New method to study inhibition of mast cell degranulation by showing that soluble OX40 mimics activity of Treg interaction by binding and inhibiting MC degranulation.
Keywords: cell activation, costimulation, allergy
Abstract
Tregs play a central role in modulating FcεRI-dependent MC effector functions in the course of the allergic response. Cellular interaction depends on the constitutive expression of OX40 on Tregs and the OX40L counterpart on MCs. Study of OX40L signaling on MCs is hampered by the need of a highly purified molecule, which triggers OX40L specifically. We now report that sOX40 mimics the physiological activity of Treg interaction by binding to activated MCs. When treated with sOX40, activated MCs showed decreased degranulation and Ca++ influx, whereas PLC-γ2 phosphorylation remained unaffected. Once injected into experimental animals, sOX40 not only located within the endothelium but also in parenchyma, where it could be found in close proximity and apparently bound to MCs. This soluble molecule triggers MC-OX40L without the requirement of Tregs, thus allowing study of OX40L signaling pathways in MCs and in other OX40L-expressing cell populations. Importantly, as sOX40 inhibits MC degranulation, it may provide an in vivo therapeutic tool in allergic disease.
Introduction
Allergy is one of the most widespread pathologies in Western countries. MCs are the primary responders in atopic reactions, such as anaphylaxis and asthma [1], and are primarily triggered when encountering an allergen that binds the allergen-specific, IgE-engaged FcεRI. This causes the release of early, preformed mediators, such as histamine and neutral proteases, and later, synthesized arachidonic acid molecules, cytokines, and chemokines [2, 3]. MCs are widely distributed in all vascularized tissues and are abundant in anatomical sites at the boundary of the external environment, such as skin, airways, and gastrointestinal tract [4], exerting a protective role from environmental antigens. MCs also interact with several cell types of the acquired immune system, revealing MC-effector and immunoregulatory roles during physiological and pathological conditions [5, 6].
MCs are in close proximity to T cells in the periphery, and they can interact directly with CD4+CD25+forkhead box p3+ Tregs, which are crucial in maintaining self-tolerance and in regulating the development and the intensity of the immune response to foreign antigens, including allergens [7]. For these reasons, MCs appear to play a role in immune homeostasis [8–10].
We have recently demonstrated a bidirectional, functional interaction between MCs and Tregs, which exert an inhibitory role for MC degranulation and on in vivo anaphylaxis, whereas MCs reduce Treg-inhibitory functions on Teffs [8, 11]. These events are tightly controlled by the interaction between OX40 (CD134) on Tregs and OX40L (CD134L) adhesion molecules on MCs. We demonstrated that the dampening of MC degranulation was associated with reduced Ca++ uptake but not reduced PLC-γ2 activation or cytokine production [8]. It has been published that an increased mRNA level of c-jun and c-fos in an OX40L-transfected mouse epithelial cell line and in a HUVEC line [12] increased production of CCL5/RANTES chemokine in HUVECs [13], and PKC-β membrane translocation in a human airway smooth muscle cell model [14] follows OX40L triggering. Yet, the molecular mechanisms, resulting in the dampening of MC degranulation, remain to be determined.
Various approaches could be used to dissect the OX40L signaling pathways on activated MCs. However, defining what signals are generated solely through OX40L stimulation presents several challenges: OX40L agonist antibody might also bind FcRs expressed on MCs and deliver positive or negative signals [15]; supernatants from transfected cells releasing sOX40 may cause possible cross-contaminations by release of other molecules; and the use of Tregs to stimulate OX40L on MC surface is limited by the inability to distinguish the contribution of other Treg-related stimuli. Therefore, to overcome these hurdles in exploring the molecular events upon engagement of OX40L on MCs, we developed a sOX40 and tested whether it faithfully reproduced the physiological Treg-inhibitory effect on MC degranulation through the OX40L:OX40 axis. We found that treatment of FcεRI-activated MCs with sOX40 inhibits MC degranulation and extracellular Ca++ influx without affecting PLC-γ2 phosphorylation or cytokine or chemokine production. Moreover, we also found that upon i.v. injection in mice, sOX40 distributed systemically and suggests a potential, novel therapeutic strategy in treatment of allergic diseases.
MATERIALS AND METHODS
BMMC differentiation and activation
WT and Tnfsf4−/− BMMCs were obtained from in vitro differentiation of BM precursors from WT (Harlan Laboratories, Indianapolis, IN, USA) and Tnfsf4−/− (kindly gifted from Dr. Andrew S. MacDonald, University of Edinburgh, Scotland) C57BL/6 mice, as in ref. [8]. After 5 weeks, FcεRI expression in BMMCs was determined by flow cytometry. BMMCs were usually >98% FcεRI+-stained. DNP-HSA (DNP36-HSA, antigen) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Before each experiment, 1 × 106 BMMC/ml in 5% FBS and IL-3-free medium were sensitized for 3 h with DNP-specific IgE and challenged with antigen in Tyrode's buffer at 37°C [8].
sOX40 molecule production, Western blotting, and viability assay
OX40-IgG1 fusion protein was provided by Genentech (San Francisco, CA, USA). It contained the murine OX40 extracellular domain (residues 23–198) in fusion with a murine IgG1 Fc (see Fig. 1A). As the entire molecule would interfere with the MC response via FcγR, the Fc portion (murine hinge, CH2, CH3) was removed by OX40-IgG1 digestion with 10 μg papain/mg protein for 18 h at 37°C. After enzyme inactivation (30 mM iodoacetamide, 2 h, room temperature) and 16 h dialysis at 4°C, the mixture was loaded on Protein G Sepharose (GE Healthcare, Waukesha, WI, USA) for 2 h at 4°C to separate the Fc portion. The eluted fraction was loaded on a polyacrylamide gel and analyzed through Coomassie brilliant blue staining and Western blot with anti-OX40 (eBioscience, San Diego, CA, USA) and HRP-conjugated secondary antibody (Sigma-Aldrich). For viability assay, 1 × 106/ml resting BMMCs were coincubated with increasing concentrations of sOX40 (1, 10, 20, 100 μg/ml), and viability was assessed by trypan blue staining.
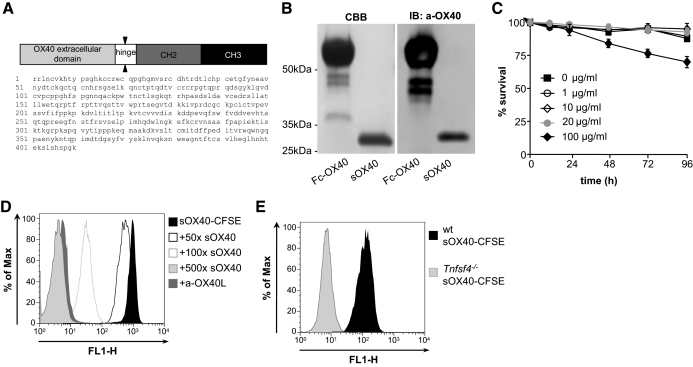
Figure 1. Characterization and specificity of sOX40 molecule.
(A) Schematic representation and amino acidic sequence of murine OX40-Fc fusion protein provided by Genentech. (B) Purity analysis of sOX40 molecule after papain digestion and comparison with undigested molecule. CBB, Coomassie brilliant blue; IB, immunoblot; a-OX40, anti-OX40. (C) Viability curve of BMMCs incubated 24, 48, 72, and 96 h with increasing concentration of sOX40 (1, 10, 20, and 100 μg/ml). All graphs show means ± sem of at least three independent experiments. (D) sOX40 binding and competition assays. BMMCs were coincubated with sOX40-CFSE for 30 min, washed, and analyzed by flow cytometry for positivity on a fluorescence 1-height (FL1-H) channel. For competition assay, BMMCs were incubated with an excess of unlabeled sOX40 (50×, 100×, and 500×) or anti-OX40L antibody, washed, incubated with sOX40-CFSE, and analyzed by flow cytometry. (E) WT or Tnfsf4−/− BMMCs were incubated with sOX40-CFSE as in D.
sOX40 fluorescein labeling, binding, and competition assay
DMSO-resuspended 5-(and-6)-CFSE/FAM (150 μg; C-1311; Invitrogen, Carlsbad, CA, USA) was added to 1 mg sOX40 for 90 min at 4°C. Labeled protein (sOX40-CFSE) was separated from free fluorescein compounds by 16 h dialysis versus PBS. WT or Tnfsf4−/− BMMCs were incubated with sOX40-CFSE (20 μg/ml) for 30 min at room temperature, washed, and analyzed by flow cytometry. In some experiments, WT BMMCs were incubated with an increasing amount of unlabeled sOX40, starting from 1000 μg/ml (50×) to 10,000 μg/ml (500×), or with 40 μg/ml anti-OX40L antibody (eBioscence). Data were acquired on a FACScan (Becton Dickinson, San Diego, CA, USA) and analyzed with FlowJo software (Tree Star, Ashland, OR, USA).
β-Hexosaminidase assay
IgE-presensitized BMMCs were challenged with antigen (50 ng/ml) for 30 min, and the extent of degranulation was determined by the percent of β-hexosaminidase released. Equal numbers of BMMCs and WT and Tnfrsf4−/− Tregs, Th1, and Th2 cells were used. In some experiments, to trigger OX40L, 20 μg/ml sOX40 was added at the same time as antigen.
Purification of CD4+CD25+ T cell subset
CD4+CD25+ cells were purified from mouse splenocytes with the CD25+ T cell isolation kit (Miltenyi Biotec, Auburn, CA, USA). Splenocytes from Tnfrsf4−/− mice were kindly provided by Dr. Mario Colombo (Instituto Nazionale dei Tumori, Milan, Italy). CD4+CD25+ isolation and OX40 expression using biotinylated anti-OX40 (eBioscence), followed by PerCP-Cy5.5-conjugated streptavidin (BD Biosciences, San Jose, CA, USA), were assessed by flow cytometry. Gated cells were always >80% CD25+.
Production of polyclonal CD4+ Th1 and Th2 cell lines
Inguinal LNs were dissected from C57BL/6 mice, and cells were collected. Cells (0.4×106) were placed on plate-bound anti-CD3 and anti-CD28 (6 μg/ml working solution, eBioscence) in 1 ml final vol RPMI 5% FBS for 7 days. For Th1 culture, medium was supplemented with mouse IL-2 (10 ng/ml, ImmunoTools, Germany), anti-IL-4 (20 μg/ml, eBioscence), and mouse IL-12 (20 ng/ml, PeproTech, Rocky Hill, NJ, USA). For Th2 culture, medium was supplemented with mouse IL-2 (10 ng/ml, ImmunoTools), IL-4 (4 ng/ml, PeproTech), anti-IFN-γ (20 μg/ml, eBioscence), and anti-IL-12 (20 ng/ml, eBioscence) [16].
BMMC lysis and PLC-γ2 antibodies
BMMC lysates were prepared in borate-buffered saline (BBS) with a final concentration of 1% Nonidet P-40, 60 mM octyl pyranoside, 1 mM Na3VO4, 5 mM Na4P2O7, 50 mM NaF, and protease inhibitor cocktail (Roche Diagnostic GmbH, Mannheim, Germany). Antiphospho-PLC-γ2 (pY759) was from Cell Signaling Technology (Beverly, MA, USA), and anti-PLC-γ2 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Intracellular Ca++ measurement
WT or Tnfsf4−/− BMMCs were loaded with Fluo-4 AM (2 μM) and Fura Red AM (10 μM; both from Molecular Probes, Eugene, OR, USA) for 30 min at 37°C. A Zeiss LSM-510 Meta confocal microscope with a 488-nm wavelength argon-laser light was used for ratio-metric measurements. Fluorescence data were collected every 5 s, and the intensity of fluorescence was quantified using Zeiss LSM-510 Meta software [17].
Mouse injection, tissue collection, and analysis
C57BL/6 mice were i.v.-injected with 150 μg CFSE-conjugated sOX40 and killed 24 h later. To assess sOX40-CFSE distribution, tissue samples were collected for confocal immunofluorescence and immunohistochemistry analysis using anti-FcεRIβ antibody (clone JRK [18]).
Statistical analysis
Results are expressed as mean ± sem. Data were analyzed with a nonpaired Student's t test (Prism, GraphPad Software, La Jolla, CA, USA).
Online supplemental material
Th1 and Th2 cell line differentiation achievement.
Polyclonal anti-CD3- and anti-CD28-activated Teff or Th1 or Th2 cell supernatants were taken 72 h after stimulation with IL-2 (Teff); IL-2, anti-IL-4, and IL-12 (Th1); or IL-4, anti-IFN-γ, and anti-IL-12 (Th2) and tested for IFN-γ and IL-4 production by ELISA (eBioscence). OX40 expression on unstimulated T cells, Tregs, Th1, and Th2 was assessed by flow cytometry; data were acquired on a FACScan (Becton Dickinson) and analyzed with FlowJo software (Tree Star).
PLC-γ2 Western blotting.
Tnfsf4−/− BMMC lysates were prepared as for WT BMMCs (see Materials and Methods). Blotted extracts were probed with antiphospho-PLC-γ2 (pY759; Cell Signaling Technology) or anti-actin (Sigma-Aldrich).
BMMC cytokine and chemokine production.
IgE/antigen-stimulated BMMCs (2×106/ml) were treated with sOX40 (20 μg/ml) or incubated with equal numbers of WT or Tnfrsf4−/− Tregs for 24 h. Supernatants were tested for the presence of IL-5, IL-6, TNF-α, and MCP-1 (all from eBioscence).
RESULTS AND DISCUSSION
sOX40 charaterization and specific binding to BMMCs
sOX40 molecule, obtained by Genentech (sequence in Fig. 1A), was digested and purified to remove the Fc portion, as described in Materials and Methods. To determine the purity and specificity of the eluted fraction, Coomassie staining and Western blot analysis were performed (Fig. 1B). The obtained digested band was ∼30 kDa. To use this molecule for in vitro and in vivo applications, sOX40 was tested in viability assay. BMMC treatment with 1, 10, and 20 μg/ml sOX40 did not influence cell viability, whereas 100 μg/ml impaired cell viability at 72 h and 96 h of culture (Fig. 1C). Thus, for further studies, 20 μg/ml sOX40 was used.
To test whether sOX40 was targeting OX40L-expressing MCs specifically, sOX40-CFSE was tested for its ability to bind MCs in vitro. sOX40-CFSE bound to BMMCs (Fig. 1D). This binding was specific, as cells pretreated with an increasing amount of unlabeled sOX40 competitor showed decreased sOX40-CFSE staining, pretreatment with anti-OX40L antibody completely abrogated sOX40-CFSE binding (Fig. 1D), and Tnfsf4−/− BMMCs did not bind this molecule (Fig. 1E).
sOX40 specifically decreases MC degranulation
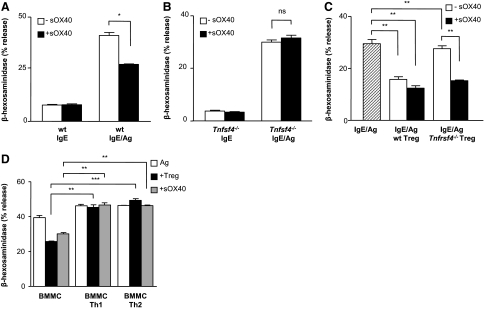
To validate the efficacy of the purified sOX40 molecule on MC degranulation, the effect of sOX40 was tested on IgE/antigen-stimulated BMMCs. Degranulation was assessed by measuring MC granule-associated enzyme β-hexosaminidase release. As shown in Fig. 2A, sOX40 reduced BMMC degranulation (from 41.1%±1.4% to 27.3%±0.2%; P=0.011) significantly. Degranulation in the absence of antigen was not affected by sOX40, thus indicating that sOX40 exerts its action only when the cells are activated by antigen. Moreover, sOX40 did not influence degranulation of Tnfsf4−/− BMMCs (Fig. 2B). The extent of sOX40-mediated inhibition on BMMC degranulation was comparable with that observed in MC:Treg coculture (from 30.3%±0.8% of IgE/antigen-stimulated BMMCs to 17.5%±0.6% and 13.5%±1.1% of IgE/antigen BMMCs, stimulated with WT Tregs or WT Tregs together with sOX40, respectively; Fig. 2C). sOX40 is a specific target of OX40L on MCs, as activated MCs in coculture with Tnfrsf4−/− Tregs showed a significantly reduced degranulation only when sOX40 was added. Indeed, no significant difference in MC degranulation was seen when BMMCs were activated in the presence or absence of Tnfrsf4−/− Tregs (28.2%±0.6% vs. 30.3%±0.8%). However, when sOX40 was added to the mixed coculture containing the Tnfrsf4−/− Tregs, BMMCs released 15.3% ± 0.1% (P=0.003) of β-hexosaminidase (Fig. 2C).
Figure 2. Dampening of IgE-mediated BMMC degranulation by sOX40.
(A) WT IgE-presensitized BMMCs were challenged with 50 ng/ml antigen in the absence or presence of 20 μg/ml sOX40 (–sOX40 and +sOX40, respectively) and were examined for β-hexosaminidase release. (B) Tnfsf4−/− BMMCs were challenged as in A. (C) IgE/antigen challenged BMMCs in coculture with equal numbers of WT, and Tnfrsf4−/− Tregs were examined for β-hexosaminidase release in the absence or presence of 20 μg/ml sOX40. (D) IgE/antigen challenged BMMCs in coculture with equal numbers of Tregs, Th1, or Th2 cells, and sOX40 (20 μg/ml) was examined for β-hexosaminidase release. All graphs show means ± sem of at least two independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Activated CD4+ T cells, although expressing the OX40 molecule, significantly enhanced several costimulatory molecules, which by binding on IgE/antigen-stimulated MCs, could influence their degranulation activity [6, 8, 19]. Indeed, LFA-1 [19], 4-1BB [20], CD226 [21], and CCR1 [22] triggering has been shown to up-regulate MC effector functions. To establish the effect of different T cell subsets, namely Th1 and Th2, on FcεRI-initiated MC degranulation, we set up a coculture assay with BMMCs and Th1 or Th2 cells in the absence or presence of Tregs. Th1 and Th2 differentiation process was tested by IFN-γ and IL-4 release, respectively (Supplemental Fig. 1A and B). OX40 expression was similar between Th1 and Th2 cell types (Supplemental Fig. 1C), as reported previously [23]. Degranulation was slightly up-regulated by Th1 or Th2 cells (46.2%±1.2% and 46.4%±0.2%, respectively, compared with 39.4%±1.7% of activated BMMCs alone). The presence of Tregs did not affect the trend of BMMC degranulation (45.4%±2% and 49.4%±0.9% in Treg coculture with Th1 and Th2, respectively). Importantly, addition of sOX40 in BMMC:Th1 or BMMC:Th2 cocultures did not interfere with MC degranulation (Fig. 2D). Thus, it seems that sOX40 acts on OX40L-expressing BMMCs only when these cells are not engaged with other T cell types regarding the early degranulation events.
Collectively, these data demonstrate that a highly purified sOX40, which specifically targets OX40L, is able to mimic Treg physiological OX40L triggering on MCs, providing a new tool to dissect OX40L-dependent pathways in MCs.
sOX40 specifically inhibits Ca++ mobility but not PLC-γ2 phosphorylation on activated MCs
Tregs exert their modulatory effect on MC degranulation by inhibiting extracellular Ca++ influx, a process that requires the OX40:OX40L axis and does not depend on PLC-γ2 activation [8]. sOX40, as well, did not affect PLC-γ2 activation, as measured by the phosphorylation level of tyrosine 759 in activated WT BMMCs (Fig. 3A) and in control Tnfsf4−/− BMMCs (Supplemental Fig. 2), thus confirming that this molecule resembles Treg physiological interaction with BMMCs. Moreover, the production of BMMC-derived cytokines IL-5, IL-6, and TNF-α and chemokine MCP-1 between sOX40-treated and -untreated cells was comparable (Supplemental Fig. 3A–D), similarly to what was observed previously in MC-Treg cocultures [8]. Thus, sOX40 does not inhibit MC activities in toto, but it selectively discriminates among MC functions, preserving activated MC capacity to release cytokines and chemokines necessary for their physiological activity in tissue remodeling and homeostasis. By acting on extracellular Ca++ uptake, OX40L-dependent signaling could modulate early degranulation events, allowing MCs to recover and to proceed with long-term effector functions, such as cytokine production. Consistently, the development of the sOX40 will enable an in-depth examination of this issue.
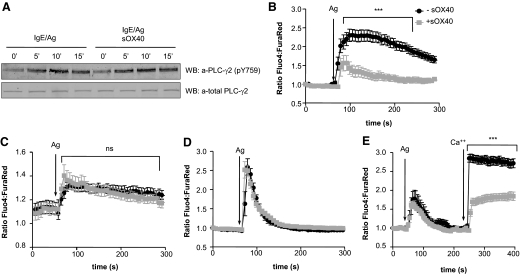
Figure 3. sOX40 does not affect PLC-γ2 activation but impairs FcεRI-dependent Ca++ influx in activated BMMCs.
(A) Time course analysis of phospho-PLC-γ2 (pY759) expression levels in IgE/antigen BMMCs in the absence or presence of sOX40 compared with total PLC-γ2 expression levels. WB, Western blot. IgE-sensitized (B) WT or (C) Tnfsf4−/− BMMCs loaded with Fluo-4 and Fura Red were untreated (black lines) or treated (gray lines) with sOX40 in the presence of extracellular Ca++ and triggered with antigen (arrows). Fluorescence emission was monitored for up to 5 min. (D) WT IgE-sensitized BMMCs were treated the same as above in the absence of extracellular Ca++. (E) WT IgE-sensitized BMMCs, initially cultured in the absence of Ca++, were antigen-challenged, and extracellular Ca++ was restored 3 min thereafter. Graphs were generated by capturing the fluorescence intensity of a single cell over time. All of the graphs show means ± sem of one representative experiment in which at least 10 cells were monitored. ***P < 0.001.
To assess MC calcium response after sOX40 treatment, a single-cell fluorescence analysis was performed. As shown in Fig. 3B, the extent of Ca++ mobilization in sOX40-treated, activated WT BMMCs was weaker than the one measured in activated BMMCs alone. Moreover, sOX40 did not affect Ca++ flux within Tnfsf4−/−-activated BMMCs (Fig. 3C). In the absence of extracellular Ca++, antigen challenge of sOX40-treated or -untreated WT BMMCs did not reveal any significant difference in mobilization of intracellular Ca++ stores (Fig. 3D), indicating that sOX40 treatment of BMMCs did not alter Ca++ mobilization from endoplasmic reticular stores. To assess if the dampening of Ca++ responses was a result of impaired Ca++ influx, experiments were first conducted in the absence of extracellular Ca++, thus monitoring the extent of intracellular-stored Ca++ release, and then extracellular Ca++ was replenished 3 min after antigen challenge, revealing reduced Ca++ influx only in sOX40-treated WT BMMCs (Fig. 3E). This demonstrated that similarly to the effect of Tregs cocultured with MCs, sOX40 treatment of MCs caused inhibition of extracellular Ca++ influx.
As MCs require sustained Ca++ influx to allow degranulation of allergic mediators [24], and sOX40 modulates MC response by reducing Ca++ influx, this molecule could find an in vivo application as a therapeutic tool to control MC degranulation and aberrant Ca++ responses that characterize MC activity in anaphylaxis [25] or skin diseases [26]. This use would be akin to Ca++ antagonists or antihypertensive calcium-channel blockers [27] but with the advantage that it specifically intervenes on OX40:OX40L-dependent Ca++ modulation in MCs.
sOX40 in vivo distribution upon systemic challenge
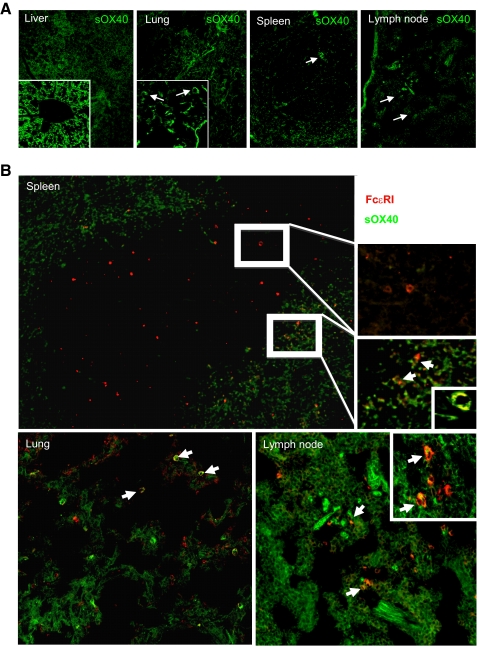
Upon systemic i.v. injection of mice, sOX40-CFSE was found to be widely distributed in all of the tissues analyzed, including liver, lung, LNs, and spleen (Fig. 4). In these tissues, it differed in its pattern of localization, with a predominance of sOX40 localized in the vascular endothelia of the portal veins and in the cytoplasm of hepatocytes in the liver (Fig. 4A, left panel and inset); in the lung vasculature and in the surface of immune cells populating the alveolar interstitium in the lung (Fig. 4A, second panel from left and inset); and mainly confined to outer layers of the white pulp in the spleen, marking cells with lymphoid (round), histiocytic (stellate), or dendritic morphology (spindle-to-stellate; Fig. 4A, second panel from right). In most of the analyzed tissues (except for the liver), sOX40-CFSE was found on the surface of cells with monocytoid appearance, which was suggestive of MCs (Fig. 4A, middle panels and right panel, arrows). To confirm that among other cell types, sOX40-CFSE was indeed on the MC surface, immunofluorescence, using a primary antibody against the FcεRIβ subunit, was performed on sections from tissues analyzed for sOX40-CFSE distribution. Counts of single-stained sOX40-CFSE+, FcεRIβ+, or double sOX40-CFSE+/FcεRIβ+ cells are summarized in Table 1. Notably, several MCs that stained with FcεRIβ+ (Fig. 4B, red signal) also showed sOX40-CFSE colocalization (Fig. 4B, arrows, and Table 1). Basophils, which are also FcεRIβ+, are extremely rare in normal tissues, and OX40L expression and function in these cells have never been proven, thus sOX40-CFSE+/FcεRIβ+-double-positive cells within analyzed tissues should be predominantly MCs.
Figure 4. sOX40 biodistribution and colocalization with BMMCs.
(A) Confocal immunofluorescence analysis of sOX40-CFSE (green) distribution within liver, lung, spleen, and LN. Arrows indicate monocytoid-like cells (original magnifications, 100×; insets, 400×). (B) Sections from spleen, lung, and LN showing localization of sOX40-CFSE and MC stained with mouse anti-rat FcεRIβ (red). FcεRIβ+ cells marked with sOX40-CFSE (green) are indicated by arrows (original magnifications, 100×, insets; 400× and 630×).
Table 1. Numbers of FcεRIβ-Positive Cells Reached by the sOX40.
| sOX40-positivea | FcεRIβ-positiveb | sOX40/FcεRI-double-positivec | |
|---|---|---|---|
| Lung | 24.3 ± 2.7 | 10 ± 1.4 | 8.0 ± 1.5 |
| Liver | 6.5 ± 2 | 2 ± 1.3 | 0.5 ± 0.7 |
| Spleen | 46.5 ± 10.3 | 20.6 ± 4.2 | 14 ± 3.6 |
| LN | 28.7 ± 5.7 | 11.5 ± 3.1 | 6.3 ± 0.6 |
sOX40-positive cells were detected and counted in five different fields at 400×magnification. Counts are relative to cells displaying a round-to-oval (monocytoid) morphology and do not include spindle-shaped stromal cells (e.g., endothelia) or epithelial cells (e.g., hepatocytes). Numbers indicate mean ± sem.
FcεRIβ-single-positive cells and sOX40/FcεRIβ-double-positive cells were counted as indicated ina. Numbers indicate mean ± sem.
These results demonstrate that sOX40 could target OX40L-expressing cells in vivo, as it binds to endothelial cells, DCs, and MCs, all of which express OX40L on their surface [10, 28–30], and could be of therapeutic value in vivo in light of its ability to leave the vascular compartment and distribute systemically into MC-populated parenchymas upon i.v. injection. Thus, we developed a tool that resembles the inhibitory effect of Tregs on FcεRI-dependent degranulation and extracellular Ca++ influx through the OX40:OX40L axis. Mimicking Treg-mediated physiological regulation of IgE/antigen-activated MCs, without physical interactions with Tregs, could be useful in several settings: to identify the unknown signaling events downstream OX40L triggering; to decipher how MC degranulation can be regulated independently of other effector functions; and to use sOX40 as a therapeutic tool, where the OX40:OX40L axis may be relevant. As sOX40 specifically triggers MCs in vitro, it provides a useful molecular tool that specifically binds to OX40L and resolves the difficulty of studying the molecular events of a single cell population in a coculture system. Given that we previously demonstrated the importance of the OX40:OX40L axis for in vivo anaphylaxis [8], we now find that sOX40 targets MCs in vivo and thus, may function to supplant Treg-mediated regulation of MCs, a hypothesis that remains to be tested.
Noteworthy, beyond anaphylaxis, the importance of sOX40 in vivo application could also be extended to other diseases, in which disruption of the OX40:OX40L axis or expansion and/or activation of MCs have been reported, such as immune-mediated colitis and nephritis, systemic lupus erythematosus, heart diseases, graft-versus-host disease, tumors (reviewed in ref. [23]), and indolent mastocytosis [31]. Recently, it has also been reported that in experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis, MCs are able to skew Teffs into IL-17-producing Teffs (Th17) through the OX40:OX40L axis, boosting a Th17-inflammatory environment [11].
The length of sOX40 treatment should be considered, as this molecule would immediately target not-engaged OX40L, constitutively expressing cells such as MCs and endothelial cells. Thus, treatment of the acute phase of anaphylaxis, in which MCs are the main effector arm, should be feasible [32]. OX40L on endothelial cells costimulates memory CD4+ T cell proliferation and stabilizes their cytokine production [33]. Therefore, long-term sOX40 administration could interfere with endothelial-CD4+ T cell interaction, resulting in the decrease of T cell inflammatory response, but could also affect endothelial physiology in a yet-unknown pathway. Similarly, binding of sOX40 on activated OX40L+ DCs could impair DC-T cell interaction, resulting in a defective Th2 skewing while promoting DC maturation [23, 34] and B cell activation [35], a hypothesis that cannot be excluded.
In this perspective, our findings suggest a potential therapeutic application in conditions in which a need to “finely tune” rather than “turn off” MC responses may be required.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from Associazione Italiana Ricerca sul Cancro (AIRC), Ministero dell′Istruzione, Università e Ricerca (PRIN 2005), Agenzia Spaziale Italiana (Progetto OSMA), LR.11 del Friuli Venezia Giulia, Fondazione Cariplo, and the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (Bethesda, MD, USA). The authors thank Drs. A. Olivera and M. Mongillo for technical assistance.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- BM
- bone marrow
- FcεRI
- high-affinity receptor for IgE
- MC
- mast cell
- OX40L
- OX40 ligand, sOX40, soluble OX40
- sOX40-CFSE
- soluble OX40 conjugated with 5(6)-CFSE/FAM
- Teff
- effector T cell
- Tnfrsf4−/−
- TNFR superfamily 4-deficient (OX40-deficient)
- Tnfsf4−/−
- TNF superfamily 4-deficient (OX40 ligand-deficient)
- Treg
- regulatory T cell
AUTHORSHIP
R.S. acquired data and analyzed, interpreted, and drafted the manuscript. G.G. performed the study supervision, interpretation of data, and revision of the manuscript. B.F. added technical support. C.T. provided technical support and interpretation of data. R.S. offered technical and material support. J.R. obtained funding and did critical revision. A.S.M. provided material support and revised the manuscript. C.E.P. performed the study design and obtained funding.
REFERENCES
- 1. Minai-Fleminger Y., Levi-Schaffer F. (2009) Mast cells and eosinophils: the two key effector cells in allergic inflammation. Inflamm. Res. 58, 631–638 [DOI] [PubMed] [Google Scholar]
- 2. Frossi B., Gri G., Tripodo C., Pucillo C. (2010) Exploring a regulatory role for mast cells: “MCregs”? Trends Immunol. 31, 97–102 [DOI] [PubMed] [Google Scholar]
- 3. Galli S. J., Nakae S., Tsai M. (2005) Mast cells in the development of adaptive immune responses. Nat. Immunol. 6, 135–142 [DOI] [PubMed] [Google Scholar]
- 4. Galli S. J., Tsai M. (2010) Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. Eur. J. Immunol. 40, 1843–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Galli S. J., Kalesnikoff J., Grimbaldeston M. A., Piliponsky A. M., Williams C. M. M., Tsai M. (2005) Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu. Rev. Immunol. 23, 749–786 [DOI] [PubMed] [Google Scholar]
- 6. Sayed B. A., Brown M. A. (2007) Mast cells as modulators of T-cell responses. Immunol. Rev. 217, 53–64 [DOI] [PubMed] [Google Scholar]
- 7. Akdis M. (2006) Healthy immune response to allergens: T regulatory cells and more. Curr. Opin. Immunol. 18, 738–744 [DOI] [PubMed] [Google Scholar]
- 8. Gri G., Piconese S., Frossi B., Manfroi V., Merluzzi S., Tripodo C., Viola A., Odom S., Rivera J., Colombo M. P., Pucillo C. E. (2008) CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity 29, 771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu L-F., Lind E. F., Gondek D. C., Bennett K. A., Gleeson M. W., Pino-Lagos K., Scott Z. A., Coyle A. J., Reed J. L., Van Snick J., Strom T. B., Zheng X. X., Noelle R. J. (2006) Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature 442, 997–1002 [DOI] [PubMed] [Google Scholar]
- 10. Nakae S., Suto H., Iikura M., Kakurai M., Sedgwick J. D., Tsai M., Galli S. J. (2006) Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J. Immunol. 176, 2238–2248 [DOI] [PubMed] [Google Scholar]
- 11. Piconese S., Gri G., Tripodo C., Musio S., Gorzanelli A., Frossi B., Pedotti R., Pucillo C. E., Colombo M. P. (2009) Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood 114, 2639–2648 [DOI] [PubMed] [Google Scholar]
- 12. Matsumura Y., Hori T., Kawamata S., Imura A., Uchiyama T. (1999) Intracellular signaling of gp34, the OX40 ligand: induction of c-jun and c-fos mRNA expression through gp34 upon binding of its receptor, OX40. J. Immunol. 163, 3007–3011 [PubMed] [Google Scholar]
- 13. Kotani A., Hori T., Matsumura Y., Uchiyama T. (2002) Signaling of gp34 (OX40 ligand) induces vascular endothelial cells to produce a CC chemokine RANTES/CCL5. Immunol. Lett. 84, 1–7 [DOI] [PubMed] [Google Scholar]
- 14. Burgess J. K., Carlin S., Pack R. A., Arndt G. M., Au W. W., Johnson P. R. A., Black J. L., Hunt N. H. (2004) Detection and characterization of OX40 ligand expression in human airway smooth muscle cells: a possible role in asthma? J. Allergy Clin. Immunol. 113, 683–689 [DOI] [PubMed] [Google Scholar]
- 15. Tkaczyk C., Okayama Y., Metcalfe D. D., Gilfillan A. M. (2004) Fcγ receptors on mast cells: activatory and inhibitory regulation of mediator release. Int. Arch. Allergy Immunol. 133, 305–315 [DOI] [PubMed] [Google Scholar]
- 16. Fitch F. W., Gajewski T. F., Hu-Li J. (2006) Production of TH1 and TH2 cell lines and clones. Curr. Protoc. Immunol. May, 13. [DOI] [PubMed] [Google Scholar]
- 17. Suzuki R., Liu X., Olivera A., Aguiniga L., Yamashita Y., Blank U., Ambudkar I., Rivera J. (2010) Loss of TRPC1-mediated Ca2+ influx contributes to impaired degranulation in Fyn-deficient mouse bone marrow-derived mast cells. J. Leukoc. Biol. 88, 863–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rivera J., Kinet J. P., Kim J., Pucillo C., Metzger H. (1988) Studies with a monoclonal antibody to the β subunit of the receptor with high affinity for immunoglobulin E. Mol. Immunol. 25, 647–661 [DOI] [PubMed] [Google Scholar]
- 19. Inamura N., Mekori Y. A., Bhattacharyya S. P., Bianchine P. J., Metcalfe D. D. (1998) Induction and enhancement of Fc(ε)RI-dependent mast cell degranulation following coculture with activated T cells: dependency on ICAM-1- and leukocyte function-associated antigen (LFA)-1-mediated heterotypic aggregation. J. Immunol. 160, 4026–4033 [PubMed] [Google Scholar]
- 20. Nishimoto H., Lee S. W., Hong H., Potter K. G., Maeda-Yamamoto M., Kinoshita T., Kawakami Y., Mittler R. S., Kwon B. S., Ware C. F., Croft M., Kawakami T. (2005) Costimulation of mast cells by 4-1BB, a member of the tumor necrosis factor receptor superfamily, with the high-affinity IgE receptor. Blood 106, 4241–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bachelet I., Munitz A., Mankutad D., Levi-Schaffer F. (2006) Mast cell costimulation by CD226/CD112 (DNAM-1/Nectin-2): a novel interface in the allergic process. J. Biol. Chem. 281, 27190–27196 [DOI] [PubMed] [Google Scholar]
- 22. Fifadara N. H., Aye C. C., Raghuwanshi S. K., Richardson R. M., Ono S. J. (2009) CCR1 expression and signal transduction by murine BMMC results in secretion of TNF-α, TGFβ-1 and IL-6. Int. Immunol. 21, 991–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Croft M. (2010) Control of immunity by the TNFR-related molecule OX40 (CD134). Annu. Rev. Immunol. 28, 57–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vig M., Kinet J-P. (2009) Calcium signaling in immune cells. Nat. Immunol. 10, 21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baba Y., Nishida K., Fujii Y., Hirano T., Hikida M., Kurosaki T. (2008) Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat. Immunol. 9, 81–88 [DOI] [PubMed] [Google Scholar]
- 26. Sicherer S. H., Leung D. Y. M. (2010) Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insects in 2009. J. Allergy Clin. Immunol. 125, 85–97 [DOI] [PubMed] [Google Scholar]
- 27. Palamaras I., Kyriakis K. (2005) Calcium antagonists in dermatology: a review of the evidence and research-based studies. Dermatol. Online J. 11, 8. [PubMed] [Google Scholar]
- 28. Kashiwakura J., Yokoi H., Saito H., Okayama Y. (2004) T cell proliferation by direct cross-talk between OX40 ligand on human mast cells and OX40 on human T cells: comparison of gene expression profiles between human tonsillar and lung-cultured mast cells. J. Immunol. 173, 5247–5257 [DOI] [PubMed] [Google Scholar]
- 29. Nakano M., Fukumoto Y., Satoh K., Ito Y., Kagaya Y., Ishii N., Sugamura K., Shimokawa H. (2010) OX40 ligand plays an important role in the development of atherosclerosis through vasa vasorum neovascularization. Cardiovasc. Res. 88, 539–546 [DOI] [PubMed] [Google Scholar]
- 30. Zaini J., Andarini S., Tahara M., Saijo Y., Ishii N., Kawakami K., Taniguchi M., Sugamura K., Nukiwa T., Kikuchi T. (2007) OX40 ligand expressed by DCs costimulates NKT and CD4+ Th cell antitumor immunity in mice. J. Clin. Invest. 117, 3330–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marone G., Spadaro G., Granata F., Triggiani M. (2001) Treatment of mastocytosis: pharmacologic basis and current concepts. Leuk. Res. 25, 583–594 [DOI] [PubMed] [Google Scholar]
- 32. Grimbaldeston M. A., Metz M., Yu M., Tsai M., Galli S. J. (2006) Effector and potential immunoregulatory roles of mast cells in IgE-associated acquired immune responses. Curr. Opin. Immunol. 18, 751–760 [DOI] [PubMed] [Google Scholar]
- 33. Mestas J., Crampton S. P., Hori T., Hughes C. C. (2005) Endothelial cell co-stimulation through OX40 augments and prolongs T cell cytokine synthesis by stabilization of cytokine mRNA. Int. Immunol. 17, 737–747 [DOI] [PubMed] [Google Scholar]
- 34. Blázquez A. B., Berin M. C. (2008) Gastrointestinal dendritic cells promote Th2 skewing via OX40L. J. Immunol. 180, 4441–4450 [DOI] [PubMed] [Google Scholar]
- 35. Stüber E., Strober W. (1996) The T cell-B cell interaction via OX40-OX40L is necessary for the T cell-dependent humoral immune response. J. Exp. Med. 183, 979–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.