C/EBPs heterodimerize with AP-1 to bind hybrid DNA elements, and occurs consequent to AP-1 induction during monopoiesis to induce FosB and likely additional targets.
Keywords: c-Jun, c-Fos, myeloid, leucine zipper
Abstract
AP-1 proteins heterodimerize via their LZ domains to bind TGACGTCA or TGACTCA, whereas C/EBPs dimerize to bind ATTGCGCAAT. We demonstrate that intact C/EBPα also heterodimerizes with c-Jun or c-Fos to bind a hybrid DNA element, TGACGCAA, or more weakly to TGATGCAA. A 2:1 ratio of c-Jun:C/EBPα or c-Fos:C/EBPα was sufficient for preferential binding. Semiquantitative Western blot analysis indicates that the summation of c-Jun, JunB, and c-Fos levels in differentiating myeloid cells is similar to or exceeds the entirety of C/EBPα and C/EBPβ, indicating the feasibility of heterodimer formation. Induction of AP-1 proteins during monocytic differentiation favored formation of C/EBP:AP-1 heterodimers, with C/EBPα homodimers more evident during granulopoiesis. Approximately 350 human and 300 murine genes contain the TGACGCAA motif between –2 kb and +1 kb of their transcription start sites. We focused on the murine Fosb promoter, which contains a C/EBP:AP-1 cis element at –56 and –253, with the hFOSB gene containing an identical site at –253 and a 1-bp mismatch at –56. C/EBPα:AP-1 heterodimers bound either site preferentially in a gel-shift assay, C/EBPα:c-Fos ER fusion proteins induced endogenous Fosb mRNA but not in the presence of CHX, C/EBP and AP-1 proteins bound the endogenous Fosb promoter, mutation of the –56 cis element reduced reporter activity fivefold, and endogenous FosB protein was expressed preferentially during monopoiesis versus granulopoiesis. Increased expression of Jun/Fos proteins elevates C/EBP:AP-1 heterodimer formation to potentially activate novel sets of genes during monopoiesis and potentially during other biologic processes.
Introduction
C/EBPs bind DNA via their BR-LZ domains [1]. The LZ forms a coiled-coil structure to mediate dimerization, and the BR contacts DNA [2]. AP-1 transcription factors also bind DNA via bZIP domains, with c-Jun, JunB, or JunD heterodimerizing with c-Fos, FosB, Fra1, or Fra2 [3]. C/EBPs bind ATTGCGCAAT, whereas AP-1 proteins bind the PMA-responsive element TGA(G/C)TCA or the cAMP-responsive element TGACGTCA.
c-Jun or c-Fos interact with the bZIP domain of C/EBPβ to prevent binding to a C/EBP cis element, and c-Jun interacts with C/EBPα to prevent its DNA binding but not if the c-Jun LZ is deleted [4–6]. We provided further evidence that C/EBPs zipper with AP-1 proteins using zipper-swap constructs and gel-shift analysis [7]. In addition, we used proteins in which acidic or basic LZs direct formation of specific homodimers or heterodimers to show that C/EBPα acid:basic LZ homodimers preferentially bind C/EBP sites, whereas C/EBPα:AP-1 acid:base LZ complexes prefer the hybrid site TGACGCAA. Moreover, C/EBPα:AP-1 acid:base LZ complexes induced monocytic differentiation of marrow progenitors. Also, ChIP of C/EBPβ from macrophages identifies frequent, nearby motifs similar to the C/EBPα:AP-1 consensus in addition to C/EBP homodimer consensus sites [8].
We now demonstrate that WT C/EBPα zippers with c-Jun or c-Fos, a preference for C/EBPα:AP-1 complex formation occurs when c-Jun or c-Fos and C/EBPα are expressed at a 2:1 ratio, endogenous C/EBP and AP-1 proteins are present at approximately equal levels in myeloid cells, and endogenous C/EBP:AP-1 complexes are readily detected. In addition, increased expression of AP-1 proteins during monopoiesis leads to formation of C/EBP:AP-1 complexes, largely to the exclusion of C/EBP homodimers. Analysis of the human and murine genomes identifies >300 genes with the TGACGCAA element in their promoter regions, and we provide evidence that the gene encoding FosB is bound and activated by C/EBP:AP-1 proteins via this element. These findings support the idea that C/EBP:AP-1 LZ heterodimerization plays a significant role in myeloid lineage determination and potentially also other biologic process, including malignant transformation.
MATERIALS AND METHODS
Cell culture and transfection
32Dcl3 cells were cultured in IMDM with 10% HI-FBS and 1 ng/ml murine IL-3 (PeproTech, Rocky Hill, NJ, USA) [9]. HF-1 cells were cultured in IMDM with 10% HI-FBS and 2.5 ng/ml murine GM-CSF (PeproTech) [10]. HL-60 and M1 cells were cultured in RPMI 1640 with 10% HI-FBS or 10% heat-inactivated horse serum, respectively. To induce granulocytic differentiation, 32Dcl3 or HF-1 cells were washed twice with PBS and transferred to IMDM with 10% HI-FBS and 20 ng/ml human G-CSF (Amgen, Thousand Oaks, CA, USA). To induce monocytic differentiation, 100 ng/ml PMA was added to the HL-60 cultures, or 50 ng/ml human IL-6 (PeproTech) was added to the M1 cell cultures. bzATP was used at 250 μM. Ba/F3 lines expressing ER fusion proteins were cultured in phenol red-free RMPI 1640 with 10% HI-FBS and 1 ng/ml murine IL-3. E2 was used at 1 μM and CHX at 50 μg/ml. 293T cells cultured in DMEM with 10% HI-FBS were transiently transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). 32Dcl3 cells were transiently transfected using DEAE-dextran as described [11].
Plasmids, IVT, and Western blotting
The cDNAs encoding C/EBPα, C/EBPβ, c-Jun, JunB, and c-Fos were positioned in-frame with an N-terminal methionine and a single Myc tag downstream from the CMV and T7 promoters for expression in 293T cells or for IVT. Each construct was confirmed by DNA sequencing. Coupled in vitro transcription and IVT were conducted using the TnT kit (Promega, Madison, WA, USA). Western blotting was carried out as described [7] using rabbit anti-C/EBPα (AA14), C/EBPβ (C19), c-Jun (N), JunB (N-17), and c-Fos (4) antisera or mouse anti-c-Myc (A-14) antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or rabbit anti-FosB antiserum 2251 (Cell Signaling, Danvers, MA, USA). FosB(–497/+1)-Luc was generated by mouse DNA genomic amplification followed by XhoI/BglII digestion and ligation into pGL4B (Promega). Primers used were: FosB(–497): 5′-CGCGCTCGAGTAAGCAGACCTGGGATCTGGAG-3′ and FosB(+1): 5′-CGCGCTCGAGTAAGCAGACCTGGGATCTGGAG-3′. The –56 and –253 αJ sites in the promoter were mutated by site-directed mutagenesis to match the mutant gel-shift oligonucleotides.
Nuclear protein preparation and gel-shift analysis
Nuclear extract preparation and gel-shift/super-shift assays were carried out as described [12]. Oligonucleotide probes containing 5′-TCGA, 5′-GATC, or 5′-CTAG overhangs were radiolabeled to similar specific activity using Klenow enzyme and α-P32-dCTP. Sense strands of the probes, used with binding sites underlined, were: αα: 5′-TCGAGGCCAGGAATTGCGCAATACAACCCG-3′; αJ: 5′-TCGAGGCCAGGATGACGCAATACAACCCG-3′; JJ: 5′-GATCCAGGTGTCTGAGTCAGGTTTGG-3′; PU.1(–68): 5′-CTAGGACTTCCTGTAGCGCAAGAGATTTATG-3′; murine FosB(–56): 5′-TCGAGAGTTGCGGGTGACGCAAGCGCGGGGGC-3′; mutant murine FosB(–56): 5′-TCGAGAGTTGCGGGTGACGCGTGCGCGGGGGC-3′; hFosB(–56): 5′-TCGAAAGGGGCGGGTGACGTAAGCAGGGGGGCG-3′; FosB(–253): 5′-TCGAATATGGCTAATTGCGTCACAGGAACTCC-3′; mutant FosB(–253): 5′-TCGAATATGGCTAAACGCGTCACAGGAACTCC-3′.
Oligonucleotide pull-down and ChIP
Unlabeled sense strands αα, αJ, or JJ oligonucleotides listed above were annealed to antisense strands without overlap and containing biotin linked to the 3′-nucleotide. Each annealed double-stranded oligonucleotide (1 μg) was incubated with 100 μg nuclear protein, isolated as for the gel-shift assay, for 20 min at room temperature in binding buffer containing 12% w/v glycerol, 12 mM Hepes, and 4 mM Tris, pH 7.9, 150 mM KCl, 1 mM EDTA, 1 mM DTT, and 2 μg poly-dI:dC competitor. Following the incubation, 20 μl streptavidin-agarose, preabsorbed with 500 μg BSA, 50 μg poly-dI:dC, and 50 μg sheared salmon sperm DNA for 30 min at 25°C, was added to the reaction and further incubated at 4°C for 4 h. The protein-DNA-streptavidin-agarose complex was washed three times with binding buffer and loaded onto a 10% SDS-PAGE, followed by Western blot analysis. ChIP was conducted as described [13] using the following genomic DNA PCR primers: hFosB(–211)-F: 5′-GAGTGGCCCGGAACGCAAC, hFosB(–103)-R: 5′-CAAATCGAGTCCCGCACTG; hFosB(–5321)-F: 5′-TTCGAAGACCGCCATCACTC, hFosB(–5219)-R: 5′-ACACCACGTCCTTCCCACCT.
Quantitative RT-PCR
RNA was prepared from Ba/F3 lines using the Nucleospin RNA II kit (Macherey-Nagel, Bethlehem, PA, USA), and first-strand cDNA was generated from 2 μg RNA using the ImProm-II RT System (Promega). Quantitative PCR was carried out using 10 ng each cDNA with the iQ-SYBR Green supermix (Bio-Rad, Hercules, CA, USA). Primers used were: FosB-F: 5′-ACCAGCTACTCAACCCCAG-3′, FosB-R: 5′-GGGTAAGTGTCTCTTCTCGGG-3′; mS16-F: 5′-CTTGGAGGCTTCATCCACAT-3′, mS16-R: 5′-ATATTCGGGTCCGTGTGAAG-3′.
Genomics analysis
Promoters with C/EBP:AP-1 binding sites were found by scanning from –2 kb to +1 kb each transcription start site in the human or mouse genome for matches to the consensus sequence TGACGCAA. Mouse build mm8 and human build GRCh37 were used in this analysis. Perl scripts are available upon request. Analysis of functional annotation was performed using R code (L. Marchionni, personal communication, July 2010). The Wilcoxon rank-sum test is used to compute a P value for enrichment of the gene list in any of the known annotated functional gene sets. Resulting enriched sets are filtered by a P value and comparison of the relative ranks of the candidate gene list in that set. A cutoff of P < 0.001 was used after correction for multiple testing [14].
NIH ImageJ software was used to quantify band intensities. Statistical analysis of experimental data was done using the Student's t test.
Online Supplemental Material
Supplemental Figs. 1–3 provide additional gel-shift analyses. Supplemental Tables 1 and 2 list human and murine genes containing binding sites for C/EBP:AP-1 heterodimers located between –2 kb and + 1 kb. Supplemental Table 3 describes ontogeny of human genes with such sites.
RESULTS
C/EBPα zippers with c-Jun or c-Fos to bind a hybrid DNA element
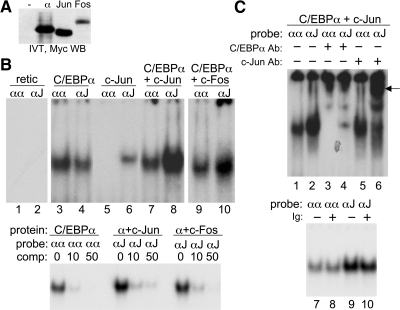
We first sought to determine whether C/EBPα could zipper with c-Jun or c-Fos. To allow comparison of relative protein expression levels, a Myc tag was positioned at the N terminus of the C/EBPα, c-Jun, or c-Fos cDNAs. Myc-tagged C/EBPα, c-Jun, and c-Fos were expressed by coupled in vitro transcription/IVT, and relative expression was assessed by Western blot analysis (Fig. 1A). C/EBPα and c-Jun were expressed similarly, whereas c-Fos was expressed approximately fourfold less efficiently, based on image scanning. C/EBPα, generated by IVT, was subjected to gel-shift assay using radiolabeled probes containing a consensus, C/EBPα site, ATTGCGCAAT (αα), or a hybrid AP-1:C/EBPα site, TGACGCAAT (αJ). No binding to either probe was seen with control reticulocyte lysate (Fig. 1B, lanes 1 and 2). C/EBPα preferentially bound the αα probe, the combination of C/EBPα and c-Jun at a 1:8 ratio preferentially bound the αJ probe, whereas an equivalent amount of c-Jun alone only weakly bound the αJ probe (Fig. 1B, lanes 3–8). When C/EBPα and c-Fos were combined at a C/EBPα:c-Fos mol ratio of ∼1:2, preferential binding to the αJ probe was again observed (Fig. 1B, lanes 9 and 10). Specificity of gel-shift bands was confirmed by competition of 1 ng labeled probe with 0, 10, or 50 ng unlabeled probe (Fig. 1B, lower panel).
Figure 1. C/EBPα zippers with c-Jun or c-Fos when expressed by IVT.
(A) Equal volumes of IVT reaction programmed to encode no protein or Myc-tagged C/EBPα, c-Jun, or c-Fos were subjected to Western blotting (WB) using Myc antibody. (B) Gel-shift assay was carried out using 1 ng of the indicated αα- or αJ-radiolabeled oligonucleotide probes and the indicated C/EBPα, C/EBPα + c-Jun (1:8 mol ratio), or C/EBPα + c-Fos (1:2 mol ratio) protein combinations (upper). retic, Reticulocyte lysate. Specificity was confirmed by competition with 0, 10, or 50 ng unlabeled αα or αJ probe (lower). (C) Gel-shift/super-shift assays were carried out using the αα or αJ probes, C/EBPα + c-Jun (1:8 mol ratio), and 200 ng C/EBPα or c-Jun antiserum. An arrow indicates the super-shift species (upper). Lack of super-shift by normal rabbit Ig was confirmed as a control (lower).
Super-shift assay was then conducted with the C/EBPα and c-Jun combination (Fig. 1C). In the absence of antisera, preferential binding to the αJ oligonucleotide relative to the αα probe was again seen (lanes 1 and 2), the complex bound to the αα site was super-shifted by the C/EBPα but not the c-Jun antiserum (lanes 3 and 5), whereas the complex bound to the αJ probe was super-shifted by both antisera (lanes 4 and 6). The residual gel-shift complex that did not super-shift in lane 6 may reflect partial protein degradation with removal of the N-terminal c-Jun antibody epitope. Normal rabbit Ig did not induce a super-shift species (lanes 7–10).
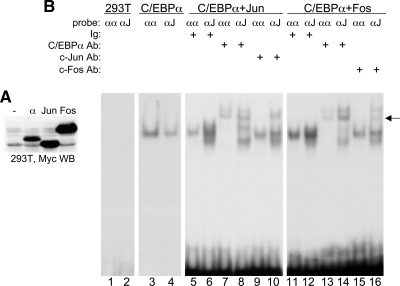
We sought to confirm these findings in mammalian 293T cells. Relative expression of each protein was assessed with Myc-tag antibody (Fig. 2A). c-Jun was expressed approximately twofold higher than C/EBPα, and c-Fos was expressed approximately fourfold higher. No binding to the αα or αJ gel-shift probes occurred using control nuclear extract (Fig. 2B, lanes 1 and 2). Exogenous C/EBPα bound the αα oligonucleotide with two- to threefold higher affinity than the αJ oligonucleotide (lanes 3 and 4). When C/EBPα and c-Jun were combined at a C/EBPα:c-Jun ratio of 1:8, binding to the αJ probe was detected with several-fold higher affinity (Fig. 2B, lanes 5 and 6). The complex bound to the αα probe was super-shifted by the C/EBPα antiserum but not by the c-Jun antiserum (lanes 5, 7, and 9), whereas both antisera super-shifted the complex bound to the αJ probe (lanes 6, 8, and 10). The C/EBPα and c-Fos extracts were combined similarly and assessed by gel-shift and super-shift assay. Again, preferential binding was seen with the αJ probe (compare lanes 11 and 12). The complex bound to the αα probe was super-shifted by C/EBPα but not c-Fos antiserum (lanes 13 and 15), whereas the complex bound to the αJ probe was super-shifted by both antisera (lanes 14 and 16). The residual gel-shift complexes evident in lanes 10 and 16 may represent endogenous AP-1 proteins bound to the probe after zippering with expressed C/EBPα. To verify that expressed c-Jun and c-Fos could zipper and bind a consensus AP-1 site, these extracts were subject to gel-shift assay with the JJ probe TGAGTCA. A complex was observed that super-shifted with c-Jun or c-Fos antisera (Supplemental Fig. 1).
Figure 2. C/EBPα zippers with c-Jun or c-Fos when expressed in mammalian cells.
(A) Nuclear extracts (12 μg) prepared from 293T cells transiently transfected with CMV vectors encoding no protein (–) or Myc-tagged C/EBPα, c-Jun, or c-Fos were subjected to Western blotting using Myc antibody. (B) Gel-shift/super-shift assay was carried out using 1 ng of the indicated αα- or αJ-radiolabeled oligonucleotide probes labeled to similar specific activity, the indicated C/EBPα, C/EBPα + c-Jun (1:8 mol ratio), C/EBPα + c-Fos (1:8 mol ratio) protein combinations, and 200 ng of the indicated C/EBPα, c-Jun, or c-Fos antiserum or rabbit Ig. An arrow indicates the super-shift species.
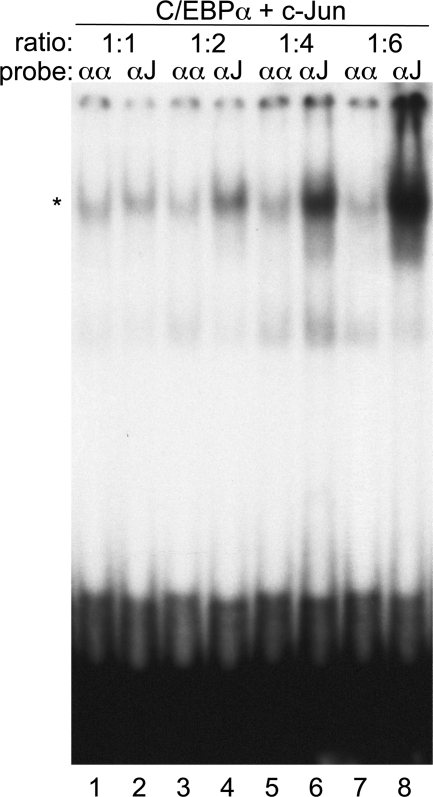
A titration was conducted in which C/EBPα and c-Jun produced by IVT were combined at 1:1, 1:2, 1:4, or 1:6 mol ratios and subjected to gel-shift analysis (Fig. 3). Strong preference for the αJ probe in comparison with the αα probe was evident at a C/EBPα:c-Jun ratio of 1:4 or 1:6, a modest preference was evident at a ratio of 2:1, and similar affinity for the two probes was evident at a 1:1 ratio. A nonspecific, more rapidly migrating band was noted in each lane. These data demonstrate that intact C/EBPα has the capacity to zipper with intact c-Jun or c-Fos to preferentially bind a hybrid DNA element.
Figure 3. Formation of a DNA-binding C/EBPα:c-Jun complex at different mol ratios.
Gel-shift assay was carried out using the αα or αJ oligonucleotide-radiolabeled probes and IVT C/EBPα + c-Jun at the indicated mol ratios. The specific gel-shift complex is indicated (*).
Quantification of C/EBP and AP-1 protein levels in myeloid cells
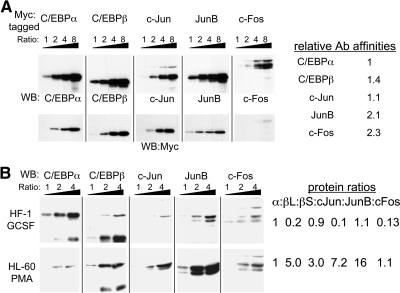
To determine whether endogenous C/EBP and AP-1 proteins are expressed at levels that might allow heterodimerization, we undertook a semiquantitative analysis using two cell lines capable of granulocytic differentiation in response to G-CSF (HF-1 or 32Dcl3) or two lines capable of monocytic differentiation in response to phorbol ester or IL-6 (HL-60 or M1). To estimate relative antibody affinities, Myc-tagged C/EBPα, C/EBPβ, c-Jun, JunB, or c-Fos were first expressed by IVT, and increasing amounts of these samples were subjected to Western blotting with the specific antisera or with Myc antibody (Fig. 4A). All five blots used for the individual antisera or with the Myc antibody were exposed to the same mix of secondary antibody for the same time period followed by simultaneous exposure to the chemiluminescence reagents and subsequent simultaneous autoradiography. Relative band intensities obtained with the specific antisera, quantified using NIH ImageJ software, were then normalized to the expression levels evident with the Myc antibody. For example, c-Jun gave stronger band intensities than did JunB with the Myc antibody, indicating higher absolute expression, and yet weaker band intensities with the specific antiserum, indicating that the JunB antibody has a higher affinity for its epitope.
Figure 4. Semiquantitative analysis of C/EBP and AP-1 family protein levels in myeloid cells.
(A) Increasing volumes (ratio 1:2:4:8) of IVT Myc-tagged C/EBPα, C/EBPβ, c-Jun, JunB, or c-Fos were subjected to Western blotting with protein-specific antisera (upper panels) or Myc antibody (lower panels). The upper or lower sets of blots were exposed to the same secondary antibody solution for an identical time period and were subjected simultaneously to the chemiluminescence reagents and autoradiography. Relative antibody affinities were estimated by densitometric band analysis, correcting the intensities obtained with the specific antisera using the relative protein expression, as determined using the Myc antibody. (B) Nuclear extracts (2.5, 5.0, or 10.0 μg; ratio 1:2:4) from HF-1 cells cultured with G-CSF for 24 h or HL-60 cells cultured with PMA for 6 h were subjected to Western blotting with the indicated antisera. For each cell line, the series of blots were exposed to the same secondary antibody solution for an identical time period and were subjected simultaneously to the chemiluminescence reagents and autoradiography. Protein expression ratios, setting the C/EBPα (α) level to 1 in each cell line, were estimated from densitometric analysis, correcting intensities using the relative antibody affinities. βL and βS are the long and short C/EBPβ isoforms, LAP and LIP.
The specific antisera were then used to determine the expression of C/EBPα, C/EBPβ, c-Jun, JunB, and c-Fos in increasing amounts of nuclear extracts from HF-1 cells induced to undergo early granulocytic differentiation by exposure to G-CSF for 24 h or in HL-60 cells induced to undergo early monocytic differentiation by exposure to PMA for 6 h, respectively (Fig. 4B). Band intensities were corrected using the relative antibody affinities to estimate relative protein expression, with the level of C/EBPα arbitrarily set to 1 in each cell line. The long and short LAP and LIP C/EBPβ isoforms were analyzed separately and are designated βL and βS. Lower C/EBPα levels in HL-60 cells result in higher relative expression of the other proteins, but the absolute values of these numbers cannot be compared between cell lines. The sum of C/EBPα and the two C/EBPβ isoforms compared with the sum of the three AP-1 proteins is of similar magnitude for each cell line. In HF-1 cells, total C/EBP = 2.1 and AP-1 = 1.3, and in HL-60 cells, C/EBP = 9.0 and AP-1 = 24.3. Similar analysis found C/EBP = 5.0 and AP-1 = 4.2 in 32Dcl3 cells cultured in G-CSF, and C/EBP = 15.5 and AP-1 = 15.6 in M1 cells transferred to IL-6 for 1 day (not shown). Although the estimated protein ratios are semiquantitative, these findings, together with our gel-shift analysis of the C/EBPα:AP-1 interaction at different mol ratios, indicate that C/EBP:AP-1 heterodimer formation is feasible in these myeloid cell lines and perhaps favored during monocytic differentiation.
Endogenous C/EBP:AP-1 complexes are evident in myeloid extracts
We next sought to determine whether C/EBP:AP-1 heterodimers can be detected endogenously in myeloid nuclear extracts. First, we conducted gel-shift/super-shift analysis using the αα and αJ probes and nuclear extracts from HF-1 cells exposed to G-CSF or HL-60 cells exposed to PMA. Extracts from HF-1 cells bound both probes and were super-shifted by C/EBPα, C/EBPβ, or C/EBPε antisera, but not by c-Jun, JunB, or c-Fos antisera (Fig. 5A). C/EBPα and C/EBPβ antisera shifted different complexes (labeled as α or β), likely representing C/EBPα and C/EBPβ homodimers, respectively. The HL-60 nuclear extract again bound both probes, with the αα complexes super-shifted by C/EBPβ antiserum and the αJ complexes super-shifted by C/EBPβ, JunB, or c-Fos antisera (Fig. 5B, left and center panels). These findings suggest that the αJ site is bound by C/EBPβ:JunB and C/EBPβ:c-Fos heterodimers in HL-60 cell extracts and that the αα and αJ probes are predominantly bound by C/EBP dimers in HF-1 cell extracts. The JJ probe was bound only by JunB and c-Fos using HL-60 nuclear extracts (Fig. 5B, right panel) and was not bound by proteins in HF-1 extracts (not shown).
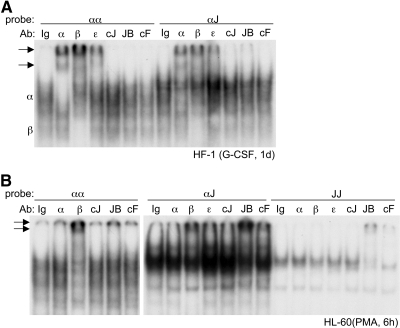
Figure 5. Gel-shift analysis of proteins in myeloid extracts using C/EBP, AP-1, or hybrid sites.
(A) Nuclear extracts (12 μg) from HF-1 cells cultured in G-CSF for 1 day were subject to gel-shift/super-shift assay using the αα- or αJ-radiolabeled probes. Antisera used were normal rabbit Ig (Ig), C/EBPα (α), C/EBPβ (β), C/EBPε (ε), c-Jun (cJ), JunB (JB), or c-Fos (cF). Arrows indicate super-shift complexes. Positions of C/EBPα:C/EBPα and C/EBPβ:C/EBPβ gel-shift complexes are also indicated (α and β). (B) Nuclear extracts (12 μg) from HL-60 cells cultured in PMA for 6 h were subjected similarly to gel-shift/super-shift assay using the αα-, αJ-, and JJ-radiolabeled probes.
As a second, more quantitative approach to detect endogenous complexes, we used αα, αJ, and JJ oligonucleotides linked to biotin. After incubation with nuclear extracts, these probes were “pulled down” with streptavidin beads, and Western blotting identified bound proteins. Input samples represent 10% of the nuclear extracts prior to probe addition. Results similar to findings shown were obtained also in a second experiment using independent extracts. In HF-1 granulocytic cells, C/EBPα protein levels were increased by addition of G-CSF (input lanes), and C/EBPα interaction with the αα probe increased (Fig. 6A); similar findings were obtained with 32Dcl3 cells (not shown). In HL-60 monocytic cells, addition of PMA induced C/EBPα protein expression, but no interaction with the αα probe was seen (Fig. 6B), and similar findings were obtained with monocytic M1 cells (not shown). Preferential interaction of C/EBPα homodimers with the αα probe during granulopoiesis but not monopoiesis may result from increased expression of AP-1 proteins in the latter context. In addition to interaction with the αα probe, C/EBPα bound the αJ probe using HF-1 but not HL-60 extracts, consistent with the gel-shift/super-shift findings. The longer LAP and shorter LIP isoforms of C/EBPβ increased during HF-1 differentiation, with binding of LIP but not LAP to the αα and αJ probes evident (Fig. 6A); unexpected, exclusive DNA binding by LIP was also seen using 32Dcl3 and M1 extracts (not shown). In HL-60 cells, which lacked LIP expression, binding of C/EBPβ to the αα probe greatly diminished after addition of PMA, perhaps again reflecting C/EBPβ:JunB and C/EBPβ:c-Fos hetereodimer formation upon induction of AP-1 proteins.
Figure 6. Oligonucleotide pull-down analysis of proteins in myeloid extracts using C/EBP, AP-1, or hybrid sites.
(A) Nuclear extracts prepared from HF-1 cells cultured with G-CSF (G) for 24 h or from noninduced HF-1 cells (NI) were incubated with biotinylated αα, αJ, or JJ oligonucleotides, followed by streptavidin beads, washed, and subjected to Western blotting for the indicated proteins. (B) Similar analysis was conducted using nuclear extracts from HL-60 cells cultured with PMA for 6 h or from noninduced HL-60 cells. Input samples represent 10% of the extract used for oligonucleotide pull-down.
In HF-1 cells, G-CSF induced c-Jun, JunB, and c-Fos, and yet only c-Jun and JunB bound the αJ probe, presumably in combination with C/EBPα, LIP, and possibly other C/EBP family members. In fact, c-Jun and JunB bound the αJ probe more readily than the JJ probe, whereas c-Fos exclusively bound the JJ probe, presumably with JunB and possibly other AP-1 proteins. Binding of c-Jun and JunB, expressed in HF-1 cells to the αJ probe in the pull-down but not the gel-shift/super-shift assay, may reflect the increased sensitivity of the pull-down assay. In HL-60 cells, PMA induced JunB and c-Fos, and very strong interaction of these proteins with the αJ and JJ probes is evident, consistent with the gel-shift assay. These findings support the conclusion that C/EBP:AP-1 heterodimers form endogenously in myeloid cells. In addition, they suggest that C/EBP homodimers are more evident during granulopoiesis, AP-1 complexes are more abundant during monopoiesis, and C/EBP:AP-1 heterodimers are also more evident during monopoiesis, although still detectable during granulopoiesis.
The FosB promoter is bound and activated by C/EBP:AP-1 complexes
We previously identified a conserved cis element at –68 in PU.1 promoter TAGCGCAA, which differs from the TGACGCAA consensus for C/EBP:AP-1 binding and binds C/EBPα and artificially generated C/EBPα:AP-1 complexes, albeit weakly [7, 15]. Using WT proteins, we find that C/EBPα:c-Jun interacts more strongly with this site than does C/EBPα alone (Supplemental Fig. 2). To identify additional genes regulated by C/EBP:AP-1 heterodimers, we analyzed the human and murine genomes for the presence of TGACGCAA from –2 kb to +1 kb annotated transcription start sites, identifying ∼350 human and 300 murine genes, which fit this criteria (Supplemental Tables 1 and 2). Pathway analysis indicates that C/EBP:AP-1 heterodimers potentially play a role of several biologic processes, including cell growth, cell transport, and gene expression (Supplemental Table 3). Among the human and murine promoter regions harboring a TGACGCAA site, 41 had such a consensus site in both species (Table 1). Among these were several Ras family members, protein phosphatases, and transport proteins. In addition, three proteins relevant to myeloid development, Csf2ra, Egr1, and Fosb, contain the hybrid motif in both species. We chose to focus on the murine Fosb promoter, which contains two perfect TGACGCAA sites at –56 and –253, with the human promoter having an identical site at –253 and a C for T change at –56 to yield TGACGTAA.
Table 1. Human and Murine Genes with TGACGCAA between –2 kb and +1 kb.
| Gene name | Descriptive name |
|---|---|
| BUD31 | BUD31 homolog |
| CALCA | calcitonin-related polypeptide α |
| CASC5 | cancer susceptibility candidate 5 |
| CCND2 | cyclin D2 |
| CHAC1 | ChaC, cation transport regulator homolog 1 |
| CHAF1A | chromatin assembly factor 1, subunit A (p150) |
| CHD2 | chromodomain helicase DNA-binding protein 2 |
| CLMN | calmin (calponin-like, transmembrane) |
| COQ5 | coenzyme Q5 homolog, methyltransferase |
| CSF2RA | colony-stimulating factor 2 receptor, α (GM-CSFRα) |
| EGR1 | early growth response-1 transcription factor |
| FOSB | FosB transcription factor |
| GRIPAP1 | GRIP1-associated protein 1 |
| HNRPH1 | heteronuclear riboprotein H1 |
| HOXB13 | homeobox B13 |
| KLHL15 | kelch-like 15 |
| LYCAT | lysocardiolipin acetyltransferase 1 |
| MIRN592 | micro-RNA 592 |
| OAZ1 | ornithine decarboxylase antizyme 1 |
| PELP1 | proline, glutamate, and leucine-rich protein 1 |
| PHF8 | PHD finger protein 8 |
| PPM1M | protein phosphatase, Mg2+/Mn2+-dependent, 1M |
| PRDM8 | PR domain-containing 8 |
| PTP4A1 | protein tyrosine phosphatase type IVA, member 1 |
| PTPRN | protein tyrosine phosphatase, receptor type, N |
| RAB7 | RAB7, member RAS oncogene family |
| RAN | RAN, member RAS oncogene family |
| RHOQ | ras homolog gene family, member Q |
| RPS6KB2 | ribosomal protein S6 kinase, 70 kDa, polypeptide 2 |
| SH3PX3 | SH3 and PX domain-containing 3 |
| SLC6A4 | solute carrier family 6 (serotonin transporter) 4 |
| SLC6A5 | solute carrier family 6 (glycine transporter) 5 |
| SMARCAD1 | SWI/SNF-related, matrix-associated actin-dependent regulator of chromatin, subfamily a, containing DEAD/H box 1 |
| SYNPO2 | synaptopodin 2 |
| SYT4 | synaptotagmin IV |
| TAC1 | tachykinin, precursor 1 |
| THRAP3 | thyroid hormone receptor-associated protein 3 |
| TSPAN31 | tetraspanin 31 |
| TWISTNB | TWIST neighbor |
| USP3 | ubiquitin-specific peptidase 3 |
| ZUBR1 | ubiquitin protein ligase 3 component |
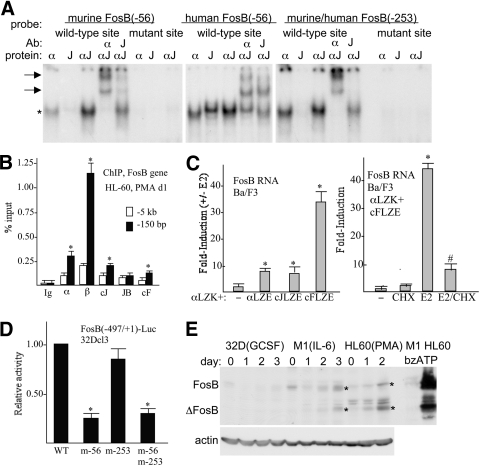
The murine –56 site bound C/EBPα weakly, did not bind c-Jun, but strongly bound the C/EBPα:c-Jun heterodimer, which was super-shifted by C/EBPα and c-Jun antisera (Fig. 7A, left panel). Mutation of this site to TGACGCGT prevented binding. The human –56-bp site and the murine/human –253-bp site also showed preferential binding by the C/EBP:c-Jun complex, although not as strikingly as the –56-bp murine site (Fig. 7A, center and right panels). C/EBPα, C/EBPβ, c-Jun, and c-Fos expressed in HL-60 cells exposed to PMA bound the endogenous FosB promoter, as detected by ChIP assay (Fig. 7B). We described previously Ba/F3 lines expressing C/EBPαLZK-ER harboring a basic LZ, together with C/EBPαLZE-ER, c-JunLZE-ER, or c-FosLZE-ER harboring complementary acid LZs. Exposure of these cell lines to E2, to activate these ER fusion proteins, led to mild Fosb mRNA induction by the C/EBPα:C/EBPα or C/EBPα:cJun complexes and more potent induction by C/EBPα:c-Fos (Fig. 7C, left panel). Induction of Fosb by C/EBPα:c-Fos occurred even in the presence of CHX, consistent with direct gene activation (Fig. 7C, right panel). Segment –497 to +1 of the Fosb promoter was linked to a luciferase reporter, and point mutations were introduced into the –56- or –253-binding sites or into both sites. Mutation of the –56 site reduced promoter activity approximately fivefold in the more-readily transfectable 32Dcl3 myeloid cell line, whereas mutation of the –253-bp site alone or in combination with the –56-bp mutation did not reduce activity (Fig. 7D). Western blot analysis of endogenous FosB expression detected basal expression in 32Dcl3, M1, or HL-60 cells and induction during monocytic differentiation of the latter two lines. Expression of the alternatively spliced form ΔFosB was also prominent in differentiating M1 or HL-60 cells (Fig. 7E). Induction of FosB and ΔFosB by bzATP, a ligand for the P2RX7 extracellular nucleotide receptor, had been noted previously in a macrophage line [16], and we find similar, striking induction in HL-60 but not M1 myeloid cells (Fig. 7E).
Figure 7. Regulation of the FosB promoter by C/EBP:AP-1 heterodimers.
(A) Gel-shift assay was conducted with the indicated radiolabeled probes with IVT, myc-tagged proteins C/EBPα (α) and eightfold c-Jun (J) on a per-mol basis, or their combination at a 1:8 mol ratio (αJ). Where indicated, super-shift was conducted by further addition of C/EBPα or c-Jun antisera. Arrows indicate super-shift complexes. (B) HL-60 cells cultured in PMA for 1 day were subjected to ChIP assay using rabbit IgG or C/EBPα, C/EBPβ, c-Jun JunB, and c-Fos antiserum. Shown is the signal obtained, expressed as percent input using primers centered on the FosB –150-bp promoter region or on a more distal –5-kb control region (mean and se). *P < 0.05 versus Ig control. (C) FosB RNA levels were evaluated in the Ba/F3 cell lines expressing C/EBPαLZK-ER (αLZK) together with empty vector (–), C/EBPαLZE-ER (αLZE), c-JunLZE-ER (cJLZE), or c-FosLZE-ER (cFLZE) 24 h after culture in the presence or absence of E2, with triplicate data expressed as an induction ratio (+E2/–E2; left, mean and se). *P < 0.05 versus empty vector cells. Induction of FosB RNA was then assessed similarly at 8 h using cells expressing C/EBPαLZK-ER and c-FosLZE-ER with no addition (–), CHX, E2, or both (E2/CHX). CHX was added 30 min prior to E2 (right, mean and se). *P < 0.05 for E2 versus no addition; #P < 0.05 for E2 versus E2/CHX. (D) FosB(–497/+1)-Luc (5 μg) or the indicated mutant variants were transiently transfected together with CMV-β-galactosidase (0.5 μg) into 5 × 106 32Dcl3 cells in IL-3, and luciferase and β-galactosidase assays were conducted 48 h later. FosB promoter activity relative to WT (set to 1.00) is shown (mean and se from three experiments). *P < 0.05 versus WT. (E) Total cellular proteins from 32Dcl3 cells cultured in G-CSF for 0–3 days, M1 cells cultured in IL-6 for 0–3 days, HL-60 cells cultured in PMA for 0–2 days, or M1 or HL-60 cells exposed to bzATP for 5 min were subjected to Western blotting for FosB and β-actin. *Locations of FosB and ΔFosB induced in the monocytic lines.
DISCUSSION
Herein, we demonstrate that C/EBPα heterodimerizes with c-Jun or c-Fos to bind a hybrid DNA element consisting of C/EBP and AP-1 half-sites. Preferential binding to such hybrid sites occurs even at a 2:1 ratio of c-Jun:C/EBPα or c-Fos:C/EBPα, and semiquantitative Western blot analysis indicates that endogenous C/EBP and AP-1 proteins exist at similar levels in myeloid cells, indicating that formation of C/EBPα:AP-1 heterodimers is feasible in vivo. Gel-shift and oligonucleotide pull-down assays using myeloid nuclear extracts demonstrate C/EBP:AP-1 complexes in differentiating granulocytic and monocytic cell lines and uncover differences in the pattern of bZIP complex expression, which may contribute to lineage commitment and maturation events, with preferential expression of C/EBPα homodimers during granulopoiesis and increased C/EBP:AP-1 and AP-1 heterodimers during monopoiesis. Beyond myeloid development, the ability of C/EBP and AP-1 proteins to heterodimerize and bind novel DNA elements offers the opportunity for cross-talk between these transcription factor families and between upstream signals that regulate their activity to generate unique patterns of gene expression during diverse biologic processes, including malignant transformation associated with elevated AP-1 [17].
Our earlier work inserting various LZ segments downstream of the BR in C/EBPα provided evidence that the C/EBPα LZ could form a coiled-coil heterodimer with the c-Jun, JunB, or c-Fos LZs [7]. In the same study, we also forced heterodimerization between C/EBPα and c-Jun by inserting LZs containing acidic or basic residues at the salt bridge positions and demonstrated that such heterodimers preferentially bound hybrid αJ cis DNA elements. We have now extended these findings by demonstrating that intact C/EBPα zippers with c-Jun or c-Fos also preferentially bind hybrid αJ sites. This is important, as the artificial hybrid proteins might have had subtle conformational changes affecting heterodimerization and DNA-binding specificity and as intact proteins are most relevant to cellular processes.
By assessing DNA-binding using different c-Jun:C/EBPα combinations, we found that even a 2:1 ratio resulted in preferentially binding to the hybrid αJ site compared with the αα homodimeric C/EBP-binding site, suggesting that C/EBPα:c-Jun binding to the αJ site might also occur at lower c-Jun:C/EBP ratios. Preferential binding to the αJ oligonucleotide was also seen with a 2:1 ratio of c-Fos:C/EBPα. Having quantified the affinity of c-Jun or c-Fos for C/EBPα using the gel-shift assay, we then sought to estimate the relative amounts of C/EBP and AP-1 proteins present in mammalian cells. In 32Dcl3 granulocytic cells or in M1 monocytic cells, the levels of C/EBPα + C/EBPβ were essentially equal to the levels of c-Jun + JunB + c-Fos; in HF-1 granulocytic cells, the C/EBP levels exceeded the AP-1 levels by 1.5-fold; and in HL-60 cells induced toward monopoiesis, AP-1 protein levels exceeded C/EBP levels by 2.5-fold. Although other C/EBP and AP-1 family members may also be present, as may other proteins capable of zippering with those proteins assayed, these findings suggest that AP-1 proteins are generally expressed at high enough levels in absolute terms relative to C/EBP proteins to allow formation of C/EBP:AP-1 heterodimers.
Having shown that C/EBPα and AP-1 proteins are expressed at sufficient levels to form heterodimeric DNA-binding complexes, we used gel-shift/super-shift and a more quantitative oligonucleotide pull-down assay to provide evidence supporting the presence of such endogenous complexes in differentiating myeloid cell lines. The αJ probe consistently bound more C/EBPα or C/EBPβ than did the αα probe in the pull-down assay, although IVT C/EBPα bound the αα probe with higher affinity than the αJ probe. If all of the C/EBP binding to the αJ probe was as a homodimer in the pull-down assay, then we might have expected even stronger C/EBP interaction with the αα probe. However, this was not the case, supporting the conclusion that binding of endogenous C/EBPα to the αJ probe occurs, at least in part, as a C/EBP:AP-1 heterodimer. Increased expression of AP-1 proteins during monopoiesis appeared to favor formation of C/EBP:AP-1 and Fos:Jun complexes, with C/EBP homodimers more evident during granulopoiesis. These biochemical findings may have functional relevance, as forced heterodimerization of C/EBPα with c-Jun or c-Fos directs murine marrow cells to form increased numbers of monocytic progenitors, whereas C/EBPα homodimers or Fos:Jun heterodimers were ineffective [7]. Future studies will focus on extending these biochemical analyses to normal myeloid progenitors, which exist in limited cell numbers.
In three of the myeloid extracts, the LAP and LIP isoforms of C/EBPβ were present, but surprisingly, only the LIP isoform bound the αJ probe, and in HL-60 cells, only the LAP isoform was present and in this case, did bind the αJ probe. These findings suggest that LIP has an increased propensity to heterodimerize with AP-1 proteins and bind DNA than does LAP. The recent finding that DNA binding by C/EBPβ is auto-inhibited by several N-terminal domains may be relevant to these observations [18].
Our genomic analysis demonstrates that more than 300 human or murine genes contain a TGACGCAA site for C/EBP:AP-1 interaction within their –2 kb to +1 kb promoter region, with 41 genes of both species harboring a perfect match to this cis element. We chose to seek data validating the relevance of two consensus sites in the Fosb promoter. We indeed found that C/EBPα:AP-1 proteins bind and activate Fosb transcription, dependent on integrity of the –56-bp promoter site, and that FosB levels increased during monocytic differentiation. C/EBP:AP-1 heterodimers may also activate gene expression through sites related to TGACGCAA, and in fact, TGATGCAA and TGTTGCAA occur frequently in the vicinity of C/EBPβ-binding sites in peritoneal macrophages and were noted by the authors as potential C/EBP:AP-1-binding sites [8]. Gel-shift analysis with these sequences replacing TGACGCAA in the αJ probe demonstrates synergy between C/EBPα and c-Jun for binding to TGATGCAA but not TGTTGCAA (Supplemental Fig. 3), although the C/EBP:c-Jun complex bound TGACGCAA with higher affinity than it did TGATGCAA. Of note, TGATGCAA differs from the consensus TGACGCAA hybrid element by only a conservative T for C pyrimidine change at the fourth position. Analysis of the murine genome identifies TGATGCAA between –2 kb and + 1 kb of ∼800 genes (not shown).
In summary, the bZIP family of transcription factors contains three subfamilies: the C/EBP, AP-1, and CREB/ATF factors. Previous work demonstrated a functional role for AP-1:ATF or C/EBP:ATF complexes [19, 20], and we now find that C/EBP:AP-1 heterodimers also exist in mammalian cells and activate Fosb and likely additional, unique target genes of potential relevance to multiple biologic processes.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants R01 HL089176, U01 HL099775, and U01 HL100397 from the National Institutes of Health (A.D.F.).
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- BR
- basic region
- bzATP
- (2′,3′)-O-(4-benzoylbenzoyl)-ATP
- bZIP
- basic region-leucine zipper
- ChIP
- chromatin immunoprecipitation
- CHX
- cycloheximide
- ER
- estradiol receptor
- F
- forward
- FosB
- Finkel-Biskis-Jinkins murine osteosarcoma viral oncogene homolog B
- hFosB
- human Finkel-Biskis-Jinkins murine osteosarcoma viral oncogene homolog B
- HI-FBS
- heat-inactivated FBS
- IVT
- in vitro translation
- LAP
- liver-enriched activator protein
- LIP
- liver-enriched inhibitory protein
- LZ
- leucine zipper
- poly-dI:dC
- polydeoxyinosinic:deoxycytidylic acid
- R
- reverse
AUTHORSHIP
S.H. designed and performed research, analyzed data, and wrote the paper. A.M.S. and S.J.W. designed and performed genomics and pathway analyses and wrote the paper. A.D.F. designed research, analyzed data, and wrote the paper.
REFERENCES
- 1. Landschulz W. H., Johnson P. F., McKnight S. L. (1989) The DNA binding domain of the rat liver nuclear protein C/EBP is bipartite. Science 243, 1681–1688 [DOI] [PubMed] [Google Scholar]
- 2. Miller M., Shuman J. D., Sebastian T., Dauter Z., Johnson P. F. (2003) Structural basis for DNA recognition by the basic region leucine zipper transcription factor C/EBPα. J. Biol. Chem. 278, 15178–15184 [DOI] [PubMed] [Google Scholar]
- 3. Mechta-Grigoriou F., Gerald D., Yaniv M. (2001) The mammalian Jun proteins: redundancy and specificity. Oncogene 20, 2378–2389 [DOI] [PubMed] [Google Scholar]
- 4. Hsu W., Kerppola T. K., Chen P. L., Curran T., Chen-Kiang S. (1994) Fos and Jun repress transcription activation by NF-IL6 through association at the basic zipper region. Mol. Cell. Biol. 14, 268–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rangatia J., Vangala R. K., Treiber N., Zhang P., Radomska H., Tenen D. G., Hiddemann W., Behre G. (2002) Downregulation of c-Jun expression by transcription factor C/EBPα is critical for granulocytic lineage commitment. Mol. Cell. Biol. 22, 8681–8694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rangatia J., Vangala R. K., Singh S. M., Peer Zada A. A., Elsasser A., Kohlmann A., Haferlach T., Tenen D. G., Hiddemann W., Behre G. (2003) Elevated c-Jun expression in acute myeloid leukemias inhibits C/EBPα DNA binding via leucine zipper domain interaction. Oncogene 22, 4760–4764 [DOI] [PubMed] [Google Scholar]
- 7. Cai D. H., Wang D., Keefer J., Yeamans C., Hensley K., Friedman A. D. (2008) C/EBPα:AP-1 leucine zipper heterodimers bind novel DNA element, activate the PU.1 promoter, and direct monocyte lineage commitment more potently than C/EBPα homodimers or AP-1. Oncogene 27, 2772–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heinz S., Benner C., Spann N., Bertolino E., Lin Y. C., Laslo P., Cheng J. X., Murre C., Singh H., Glass C. K. (2010) Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valtieri M., Tweardy D. J., Caracciolo D., Johnson K., Mavilio F., Altmann S., Santoli D., Rovera G. (1987) Cytokine-dependent granulocytic differentiation. Regulation of proliferative and differentiative responses in a murine progenitor cell line. J. Immunol. 138, 3829–3835 [PubMed] [Google Scholar]
- 10. Calvo K. R., Sykes D. B., Pasillas M., Kamps M. P. (2000) Hoxa9 immortalizes a granulocyte-macrophage colony-stimulating factor-dependent promyelocyte capable of biphenotypic differentiation to neutrophils and macrophages, independent of enforced meis expression. Mol. Cell. Biol. 20, 3274–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suzow J., Friedman A. D. (1993) The murine myeloperoxidase promoter contains multiple functional elements, one of which binds a cell type-restricted transcription factor, myeloid nuclear factor 1(MyNF1). Mol. Cell. Biol. 13, 2141–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oelgeschläger M., Nuchprayoon I., Lüscher B., Friedman A. D. (1996) C/EBP, c-Myb, and PU.1 cooperate to regulate the neutrophil elastase promoter. Mol. Cell. Biol. 16, 4717–4725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paz-Priel I., Ghosal A. K., Kowalski J., Friedman A. D. (2009) C/EBPα or C/EBPα oncoproteins regulate the intrinsic and extrinsic apoptotic pathways by direct interaction with NF-κB p50 bound to the bcl-2 and FLIP gene promoters. Leukemia 23, 365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hochberg Y., Benjamini Y. (1990) More powerful procedures for multiple significance testing. Stat. Med. 9, 811–818 [DOI] [PubMed] [Google Scholar]
- 15. Kummalue T., Friedman A. D. (2003) Cross-talk between regulators of myeloid development: C/EBPα binds and activates the promoter of the PU.1 gene. J. Leukoc. Biol. 74, 464–470 [DOI] [PubMed] [Google Scholar]
- 16. Gavala M. L., Hill L. M., Lenertz L. Y., Karta M. R., Bertics P. J. (2010) Activation of the transcription factor FosB/activating protein-1 (AP-1) is a prominent downstream signal of the extracellular nucleotide receptor P2RX7 in monocytic and osteoblastic cell. J. Biol. Chem. 285, 34288–34298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shaulian E. (2010) AP-1—the Jun proteins: oncogenes or tumor suppressors in disguise? Cell. Signal. 22, 894–899 [DOI] [PubMed] [Google Scholar]
- 18. Lee S., Shuman J. D., Johnson P. F. (2010) CCAAT/enhancer binding protein β DNA binding is auto-inhibited by multiple elements that also mediate association with p300/CBF. J. Biol. Chem. 285, 21399–21410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hai T., Curran T. (1991) Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc. Natl. Acad. Sci. USA 88, 3720–3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shuman J. D., Cheong J., Coligan J. E. (1997) ATF-2 and C/EBPα can form a heterodimeric DNA binding complex in vitro. Functional implications for transcriptional regulation. J. Biol. Chem. 272, 12793–12800 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.