [Google Scholar]
- 70. Geissmann F., Jung S., Littman D. R. (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 [DOI] [PubMed] [Google Scholar]
- 71. Asselin-Paturel C., Brizard G., Pin J. J., Briere F., Trinchieri G. (2003) Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J. Immunol. 171, 6466–6477 [DOI] [PubMed] [Google Scholar]
- 72. Blasius A. L., Giurisato E., Cella M., Schreiber R. D., Shaw A. S., Colonna M. (2006) Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 177, 3260–3265 [DOI] [PubMed] [Google Scholar]
- 73. Allman D., Dalod M., Asselin-Paturel C., Delale T., Robbins S. H., Trinchieri G., Biron C. A., Kastner P., Chan S. (2006) Ikaros is required for plasmacytoid dendritic cell differentiation. Blood 108, 4025–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Georgopoulos K., Bigby M., Wang J. H., Molnar A., Wu P., Winandy S., Sharpe A. (1994) The Ikaros gene is required for the development of all lymphoid lineages. Cell 79, 143–156 [DOI] [PubMed] [Google Scholar]
- 75. Dumortier A., Kirstetter P., Kastner P., Chan S. (2003) Ikaros regulates neutrophil differentiation. Blood 101, 2219–2226 [DOI] [PubMed] [Google Scholar]
- 76. Kirstetter P., Thomas M., Dierich A., Kastner P., Chan S. (2002) Ikaros is critical for B cell differentiation and function. Eur. J. Immunol. 32, 720–730 [DOI] [PubMed] [Google Scholar]
- 77. Dumortier A., Jeannet R., Kirstetter P., Kleinmann E., Sellars M., dos Santos N. R., Thibault C., Barths J., Ghysdael J., Punt J. A., Kastner P., Chan S. (2006) Notch activation is an early and critical event during T-cell leukemogenesis in Ikaros-deficient mice. Mol. Cell. Biol. 26, 209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cisse B., Caton M. L., Lehner M., Maeda T., Scheu S., Locksley R., Holmberg D., Zweier C., den Hollander N. S., Kant S. G., Holter W., Rauch A., Zhuang Y., Reizis B. (2008) Transcription factor E2–2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell 135, 37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Swiecki M., Gilfillan S., Vermi W., Wang Y., Colonna M. (2010) Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity 33, 955–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Takaoka A., Wang Z., Choi M. K., Yanai H., Negishi H., Ban T., Lu Y., Miyagishi M., Kodama T., Honda K., Ohba Y., Taniguchi T. (2007) DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505 [DOI] [PubMed] [Google Scholar]
- 81. Wang Z., Choi M. K., Ban T., Yanai H., Negishi H., Lu Y., Tamura T., Takaoka A., Nishikura K., Taniguchi T. (2008) Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc. Natl. Acad. Sci. USA 105, 5477–5482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Unterholzner L., Keating S. E., Baran M., Horan K. A., Jensen S. B., Sharma S., Sirois C. M., Jin T., Latz E., Xiao T. S., Fitzgerald K. A., Paludan S. R., Bowie A. G. (2010) IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 11, 997–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhong B., Yang Y., Li S., Wang Y. Y., Li Y., Diao F., Lei C., He X., Zhang L., Tien P., Shu H. B. (2008) The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29, 538–550 [DOI] [PubMed] [Google Scholar]
- 84. Ishikawa H., Barber G. N. (2008) STING is an endoplasmic reticulum adaptor that facilitates innate immune signaling. Nature 455, 674–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ishikawa H., Ma Z., Barber G. N. (2009) STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yanai H., Ban T., Wang Z., Choi M. K., Kawamura T., Negishi H., Nakasato M., Lu Y., Hangai S., Koshiba R., Savitsky D., Ronfani L., Akira S., Bianchi M. E., Honda K., Tamura T., Kodama T., Taniguchi T. (2009) HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature 462, 99–103 [DOI] [PubMed] [Google Scholar]
- 87. Kim T., Pazhoor S., Bao M., Zhang Z., Hanabuchi S., Facchinetti V., Bover L., Plumas J., Chaperot L., Qin J., Liu Y. J. (2010) Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA 107, 15181–15186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhang Z., Kim T., Bao M., Facchinetti V., Jung S. Y., Ghaffari A. A., Qin J., Cheng G., Liu Y. J. (2011) DDX1, DDX21, and DHX36 helicases form a complex with the adaptor molecule TRIF to sense dsRNA in dendritic cells. Immunity 34, 866–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yoneyama M., Fujita T. (2009) RNA recognition and signal transduction by RIG-I-like receptors. Immunol. Rev. 227, 54–65 [DOI] [PubMed] [Google Scholar]
- 90. Takeuchi O., Akira S. (2007) Recognition of viruses by innate immunity. Immunol. Rev. 220, 214–224 [DOI] [PubMed] [Google Scholar]
- 91. Wilkins C., Gale M., Jr. (2010) Recognition of viruses by cytoplasmic sensors. Curr. Opin. Immunol. 22, 41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pichlmair A., Reis e Sousa C. (2007) Innate recognition of viruses. Immunity 27, 370–383 [DOI] [PubMed] [Google Scholar]
- 93. Meylan E., Tschopp J., Karin M. (2006) Intracellular pattern recognition receptors in the host response. Nature 442, 39–44 [DOI] [PubMed] [Google Scholar]
- 94. Schulz O., Diebold S. S., Chen M., Naslund T. I., Nolte M. A., Alexopoulou L., Azuma Y. T., Flavell R. A., Liljestrom P., Reis e Sousa C. (2005) Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433, 887–892 [DOI] [PubMed] [Google Scholar]
- 95. Yamamoto M., Sato S., Mori K., Hoshino K., Takeuchi O., Takeda K., Akira S. (2002) Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-β promoter in the Toll-like receptor signaling. J. Immunol. 169, 6668–6672 [DOI] [PubMed] [Google Scholar]
- 96. Oshiumi H., Matsumoto M., Funami K., Akazawa T., Seya T. (2003) TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-β induction. Nat. Immunol. 4, 161–167 [DOI] [PubMed] [Google Scholar]
- 97. Hardarson H. S., Baker J. S., Yang Z., Purevjav E., Huang C. H., Alexopoulou L., Li N., Flavell R. A., Bowles N. E., Vallejo J. G. (2007) Toll-like receptor 3 is an essential component of the innate stress response in virus-induced cardiac injury. Am. J. Physiol. Heart Circ. Physiol. 292, H251–H258 [DOI] [PubMed] [Google Scholar]
- 98. Alexopoulou L., Holt A. C., Medzhitov R., Flavell R. A. (2001) Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 [DOI] [PubMed] [Google Scholar]
- 99. Daffis S., Samuel M. A., Suthar M. S., Gale M., Jr., Diamond M. S. (2008) Toll-like receptor 3 has a protective role against West Nile virus infection. J. Virol. 82, 10349–10358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Le Goffic R., Balloy V., Lagranderie M., Alexopoulou L., Escriou N., Flavell R., Chignard M., Si-Tahar M. (2006) Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2, e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Richer M. J., Lavallee D. J., Shanina I., Horwitz M. S. (2009) Toll-like receptor 3 signaling on macrophages is required for survival following coxsackievirus B4 infection. PLoS ONE 4, e4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Guillot L., Le Goffic R., Bloch S., Escriou N., Akira S., Chignard M., Si-Tahar M. (2005) Involvement of Toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 280, 5571–5580 [DOI] [PubMed] [Google Scholar]
- 103. Wang T., Town T., Alexopoulou L., Anderson J. F., Fikrig E., Flavell R. A. (2004) Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 10, 1366–1373 [DOI] [PubMed] [Google Scholar]
- 104. Wang Q., Nagarkar D. R., Bowman E. R., Schneider D., Gosangi B., Lei J., Zhao Y., McHenry C. L., Burgens R. V., Miller D. J., Sajjan U., Hershenson M. B. (2009) Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J. Immunol. 183, 6989–6997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Slater L., Bartlett N. W., Haas J. J., Zhu J., Message S. D., Walton R. P., Sykes A., Dahdaleh S., Clarke D. L., Belvisi M. G., Kon O. M., Fujita T., Jeffery P. K., Johnston S. L., Edwards M. R. (2010) Co-ordinated role of TLR3, RIG-I and MDA5 in the innate response to rhinovirus in bronchial epithelium. PLoS Pathog. 6, e1001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Nasirudeen A. M., Wong H. H., Thien P., Xu S., Lam K. P., Liu D. X. (2011) RIG-I, MDA5 and TLR3 synergistically play an important role in restriction of Dengue virus infection. PLoS Negl. Trop. Dis. 5, e926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tabeta K., Georgel P., Janssen E., Du X., Hoebe K., Crozat K., Mudd S., Shamel L., Sovath S., Goode J., Alexopoulou L., Flavell R. A., Beutler B. (2004) Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 101, 3516–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhang S. Y., Jouanguy E., Sancho-Shimizu V., von Bernuth H., Yang K., Abel L., Picard C., Puel A., Casanova J. L. (2007) Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses. Immunol. Rev. 220, 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhang S. Y., Jouanguy E., Ugolini S., Smahi A., Elain G., Romero P., Segal D., Sancho-Shimizu V., Lorenzo L., Puel A., et al. (2007) TLR3 deficiency in patients with herpes simplex encephalitis. Science 317, 1522–1527 [DOI] [PubMed] [Google Scholar]
- 110. Casanova J. L., Abel L., Quintana-Murci L. (2011) Human TLRs and IL-1Rs in host defense: natural insights from evolutionary, epidemiological, and clinical genetics. Annu. Rev. Immunol. 29, 447–491 [DOI] [PubMed] [Google Scholar]
- 111. Sancho-Shimizu V., Zhang S. Y., Abel L., Tardieu M., Rozenberg F., Jouanguy E., Casanova J. L. (2007) Genetic susceptibility to herpes simplex virus 1 encephalitis in mice and humans. Curr. Opin. Allergy Clin. Immunol. 7, 495–505 [DOI] [PubMed] [Google Scholar]
- 112. Kawai T., Takahashi K., Sato S., Coban C., Kumar H., Kato H., Ishii K. J., Takeuchi O., Akira S. (2005) IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6, 981–988 [DOI] [PubMed] [Google Scholar]
- 113. Seth R. B., Sun L., Ea C. K., Chen Z. J. (2005) Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF 3. Cell 122, 669–682 [DOI] [PubMed] [Google Scholar]
- 114. Xu L. G., Wang Y. Y., Han K. J., Li L. Y., Zhai Z., Shu H. B. (2005) VISA is an adapter protein required for virus-triggered IFN-β signaling. Mol. Cell 19, 727–740 [DOI] [PubMed] [Google Scholar]
- 115. Kumar H., Kawai T., Kato H., Sato S., Takahashi K., Coban C., Yamamoto M., Uematsu S., Ishii K. J., Takeuchi O., Akira S. (2006) Essential role of IPS-1 in innate immune responses against RNA viruses. J. Exp. Med. 203, 1795–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sharma S., tenOever B. R., Grandvaux N., Zhou G. P., Lin R., Hiscott J. (2003) Triggering the interferon antiviral response through an IKK-related pathway. Science 300, 1148–1151 [DOI] [PubMed] [Google Scholar]
- 117. Saha S. K., Pietras E. M., He J. Q., Kang J. R., Liu S. Y., Oganesyan G., Shahangian A., Zarnegar B., Shiba T. L., Wang Y., Cheng G. (2006) Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J. 25, 3257–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Oganesyan G., Saha S. K., Guo B., He J. Q., Shahangian A., Zarnegar B., Perry A., Cheng G. (2006) Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature 439, 208–211 [DOI] [PubMed] [Google Scholar]
- 119. Balachandran S., Thomas E., Barber G. N. (2004) A FADD-dependent innate immune mechanism in mammalian cells. Nature 432, 401–405 [DOI] [PubMed] [Google Scholar]
- 120. Takahashi K., Kawai T., Kumar H., Sato S., Yonehara S., Akira S. (2006) Roles of caspase-8 and caspase-10 in innate immune responses to double-stranded RNA. J. Immunol. 176, 4520–4524 [DOI] [PubMed] [Google Scholar]
- 121. Dixit E., Boulant S., Zhang Y., Lee A. S., Odendall C., Shum B., Hacohen N., Chen Z. J., Whelan S. P., Fransen M., Nibert M. L., Superti-Furga G., Kagan J. C. (2010) Peroxisomes are signaling platforms for antiviral innate immunity. Cell 141, 668–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pichlmair A., Schulz O., Tan C. P., Naslund T. I., Liljestrom P., Weber F., Reis e Sousa C. (2006) RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314, 997–1001 [DOI] [PubMed] [Google Scholar]
- 123. Hornung V., Ellegast J., Kim S., Brzozka K., Jung A., Kato H., Poeck H., Akira S., Conzelmann K. K., Schlee M., Endres S., Hartmann G. (2006) 5′-Triphosphate RNA is the ligand for RIG-I. Science 314, 994–997 [DOI] [PubMed] [Google Scholar]
- 124. Pichlmair A., Schulz O., Tan C. P., Rehwinkel J., Kato H., Takeuchi O., Akira S., Way M., Schiavo G., Reis e Sousa C. (2009) Activation of MDA5 requires higher-order RNA structures generated during virus infection. J. Virol. 83, 10761–10769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Schlee M., Roth A., Hornung V., Hagmann C. A., Wimmenauer V., Barchet W., Coch C., Janke M., Mihailovic A., Wardle G., Juranek S., Kato H., Kawai T., Poeck H., Fitzgerald K. A., Takeuchi O., Akira S., Tuschl T., Latz E., Ludwig J., Hartmann G. (2009) Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity 31, 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kato H., Takeuchi O., Mikamo-Satoh E., Hirai R., Kawai T., Matsushita K., Hiiragi A., Dermody T. S., Fujita T., Akira S. (2008) Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 205, 1601–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cui S., Eisenacher K., Kirchhofer A., Brzozka K., Lammens A., Lammens K., Fujita T., Conzelmann K. K., Krug A., Hopfner K. P. (2008) The C-terminal regulatory domain is the RNA 5′-triphosphate sensor of RIG-I. Mol. Cell 29, 169–179 [DOI] [PubMed] [Google Scholar]
- 128. Saito T., Owen D. M., Jiang F., Marcotrigiano J., Gale M., Jr. (2008) Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454, 523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Miyagishi M., Taira K., Akira S., Fujita T. (2004) The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737 [DOI] [PubMed] [Google Scholar]
- 130. Yoneyama M., Kikuchi M., Matsumoto K., Imaizumi T., Miyagishi M., Taira K., Foy E., Loo Y. M., Gale M., Jr., Akira S., Yonehara S., Kato A., Fujita T. (2005) Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 175, 2851–2858 [DOI] [PubMed] [Google Scholar]
- 131. Yoneyama M., Fujita T. (2007) Function of RIG-I-like receptors in antiviral innate immunity. J. Biol. Chem. 282, 15315–15318 [DOI] [PubMed] [Google Scholar]
- 132. Kato H., Takeuchi O., Sato S., Yoneyama M., Yamamoto M., Matsui K., Uematsu S., Jung A., Kawai T., Ishii K. J., Yamaguchi O., Otsu K., Tsujimura T., Koh C. S., Reis e Sousa C., Matsuura Y., Fujita T., Akira S. (2006) Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 [DOI] [PubMed] [Google Scholar]
- 133. Plumet S., Herschke F., Bourhis J. M., Valentin H., Longhi S., Gerlier D. (2007) Cytosolic 5′-triphosphate ended viral leader transcript of measles virus as activator of the RIG I-mediated interferon response. PLoS ONE 2, e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ikegame S., Takeda M., Ohno S., Nakatsu Y., Nakanishi Y., Yanagi Y. (2010) Both RIG-I and MDA5 RNA helicases contribute to the induction of α/β interferon in measles virus-infected human cells. J. Virol. 84, 372–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Loo Y. M., Owen D. M., Li K., Erickson A. K., Johnson C. L., Fish P. M., Carney D. S., Wang T., Ishida H., Yoneyama M., Fujita T., Saito T., Lee W. M., Hagedorn C. H., Lau D. T., Weinman S. A., Lemon S. M., Gale M., Jr. (2006) Viral and therapeutic control of IFN-β promoter stimulator 1 during hepatitis C virus infection. Proc. Natl. Acad. Sci. USA 103, 6001–6006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Cárdenas W. B., Loo Y. M., Gale M., Jr., Hartman A. L., Kimberlin C. R., Martinez-Sobrido L., Saphire E. O., Basler C. F. (2006) Ebola virus VP35 protein binds double-stranded RNA and inhibits α/β interferon production induced by RIG-I signaling. J. Virol. 80, 5168–5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sumpter R., Jr., Loo Y. M., Foy E., Li K., Yoneyama M., Fujita T., Lemon S. M., Gale M., Jr. (2005) Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 79, 2689–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Fredericksen B. L., Gale M., Jr. (2006) West Nile virus evades activation of interferon regulatory factor 3 through RIG-I-dependent and -independent pathways without antagonizing host defense signaling. J. Virol. 80, 2913–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Loo Y. M., Fornek J., Crochet N., Bajwa G., Perwitasari O., Martinez-Sobrido L., Akira S., Gill M. A., Garcia-Sastre A., Katze M. G., Gale M., Jr. (2008) Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 82, 335–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Holm G. H., Zurney J., Tumilasci V., Leveille S., Danthi P., Hiscott J., Sherry B., Dermody T. S. (2007) Retinoic acid-inducible gene-I and interferon-β promoter stimulator-1 augment proapoptotic responses following mammalian reovirus infection via interferon regulatory factor-3. J. Biol. Chem. 282, 21953–21961 [DOI] [PubMed] [Google Scholar]
- 141. Opitz B., Rejaibi A., Dauber B., Eckhard J., Vinzing M., Schmeck B., Hippenstiel S., Suttorp N., Wolff T. (2007) IFNβ induction by influenza A virus is mediated by RIG-I which is regulated by the viral NS1 protein. Cell. Microbiol. 9, 930–938 [DOI] [PubMed] [Google Scholar]
- 142. Yoboua F., Martel A., Duval A., Mukawera E., Grandvaux N. (2010) Respiratory syncytial virus-mediated NF-κ B p65 phosphorylation at serine 536 is dependent on RIG-I, TRAF6, and IKK β. J. Virol. 84, 7267–7277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Liao S., Bao X., Liu T., Lai S., Li K., Garofalo R. P., Casola A. (2008) Role of retinoic acid inducible gene-I in human metapneumovirus-induced cellular signalling. J. Gen. Virol. 89, 1978–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Loo Y. M., Gale M., Jr. (2011) Immune signaling by RIG-I-like receptors. Immunity 34, 680–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Ablasser A., Bauernfeind F., Hartmann G., Latz E., Fitzgerald K. A., Hornung V. (2009) RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat. Immunol. 10, 1065–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Chiu Y. H., Macmillan J. B., Chen Z. J. (2009) RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138, 576–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Gitlin L., Barchet W., Gilfillan S., Cella M., Beutler B., Flavell R. A., Diamond M. S., Colonna M. (2006) Essential role of MDA-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA 103, 8459–8464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. McCartney S. A., Thackray L. B., Gitlin L., Gilfillan S., Virgin H. W., Colonna M. (2008) MDA-5 recognition of a murine norovirus. PLoS Pathog. 4, e1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wang J. P., Cerny A., Asher D. R., Kurt-Jones E. A., Bronson R. T., Finberg R. W. (2010) MDA5 and MAVS mediate type I interferon responses to coxsackie B virus. J. Virol. 84, 254–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Fredericksen B. L., Keller B. C., Fornek J., Katze M. G., Gale M., Jr. (2008) Establishment and maintenance of the innate antiviral response to West Nile virus involves both RIG-I and MDA5 signaling through IPS-1. J. Virol. 82, 609–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Roth-Cross J. K., Bender S. J., Weiss S. R. (2008) Murine coronavirus mouse hepatitis virus is recognized by MDA5 and induces type I interferon in brain macrophages/microglia. J. Virol. 82, 9829–9838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Gitlin L., Benoit L., Song C., Cella M., Gilfillan S., Holtzman M. J., Colonna M. (2010) Melanoma differentiation-associated gene 5 (MDA5) is involved in the innate immune response to paramyxoviridae infection in vivo. PLoS Pathog. 6, e1000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Zhou S., Cerny A. M., Zacharia A., Fitzgerald K. A., Kurt-Jones E. A., Finberg R. W. (2010) Induction and inhibition of type I interferon responses by distinct components of lymphocytic choriomeningitis virus. J. Virol. 84, 9452–9462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Satoh T., Kato H., Kumagai Y., Yoneyama M., Sato S., Matsushita K., Tsujimura T., Fujita T., Akira S., Takeuchi O. (2010) LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses. Proc. Natl. Acad. Sci. USA 107, 1512–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Venkataraman T., Valdes M., Elsby R., Kakuta S., Caceres G., Saijo S., Iwakura Y., Barber G. N. (2007) Loss of DExD/H box RNA helicase LGP2 manifests disparate antiviral responses. J. Immunol. 178, 6444–6455 [DOI] [PubMed] [Google Scholar]
- 156. Rothenfusser S., Goutagny N., DiPerna G., Gong M., Monks B. G., Schoenemeyer A., Yamamoto M., Akira S., Fitzgerald K. A. (2005) The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J. Immunol. 175, 5260–5268 [DOI] [PubMed] [Google Scholar]
- 157. Ishii K. J., Koyama S., Nakagawa A., Coban C., Akira S. (2008) Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe 3, 352–363 [DOI] [PubMed] [Google Scholar]
- 158. Matsumoto M., Seya T. (2008) TLR3: interferon induction by double-stranded RNA including poly(I:C). Adv. Drug Deliv. Rev. 60, 805–812 [DOI] [PubMed] [Google Scholar]
- 159. Longhi M. P., Trumpfheller C., Idoyaga J., Caskey M., Matos I., Kluger C., Salazar A. M., Colonna M., Steinman R. M. (2009) Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J. Exp. Med. 206, 1589–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Trumpfheller C., Caskey M., Nchinda G., Longhi M. P., Mizenina O., Huang Y., Schlesinger S. J., Colonna M., Steinman R. M. (2008) The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc. Natl. Acad. Sci. USA 105, 2574–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Pulendran B., Ahmed R. (2006) Translating innate immunity into immunological memory: implications for vaccine development. Cell 124, 849–863 [DOI] [PubMed] [Google Scholar]
- 162. Stahl-Hennig C., Eisenblatter M., Jasny E., Rzehak T., Tenner-Racz K., Trumpfheller C., Salazar A. M., Uberla K., Nieto K., Kleinschmidt J., Schulte R., Gissmann L., Muller M., Sacher A., Racz P., Steinman R. M., Uguccioni M., Ignatius R. (2009) Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog. 5, e1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ngoi S. M., Tovey M. G., Vella A. T. (2008) Targeting poly(I:C) to the TLR3-independent pathway boosts effector CD8 T cell differentiation through IFN-α/β. J. Immunol. 181, 7670–7680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Kumar H., Koyama S., Ishii K. J., Kawai T., Akira S. (2008) Cutting edge: cooperation of IPS-1- and TRIF-dependent pathways in poly IC-enhanced antibody production and cytotoxic T cell responses. J. Immunol. 180, 683–687 [DOI] [PubMed] [Google Scholar]
- 165. Wang Y., Cella M., Gilfillan S., Colonna M. (2010) Cutting edge: polyinosinic:polycytidylic acid boosts the generation of memory CD8 T cells through melanoma differentiation-associated protein 5 expressed in stromal cells. J. Immunol. 184, 2751–2755 [DOI] [PubMed] [Google Scholar]
- 166. Schmidt K. N., Leung B., Kwong M., Zarember K. A., Satyal S., Navas T. A., Wang F., Godowski P. J. (2004) APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J. Immunol. 172, 138–143 [DOI] [PubMed] [Google Scholar]
- 167. Sivori S., Falco M., Della Chiesa M., Carlomagno S., Vitale M., Moretta L., Moretta A. (2004) CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc. Natl. Acad. Sci. USA 101, 10116–10121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hart O. M., Athie-Morales V., O'Connor G. M., Gardiner C. M. (2005) TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-γ production. J. Immunol. 175, 1636–1642 [DOI] [PubMed] [Google Scholar]
- 169. Lauzon N. M., Mian F., MacKenzie R., Ashkar A. A. (2006) The direct effects of Toll-like receptor ligands on human NK cell cytokine production and cytotoxicity. Cell. Immunol. 241, 102–112 [DOI] [PubMed] [Google Scholar]
- 170. Akazawa T., Ebihara T., Okuno M., Okuda Y., Shingai M., Tsujimura K., Takahashi T., Ikawa M., Okabe M., Inoue N., Okamoto-Tanaka M., Ishizaki H., Miyoshi J., Matsumoto M., Seya T. (2007) Antitumor NK activation induced by the Toll-like receptor 3-TICAM-1 (TRIF) pathway in myeloid dendritic cells. Proc. Natl. Acad. Sci. USA 104, 252–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Miyake T., Kumagai Y., Kato H., Guo Z., Matsushita K., Satoh T., Kawagoe T., Kumar H., Jang M. H., Kawai T., Tani T., Takeuchi O., Akira S. (2009) Poly I:C-induced activation of NK cells by CD8 α+ dendritic cells via the IPS-1 and TRIF-dependent pathways. J. Immunol. 183, 2522–2528 [DOI] [PubMed] [Google Scholar]
- 172. McCartney S., Vermi W., Gilfillan S., Cella M., Murphy T. L., Schreiber R. D., Murphy K. M., Colonna M. (2009) Distinct and complementary functions of MDA5 and TLR3 in poly(I:C)-mediated activation of mouse NK cells. J. Exp. Med. 206, 2967–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Gilliet M., Cao W., Liu Y. J. (2008) Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 8, 594–606 [DOI] [PubMed] [Google Scholar]
- 174. Palucka A. K., Blanck J. P., Bennett L., Pascual V., Banchereau J. (2005) Cross-regulation of TNF and IFN-α in autoimmune diseases. Proc. Natl. Acad. Sci. USA 102, 3372–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Rönnblom L., Alm G. V., Eloranta M. L. (2011) The type I interferon system in the development of lupus. Semin. Immunol. 23, 113–121 [DOI] [PubMed] [Google Scholar]
- 176. Marshak-Rothstein A. (2006) Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 6, 823–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Lehuen A., Diana J., Zaccone P., Cooke A. (2010) Immune cell crosstalk in type 1 diabetes. Nat. Rev. Immunol. 10, 501–513 [DOI] [PubMed] [Google Scholar]
- 178. Castaño L., Eisenbarth G. S. (1990) Type-I diabetes: a chronic autoimmune disease of human, mouse, and rat. Annu. Rev. Immunol. 8, 647–679 [DOI] [PubMed] [Google Scholar]
- 179. Bach J. F. (1994) Insulin-dependent diabetes mellitus as an autoimmune disease. Endocr. Rev. 15, 516–542 [DOI] [PubMed] [Google Scholar]
- 180. Stewart T. A., Hultgren B., Huang X., Pitts-Meek S., Hully J., MacLachlan N. J. (1993) Induction of type I diabetes by interferon-α in transgenic mice. Science 260, 1942–1946 [DOI] [PubMed] [Google Scholar]
- 181. Alba A., Puertas M. C., Carrillo J., Planas R., Ampudia R., Pastor X., Bosch F., Pujol-Borrell R., Verdaguer J., Vives-Pi M. (2004) IFN β accelerates autoimmune type 1 diabetes in nonobese diabetic mice and breaks the tolerance to β cells in nondiabetes-prone mice. J. Immunol. 173, 6667–6675 [DOI] [PubMed] [Google Scholar]
- 182. Li Q., Xu B., Michie S. A., Rubins K. H., Schreriber R. D., McDevitt H. O. (2008) Interferon-α initiates type 1 diabetes in nonobese diabetic mice. Proc. Natl. Acad. Sci. USA 105, 12439–12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Mathis D., Vence L., Benoist C. (2001) β-Cell death during progression to diabetes. Nature 414, 792–798 [DOI] [PubMed] [Google Scholar]
- 184. Li Q., McDevitt H. O. (2011) The role of interferon α in initiation of type I diabetes in the NOD mouse. Clin. Immunol. 140, 3–7 [DOI] [PubMed] [Google Scholar]
- 185. Allen J. S., Pang K., Skowera A., Ellis R., Rackham C., Lozanoska-Ochser B., Tree T., Leslie R. D., Tremble J. M., Dayan C. M., Peakman M. (2009) Plasmacytoid dendritic cells are proportionally expanded at diagnosis of type 1 diabetes and enhance islet autoantigen presentation to T-cells through immune complex capture. Diabetes 58, 138–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Moriyama H., Wen L., Abiru N., Liu E., Yu L., Miao D., Gianani R., Wong F. S., Eisenbarth G. S. (2002) Induction and acceleration of insulitis/diabetes in mice with a viral mimic (polyinosinic-polycytidylic acid) and an insulin self-peptide. Proc. Natl. Acad. Sci. USA 99, 5539–5544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Devendra D., Jasinski J., Melanitou E., Nakayama M., Li M., Hensley B., Paronen J., Moriyama H., Miao D., Eisenbarth G. S., Liu E. (2005) Interferon-α as a mediator of polyinosinic:polycytidylic acid-induced type 1 diabetes. Diabetes 54, 2549–2556 [DOI] [PubMed] [Google Scholar]
- 188. von Herrath M. (2009) Diabetes: a virus-gene collaboration. Nature 459, 518–519 [DOI] [PubMed] [Google Scholar]
- 189. Nejentsev S., Walker N., Riches D., Egholm M., Todd J. A. (2009) Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science 324, 387–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Smyth D. J., Cooper J. D., Bailey R., Field S., Burren O., Smink L. J., Guja C., Ionescu-Tirgoviste C., Widmer B., Dunger D. B., Savage D. A., Walker N. M., Clayton D. G., Todd J. A. (2006) A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat. Genet. 38, 617–619 [DOI] [PubMed] [Google Scholar]
- 191. Shigemoto T., Kageyama M., Hirai R., Zheng J., Yoneyama M., Fujita T. (2009) Identification of loss of function mutations in human genes encoding RIG-I and MDA5: implications for resistance to type I diabetes. J. Biol. Chem. 284, 13348–13354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Downes K., Pekalski M., Angus K. L., Hardy M., Nutland S., Smyth D. J., Walker N. M., Wallace C., Todd J. A. (2010) Reduced expression of IFIH1 is protective for type 1 diabetes. PLoS One 5, e12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Dotta F., Censini S., van Halteren A. G., Marselli L., Masini M., Dionisi S., Mosca F., Boggi U., Muda A. O., Prato S. D., Elliott J. F., Covacci A., Rappuoli R., Roep B. O., Marchetti P. (2007) Coxsackie B4 virus infection of β cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc. Natl. Acad. Sci. USA 104, 5115–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Miyazaki A., Hanafusa T., Yamada K., Miyagawa J., Fujino-Kurihara H., Nakajima H., Nonaka K., Tarui S. (1985) Predominance of T lymphocytes in pancreatic islets and spleen of pre-diabetic non-obese diabetic (NOD) mice: a longitudinal study. Clin. Exp. Immunol. 60, 622–630 [PMC free article] [PubMed] [Google Scholar]
- 195. Alba A., Planas R., Clemente X., Carrillo J., Ampudia R., Puertas M. C., Pastor X., Tolosa E., Pujol-Borrell R., Verdaguer J., Vives-Pi M. (2008) Natural killer cells are required for accelerated type 1 diabetes driven by interferon-β. Clin. Exp. Immunol. 151, 467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Gur C., Porgador A., Elboim M., Gazit R., Mizrahi S., Stern-Ginossar N., Achdout H., Ghadially H., Dor Y., Nir T., Doviner V., Hershkovitz O., Mendelson M., Naparstek Y., Mandelboim O. (2010) The activating receptor NKp46 is essential for the development of type 1 diabetes. Nat. Immunol. 11, 121–128 [DOI] [PubMed] [Google Scholar]
- 197. Serreze D. V., Hamaguchi K., Leiter E. H. (1989) Immunostimulation circumvents diabetes in NOD/Lt mice. J. Autoimmun. 2, 759–776 [DOI] [PubMed] [Google Scholar]
- 198. Tanaka-Kataoka M., Kunikata T., Takayama S., Iwaki K., Fujii M., Ohashi K., Ikeda M., Kurimoto M. (1999) Oral use of interferon-α delays the onset of insulin-dependent diabetes mellitus in nonobese diabetes mice. J. Interferon Cytokine Res. 19, 877–879 [DOI] [PubMed] [Google Scholar]
- 199. Sobel D. O., Ahvazi B. (1998) α-Interferon inhibits the development of diabetes in NOD mice. Diabetes 47, 1867–1872 [DOI] [PubMed] [Google Scholar]
- 200. Smith K. A., Efstathiou S., Cooke A. (2007) Murine γherpesvirus-68 infection alters self-antigen presentation and type 1 diabetes onset in NOD mice. J. Immunol. 179, 7325–7333 [DOI] [PubMed] [Google Scholar]
- 201. Feillet H., Bach J. F. (2004) On the mechanisms of the protective effect of infections on type 1 diabetes. Clin. Dev. Immunol. 11, 191–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Filippi C. M., Estes E. A., Oldham J. E., von Herrath M. G. (2009) Immunoregulatory mechanisms triggered by viral infections protect from type 1 diabetes in mice. J. Clin. Invest. 119, 1515–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Diana J., Griseri T., Lagaye S., Beaudoin L., Autrusseau E., Gautron A. S., Tomkiewicz C., Herbelin A., Barouki R., von Herrath M., Dalod M., Lehuen A. (2009) NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity 30, 289–299 [DOI] [PubMed] [Google Scholar]
- 204. Diana J., Brezar V., Beaudoin L., Dalod M., Mellor A., Tafuri A., von Herrath M., Boitard C., Mallone R., Lehuen A. (2011) Viral infection prevents diabetes by inducing regulatory T cells through NKT cell-plasmacytoid dendritic cell interplay. J. Exp. Med. 208, 729–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Boettler T., von Herrath M. (2011) Protection against or triggering of type 1 diabetes? Different roles for viral infections. Expert Rev. Clin. Immunol. 7, 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Ludewig B., Odermatt B., Landmann S., Hengartner H., Zinkernagel R. M. (1998) Dendritic cells induce autoimmune diabetes and maintain disease via de novo formation of local lymphoid tissue. J. Exp. Med. 188, 1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Lang P. A., Cervantes-Barragan L., Verschoor A., Navarini A. A., Recher M., Pellegrini M., Flatz L., Bergthaler A., Honda K., Ludewig B., Ohashi P. S., Lang K. S. (2009) Hematopoietic cell-derived interferon controls viral replication and virus-induced disease. Blood 113, 1045–1052 [DOI] [PubMed] [Google Scholar]
- 208. Yoon J. W., McClintock P. R., Onodera T., Notkins A. L. (1980) Virus-induced diabetes mellitus. XVIII. Inhibition by a nondiabetogenic variant of encephalomyocarditis virus. J. Exp. Med. 152, 878–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Hühn M. H., McCartney S. A., Lind K., Svedin E., Colonna M., Flodstrom-Tullberg M. (2010) Melanoma differentiation-associated protein-5 (MDA-5) limits early viral replication but is not essential for the induction of type 1 interferons after coxsackievirus infection. Virology 401, 42–48 [DOI] [PubMed] [Google Scholar]
- 210. Horwitz M. S., Bradley L. M., Harbertson J., Krahl T., Lee J., Sarvetnick N. (1998) Diabetes induced by coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat. Med. 4, 781–785 [DOI] [PubMed] [Google Scholar]
- 211. Baek H. S., Yoon J. W. (1991) Direct involvement of macrophages in destruction of β-cells leading to development of diabetes in virus-infected mice. Diabetes 40, 1586–1597 [DOI] [PubMed] [Google Scholar]
- 212. Hober D., Sauter P. (2010) Pathogenesis of type 1 diabetes mellitus: interplay between enterovirus and host. Nat. Rev. Endocrinol. 6, 279–289 [DOI] [PubMed] [Google Scholar]
- 213. Tauriainen S., Salminen K., Hyoty H. (2003) Can enteroviruses cause type 1 diabetes? Ann. N. Y. Acad. Sci. 1005, 13–22 [DOI] [PubMed] [Google Scholar]
- 214. Hultcrantz M., Huhn M. H., Wolf M., Olsson A., Jacobson S., Williams B. R., Korsgren O., Flodstrom-Tullberg M. (2007) Interferons induce an antiviral state in human pancreatic islet cells. Virology 367, 92–101 [DOI] [PubMed] [Google Scholar]
- 215. McCartney S. A., Vermi W., Lonardi S., Rossini C., Otero K., Calderon B., Gilfillan S., Diamond M. S., Unanue E. R., Colonna M. (2011) RNA sensor-induced type I IFN prevents diabetes caused by a β cell-tropic virus in mice. J. Clin. Invest. 121, 1497–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Iannacone M., Moseman E. A., Tonti E., Bosurgi L., Junt T., Henrickson S. E., Whelan S. P., Guidotti L. G., von Andrian U. H. (2010) Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature 465, 1079–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Eloranta M. L., Alm G. V. (1999) Splenic marginal metallophilic macrophages and marginal zone macrophages are the major interferon-α/β producers in mice upon intravenous challenge with herpes simplex virus. Scand. J. Immunol. 49, 391–394 [DOI] [PubMed] [Google Scholar]
- 218. Louten J., van Rooijen N., Biron C. A. (2006) Type 1 IFN deficiency in the absence of normal splenic architecture during lymphocytic choriomeningitis virus infection. J. Immunol. 177, 3266–3272 [DOI] [PubMed] [Google Scholar]
- 219. Lang P. A., Recher M., Honke N., Scheu S., Borkens S., Gailus N., Krings C., Meryk A., Kulawik A., Cervantes-Barragan L., Van Rooijen N., Kalinke U., Ludewig B., Hengartner H., Harris N., Haussinger D., Ohashi P. S., Zinkernagel R. M., Lang K. S. (2010) Tissue macrophages suppress viral replication and prevent severe immunopathology in an interferon-I-dependent manner in mice. Hepatology 52, 25–32 [DOI] [PubMed] [Google Scholar]
- 220. Barbalat R., Lau L., Locksley R. M., Barton G. M. (2009) Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat. Immunol. 10, 1200–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Fejer G., Drechsel L., Liese J., Schleicher U., Ruzsics Z., Imelli N., Greber U. F., Keck S., Hildenbrand B., Krug A., Bogdan C., Freudenberg M. A. (2008) Key role of splenic myeloid DCs in the IFN-αβ response to adenoviruses in vivo. PLoS Pathog. 4, e1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Andoniou C. E., van Dommelen S. L., Voigt V., Andrews D. M., Brizard G., Asselin-Paturel C., Delale T., Stacey K. J., Trinchieri G., Degli-Esposti M. A. (2005) Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat. Immunol. 6, 1011–1019 [DOI] [PubMed] [Google Scholar]
- 223. Stockinger S., Kastner R., Kernbauer E., Pilz A., Westermayer S., Reutterer B., Soulat D., Stengl G., Vogl C., Frenz T., Waibler Z., Taniguchi T., Rulicke T., Kalinke U., Muller M., Decker T. (2009) Characterization of the interferon-producing cell in mice infected with Listeria monocytogenes. PLoS Pathog. 5, e1000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Bao Y., Han Y., Chen Z., Xu S., Cao X. (2011) IFN-α-producing PDCA-1(+) Siglec-H(–) B cells mediate innate immune defense by activating NK cells. Eur. J. Immunol. 41, 657–668 [DOI] [PubMed] [Google Scholar]
- 225. Mancuso G., Gambuzza M., Midiri A., Biondo C., Papasergi S., Akira S., Teti G., Beninati C. (2009) Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat. Immunol. 10, 587–594 [DOI] [PubMed] [Google Scholar]
- 226. Kang D. C., Gopalkrishnan R. V., Wu Q., Jankowsky E., Pyle A. M., Fisher P. B. (2002) MDA-5: an interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc. Natl. Acad. Sci. USA 99, 637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]