Abstract
OBJECTIVE
While metabolic syndrome (MetS) and diabetes confer greater cardiovascular disease (CVD) risk, recent evidence suggests that individuals with these conditions have a wide range of risk. We evaluated whether screening for coronary artery calcium (CAC) and carotid intimal-medial thickness (CIMT) can improve CVD risk stratification over traditional risk factors (RFs) in people with MetS and diabetes.
RESEARCH DESIGN AND METHODS
We assessed CAC and CIMT in 6,603 people aged 45–84 years in the Multi-Ethnic Study of Atherosclerosis (MESA). Cox regression examined the association of CAC and CIMT with coronary heart disease (CHD) and CVD over 6.4 years in MetS and diabetes.
RESULTS
Of the subjects, 1,686 (25%) had MetS but no diabetes and 881 (13%) had diabetes. Annual CHD event rates were 1.0% among MetS and 1.5% for diabetes. Ethnicity and RF-adjusted hazard ratios for CHD for CAC 1–99 to ≥400 vs. 0 in subjects with neither MetS nor diabetes ranged from 2.6 to 9.5; in those with MetS, they ranged from 3.9 to 11.9; and in those with diabetes, they ranged from 2.9 to 6.2 (all P < 0.05 to P < 0.001). Findings were similar for CVD. CAC increased the C-statistic for events (P < 0.001) over RFs and CIMT in each group while CIMT added negligibly to prediction over RFs.
CONCLUSIONS
Individuals with MetS or diabetes have low risks for CHD when CAC or CIMT is not increased. Prediction of CHD and CVD events is improved by CAC more than by CIMT. Screening for CAC or CIMT can stratify risk in people with MetS and diabetes and support the latest recommendations regarding CAC screening in those with diabetes.
Individuals with diabetes and/or metabolic syndrome (MetS) are more likely to have coronary heart disease (CHD) (1,2) and a poorer prognosis compared with those without these conditions (3,4). Those with diabetes without prior myocardial infarction (MI) were originally reported to have the same risk of subsequent MI as those without diabetes but before MI (5), suggesting that diabetes is a CHD risk equivalent. However, recently a large meta-analysis showed that those with diabetes without prior MI had a 43% lower risk of developing CHD events compared with those without diabetes but with a previous MI (6). Both coronary artery calcium (CAC) and carotid intimal-medial thicknesses (CIMTs) are increased in those with MetS and diabetes (7–9). Although the incremental value of CAC and CIMT over traditional risk factors (RFs) has been shown in the general population (10,11), thus reclassifying more individuals in a higher risk category (12), the utility of CAC and CIMT in those with diabetes and/or MetS is unclear (13,14), and screening has not been traditionally recommended in those with diabetes, given their status as having a CHD risk equivalent. We examined in the Multi-Ethnic Study of Atherosclerosis (MESA), a prospective population-based study of cardiovascular disease (CVD), whether CAC and CIMT add predictive value for CVD events in MetS and diabetes and if there is a role for these tests in risk stratification of these populations. Our hypothesis was that CAC and CIMT levels would show a wide range in risks in individuals with MetS and diabetes, as in those without these conditions, and that many people with diabetes would not be at customarily assumed CHD risk equivalents.
RESEARCH DESIGN AND METHODS
Study population and definitions
The design of MESA has been previously published (15). A total of 6,814 participants aged 45–84 years, free of clinical CVD and identified as white, African American, Hispanic, or Chinese were recruited from six U.S. communities (Forsyth County, NC; Northern Manhattan and the Bronx, NY; Baltimore City and Baltimore County, MD; St. Paul, MN; Chicago, IL; and Los Angeles County, CA) in 2000–2002. Recruitment included lists of residents, dwellings, telephone exchanges, lists of Medicare beneficiaries, and referrals by participants. Approximately equal numbers of men and women were recruited according to prespecified quotas. All participants gave informed consent.
We included 6,603 (97%) individuals who had information on RFs, CAC, and CIMT. Diabetes was based on a fasting glucose ≥7.0 mmol/L (126 mg/dL) or whether the individual was taking insulin or oral hypoglycemic medications, and MetS was based on having ≥3 of the following characteristics: 1) waist circumference >88 cm (35 inches) for women and >102 cm (40 inches) for men; 2) HDL cholesterol (HDL-C) <1.0 mmol/L (40 mg/dL) for men or <1.3 mmol/L (50 mg/dL) for women; 3) fasting triglycerides ≥1.7 mmol/L (150 mg/dL); 4) blood pressure of ≥130 mmHg systolic or ≥85 mmHg diastolic, or on treatment; or 5) fasting glucose of 5.55–6.99 mmol/L (100–125 mg/dL) (16). We also examined a group (which overlapped with those with MetS) of those with prediabetes (fasting glucose of 100–125 mg/dL and not on diabetes medications).
Examination data and covariates
Information on demographics, smoking, medical conditions, and family history was obtained by questionnaire. Height, weight, total cholesterol and HDL-C, triglycerides, and fasting glucose levels were determined. Resting blood pressure was measured three times, with the last two measurements averaged. Use of cholesterol, blood pressure, and diabetes medications was determined by questionnaire and from medication containers brought in by the participants.
Measurement of CAC and CIMT
CAC was measured using either electron beam (three sites) or multidetector (three sites) computed tomography (CT). Participants were scanned twice consecutively, and scans were read by a single trained physician-reader at a centralized reading center; scan acquisition and interpretation methods were published previously (17). Calcium volume scores and Agatston scores were based on the average of results from each scan and adjusted using a standard calcium phantom to calibrate the X-ray attenuation level between machines. Any detectable calcium was defined as a CAC score >0. We also log-transformed the CAC score, ln CAC = ln (CAC + 1), and created a standardized CAC variable, zCAC, which was created by subtraction of the mean and division by the SD of each measurement. The resulting variable zCAC has a mean of 0 and an SD of 1.
The intimal medial thickness (IMT) of the internal carotid artery and the common carotid artery was assessed using the B-mode ultrasound (Logiq 700 ultrasound device; General Electric Medical Systems, Waukesha, WI). The maximal IMT of the internal and common carotid sites was measured as the mean of the maximal IMT of the near and far walls of the right and left sides. We used a z score for overall maximal IMT by summing the values of the two carotid IMT sides after standardization (subtraction of the mean and division by the SD of each measure) and then dividing by the SD of the sum. The resulting variable, called zIMT, has a mean of 0 and an SD of 1.
Follow-up
The cohort was followed for incident CHD and CVD events for a median of 6.4 years (maximum 7.8 years). At intervals of 9–12 months, a telephone interviewer inquired about interim hospital admissions, cardiovascular diagnoses, and deaths. An adjudication committee received copies of all death certificates and medical records for hospitalizations and outpatient cardiovascular diagnoses and conducted next-of-kin interviews for out-of-hospital cardiovascular deaths for verification. Records were obtained on 98% of reported hospitalized CVD events. Two physicians independently classified and assigned incidence dates. For disagreements, a full mortality and morbidity review committee made the final classification.
We followed participants for occurrence of all CHD end points, which included MI, angina, resuscitated cardiac arrest, or CHD death. Angina required clear documentation of chest pain or anginal equivalent and evidence of reversible myocardial ischemia or obstructive coronary artery disease or a positive stress test. All CVD events included stroke, stroke death, and other CVD death in addition to CHD events listed above. CHD death and CVD death were based on review of hospital records and interviews with families. Definite fatal CHD required an MI within 28 days of death, resuscitated cardiac arrest, chest pain within the 72 h before death, or a history of CHD and the absence of a known nonatherosclerotic or noncardiac cause of death. Neurologists reviewed and classified stroke as present if there was a focal neurologic deficit lasting 24 h or until death, with a clinically relevant lesion on brain imaging and no nonvascular cause. Two physicians from the MESA study events committee independently reviewed all medical records for end point classification and assignment of incidence dates. The reviewers were blinded to CT scan and magnetic resonance imaging results and used prespecified criteria.
Statistical analysis
CVD and CHD event rates were annualized in percent in subjects with MetS (without diabetes), with diabetes, and with neither condition. Kaplan-Meier plots showed cumulative incidence of CVD and CHD events. Clinically used cut points were chosen for CAC scores: CAC = 0, 1–99, 100–399, and 400+. For CIMT, we analyzed the data using z score maximal IMT (as defined above) in quartiles. We also analyzed CAC and CIMT scores as standardized continuous variables using zCAC and zCIMT as defined above.
Multivariable Cox regressions were run separately for those with diabetes, MetS (without diabetes), and those with neither diabetes nor MetS but included both zCIMT and zCAC as continuous variables in each model. For ease of clinical interpretation, we also ran models using categories of CAC scores and quartiles of CIMT as described above. We computed receiver-operating characteristic curves in models containing RFs only and then with the addition of CIMT or CAC to assess added incremental predictive value. We adjusted for age, sex, ethnicity, and traditional RFs used in the Framingham risk score (systolic blood pressure, smoking, total cholesterol, HDL-C, and antihypertensive medication use) for prediction of CHD (18) and CVD events (19). Secondary analyses examined the impact of dyslipidemia or microalbuminuria on the relation of CAC and CIMT to events, and separate analyses of CHD and CVD events were also conducted in a prediabetes subset. All analyses were conducted with Stata software, version 11.0 (StataCorp, College Station, TX).
RESULTS
Of 6,814 individuals initially enrolled, we included 6,603 (97% of the sample after exclusion of people who were missing IMT or CAC measures, laboratory, or follow-up data). Table 1 shows baseline characteristics among participants with MetS (without diabetes), diabetes, or neither of these conditions. In our ethnically diverse sample, 38% of those with diabetes were African American. In the current study, 1,686 (26%) were defined to have MetS without diabetes, and an additional 881 (13%) were defined to have diabetes (Table 1). A total of 299 CHD events and 410 CVD events occurred during a median follow-up of 6.4 years (range 0 to 7.8 years). Events included 134 MIs, 161 angina episodes, 17 resuscitated cardiac arrests, 111 stroke events, 12 stroke deaths, 42 CHD deaths, 1 other atherosclerotic death, and 16 other CVD deaths. The prevalence of dyslipidemia ranged from 33 to 61% and microalbuminuria from 4.5 to 21.0% according to disease groups.
Table 1.
Baseline characteristics of study sample across disease groups
| Patient characteristic | No MetS or diabetes (n = 4,036) | MetS without diabetes (n = 1,686) | Diabetes (n = 881) | P |
|---|---|---|---|---|
| Age (years) | 62 (10) | 64 (10.0) | 65 (9.6) | <0.0001 |
| Male (%) | 49 | 41 | 52 | <0.0001 |
| Ethnicity (%) | ||||
| Caucasian | 42 | 40 | 19 | <0.0001 |
| African American | 26 | 25 | 38 | <0.0001 |
| Hispanic | 19 | 26 | 31 | <0.0001 |
| Chinese | 13 | 9 | 12 | <0.0001 |
| Ten-year predicted Framingham CHD risk (%) (18) | 8.5 (6.9) | 12.3 (8.7) | 17.4 (11.5) | <0.0001 |
| Ten-year predicted Framingham CVD risk (%) (19) | 11.6 (8.7) | 16.8 (9.1) | 22.9 (8.4) | <0.0001 |
| Prediabetes (%)|| | 6 | 38 | — | <0.0001 |
| Total cholesterol (mmol/L [mg/dL]) | 5.0 [194] (34) | 5.1 [197] (37) | 4.9 [188] (39) | <0.0001 |
| LDL-C (mmol/L [mg/dL]) | 3.1 [118] (30) | 3.1 [118] (32) | 2.9 [111] (33) | <0.0001 |
| HDL-C (mmol/L [mg/dL]) | 1.4 [55] (15) | 1.1 [43] (10) | 1.2 [46] (13) | <0.0001 |
| Dyslipidemia (%)# | 33 | 34 | 61 | <0.0001 |
| History of hypertension (%)* | 32 | 64 | 66 | <0.0001 |
| Current smoker (%)† | 13 | 13 | 13 | 0.97 |
| Microalbuminuria (% 30–299 mg/L) | 4.5 | 9.8 | 21 | <0.0001 |
| Macroalbuminuria (% ≥300 mg/L) | 0.4 | 1.3 | 6.2 | <0.0001 |
| Lipid-lowering medication use (%)‡ | 12 | 19 | 27 | <0.0001 |
| Antihypertensive medication use (%)§ | 25 | 52 | 63 | <0.0001 |
| CAC | 119 (365) | 157 (417) | 255 (596) | <0.0001 |
| CAC score categories (%) | ||||
| CAC 0 | 55 | 45 | 38 | <0.0001 |
| CAC 1–99 | 25 | 28 | 27 | <0.0001 |
| CAC 100–399 | 12 | 16 | 17 | <0.0001 |
| CAC 400+ | 8 | 11 | 17 | <0.0001 |
| Common CIMT (mm) | 0.84 (0.2) | 0.90 (0.2) | 0.93 (0.2) | <0.0001 |
| Internal CIMT (mm) | 1.0 (0.5) | 1.1 (0.7) | 1.3 (0.7) | <0.0001 |
Data are means (SD) or percent. ||Defined as fasting blood glucose of 100–125 and not on glucose-lowering medications. #Defined as elevated LDL cholesterol (LDL-C )≥100 if diabetes or ≥130 otherwise or on lipid-lowering medication. *A history of hypertension and the use of blood pressure medications for hypertension were obtained from medical history. †Current smoking was defined as having smoked a cigarette in the last 30 days. ‡Included self-report and use of statin, fibrate, niacin, and bile acid resins. §A history of hypertension and the use of blood pressure medications for hypertension were obtained from medical history.
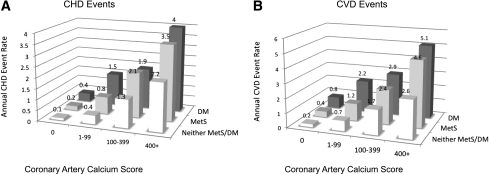
The overall unadjusted CHD rate for those with MetS was 9.9/1,000 person-years (1.0% projected annual rate); for those with diabetes, it was 15.2/1,000 (1.5% annually); and for those with neither MetS nor diabetes, it was 5.0/1,000 (0.5% annually). The highest unadjusted rates for CHD and CVD were in the highest CAC and CIMT categories for each disease group (Fig. 1). The lowest crude rate for CHD, 1.3 per 1,000 person-years (0.1% projected annual rate), was for those with neither condition and a CAC score of 0, but even those with MetS or diabetes with CAC scores of 0 had only slightly higher rates (0.2 and 0.4%, respectively) (Fig. 1). Those with MetS or who had CAC scores ≥100 had annual CHD rates of approximately 2% or higher, whereas lower CAC scores were associated with annual rates ≤1.5% for both of these groups. Those with MetS or diabetes who had CAC scores of ≥400 had the highest annual CHD rates of 3.5 and 4%, respectively. A similar relationship was observed for CVD events: those with MetS or diabetes who had CAC ≥100 had annual crude event rates exceeding 2%; however, those with lower CAC scores had lower annual rates.
Figure 1.
Annualized unadjusted CHD (A) and CVD (B) event rates in percent for individuals with neither MetS nor diabetes, MetS, or diabetes, stratified by CAC category. DM, diabetes.
For the relationship between CIMT quartiles and CHD and CVD event rates, differences were less pronounced than for CAC. Except for those with diabetes in the highest quartile of CIMT who had CHD event rates of 21.9/1,000 person-years (2.2% annual rate), CHD crude event rates were all below 20/1,000 person-years (or <2% annual rate). CVD event rates were generally higher; however, the highest rate was 28.4/1,000 person-years in those with diabetes in the highest quartile of CIMT.
In a Cox proportional hazards model, adjusting for age, sex, ethnicity, and traditional RFs from the Framingham risk score, zCAC and zCIMT, we found that CAC was predictive of CHD events (hazard ratio [HR] 2.0 [95% CI 1.5–2.6]) in those with diabetes, those with MetS (2.2 [1.7–2.9]), and those with neither diabetes nor MetS (2.3 [1.8–2.9]). Similar results were observed for prediction of CVD events (HRs 1.7–1.9, P < 0.0001). CIMT, however, was not associated with CHD or CVD events (HRs 1.0–1.1, P > 0.1) within any disease condition.
We also examined in fully adjusted models the relation of clinical CAC categories and CIMT quartiles to CHD and CVD events. Compared with a CAC score of 0, increasing CAC scores (1–99, 100–399, and ≥400) were associated with increases in CHD risk of 2.9- to 6.2-fold among those with diabetes, 3.9- to 11.9-fold among those with MetS, and 2.6- to 9.5-fold among those with neither condition (all P < 0.05 to P < 0.001) (Table 2). CIMT categories were not statistically significant predictors of CHD events for those with diabetes or MetS. However, for those with neither condition, the adjusted HR was 2.8 (95% CI 1.4–5.4) for those in the 4th quartile of CIMT compared with the 1st quartile (P < 0.01). We found similar results with CVD event risk for CAC scores in each disease group. There were, however, no statistically significant relationships between CVD events and CIMT in any of the three groups.
Table 2.
Cox proportional hazards regression examining relation of CHD and CVD events with CAC and CIMT categories among those with diabetes, MetS, or neither condition
| No MetS or diabetes (n = 4,036) | MetS without diabetes (n = 1,686) | Diabetes (n = 881) | |
|---|---|---|---|
| CHD events‡ | 123 (3.0) | 100 (5.9) | 76 (8.6) |
| CVD events§ | 168 (4.2) | 133 (7.9) | 109 (12.4) |
| CAC 1–99 vs. 0 | |||
| CHD | 2.6 (1.4–4.9)** | 3.9 (1.8–8.5)** | 2.9 (1.3–6.8)* |
| CVD | 2.3 (1.4–3.8)** | 2.5 (1.4–4.4)** | 2.0 (1.1–3.7)* |
| CAC 100–399 vs. 0 | |||
| CHD | 6.4 (3.5–12.0)*** | 8.4 (3.8–18.3)*** | 3.3 (1.4–7.8)** |
| CVD | 4.3 (2.5–7.0)*** | 4.1 (2.3–7.5)** | 2.3 (1.2–4.5)** |
| CAC 400+ vs. 0 | |||
| CHD | 9.5 (4.9–18.1)*** | 11.9 (5.2–27.0)*** | 6.2 (2.7–14.2)*** |
| CVD | 5.3 (3.1–9.1)*** | 6.7 (3.6–12.6)*** | 4.0 (2.1–7.5)*** |
| CIMT|| 2nd quartile vs. 1st quartile | |||
| CHD | 1.4 (0.7–2.8) | 0.8 (0.3–1.7) | 1.1 (0.4–3.0) |
| CVD | 1.0 (0.6–1.7) | 0.8 (0.4–1.6) | 0.6 (0.3–1.4) |
| CIMT 3rd quartile vs. 1st quartile | |||
| CHD | 1.8 (0.9–3.5) | 1.2 (0.6–2.5) | 1.0 (0.4–2.8) |
| CVD | 1.0 (0.6–1.7) | 0.9 (0.5–1.8) | 0.7 (0.3–1.4) |
| CIMT 4th quartile vs. 1st quartile | |||
| CHD | 2.8 (1.4–5.4)** | 1.6 (0.7–3.3) | 1.7 (0.7–4.3) |
| CVD | 1.3 (0.8–2.2) | 1.2 (0.6–2.2) | 1.0 (0.5–2.0) |
Data are n (%) or adjusted HRs (95% CI). HRs adjusted for age, sex, ethnicity, and traditional RFs used in the Framingham risk score (systolic blood pressure, smoking, total cholesterol, HDL-C, and antihypertensive medication use). ‡Included all CHD events, such as MI, angina, resuscitated cardiac arrest, or CHD death. §Defined as a CHD event, stroke, stroke death, other atherosclerotic death, or other CVD death. *P value < 0.05. **P < 0.01. ***P < 0.001. ||The z score maximal IMT is defined with a mean of 0 and an SD of 1. To achieve this, we used a prior constructed composite z score for overall maximal IMT by summing the values of the two carotid IMT sides after standardization (subtraction of the mean and division by the SD of each measure) and then dividing by the SD of the sum.
In subanalyses examining the impact of dyslipidemia on our results, we showed essentially no change in HRs associated with increasing CAC or CIMT groups in relation to CHD and CVD incidence in models without LDL cholesterol and lipid medication. Additional subanalyses examining CAC and CIMT relationships with CHD and CVD events among individuals with prediabetes (fasting glucose of 100–125 mg/dL and not on hypoglycemic therapy) show HRs similar to those without MetS or diabetes as follows: HRs of 7.5 for CHD and 4.6 for CVD (both P < 0.01) for those with CAC 100–399 vs. 0 and HRs of 8.0 for CHD and 5.4 for CVD (both P < 0.01) for those with CAC ≥400 vs. 0. In addition, models involving additional adjustment for microalbuminuria show consistent results, with only a negligible change in HRs.
From receiver operating characteristic analysis, in all three disease groups, CAC added incrementally to predicting CHD and CVD events over models containing age, sex, ethnicity, and traditional RFs: RF + CAC (C-statistic 0.80) versus RF alone (0.73), P < 0.0001 in those with neither MetS nor diabetes; RF + CAC (0.79) versus RF alone (0.73), P < 0.0001 in those with MetS; and RF + CAC (0.78) versus RF alone (0.72), P < 0.0001 in those with diabetes. However, although CIMT added to the prediction of CHD in all three risk groups (P < 0.05), the magnitude of increase in C-statistic was substantially less when adding CIMT compared with CAC as shown as follows: RF + CIMT (0.75) versus RF alone (0.73), P = 0.04 in those with neither MetS nor diabetes; RF + CIMT (0.74) versus RF alone (0.73), P = 0.02 in those with MetS; and RF + CIMT (0.74) versus RF alone (0.72), P = 0.002 in those with diabetes. For the prediction of CVD events (results not shown), in all three disease groups, there was also incremental prediction of events from CAC over RFs alone (C-statistic improvement from 0.73–0.74 to 0.78–0.79, all P < 0.0001); however, CIMT added only to RFs in those with neither condition nor MetS (P < 0.05), with no appreciable change in C-statistic. Models with RFs plus CAC were also superior to models with RFs plus CIMT for prediction of both CHD and CVD (P < 0.05 to P < 0.001).
CONCLUSIONS
Although guidelines currently suggest that diabetes is a CHD risk equivalent, many with diabetes in the MESA cohort did not reach the expected 2% annual CHD rate. Even when MetS or diabetes was present, unless CAC or CIMT was significant, CHD or CVD event rates were as low as in those without these conditions, questioning whether diabetes is a universal CHD risk equivalent. In fact, the observed annual rate for CHD events in those with diabetes was <1% in those without CAC (38% of those with diabetes). Our data suggest that CAC screening strongly stratifies CHD and CVD event risk in individuals with MetS and diabetes, showing that they have a wide range of risk based on the extent of CAC present, supporting the conclusion of a large meta-analysis (6) showing that many individuals with diabetes are not at a customary CHD risk equivalent status and suggesting that treatment should be based on individualized CHD risk assessment.
Our study is the first large population-based study comparing the predictive role of CAC and CIMT in those with MetS and diabetes and the first to report the utility of risk stratification with these imaging strategies in those with MetS. We found that CAC was a more powerful predictor of both CHD and CVD events in these groups as compared with CIMT. Our results are consistent with others who have shown, in a large self-referred cohort with and without diabetes, CAC to improve prediction of total mortality over Framingham risk score (14). In addition, in the overall MESA cohort, CAC was a stronger predictor of CVD events than CIMT (20). Others have also shown CAC to strongly predict short-term CVD events over 2 years, with no events occurring in those with CAC scores <10 (21), and Elkeles et al. (22), in the PREDICT cohort, showed over a median 4-year follow-up somewhat higher HRs for CHD and stroke (11.9 for CAC 401–100 vs. <11) than our study. Also reported in a recent review is that 23% of those with diabetes and low CAC are at low CVD risk, lower than our reported 38% without CAC who are at low risk (23).
Our findings corroborate the recommendations of the most current appropriateness use criteria for cardiac CT in favoring the use of CAC in assessment of CVD risk in asymptomatic adults, including those with diabetes aged ≥40 years (24). Those with the highest levels of CAC or CIMT are at highest risk, which may help motivate such individuals (and their physicians) to be more adherent to or to intensify treatment recommendations.
Strengths of MESA include its large sample size, prospective study design, ethnic diversity, community-based recruitment, and standardized protocols for CT and CIMT evaluation. However, we had limited power to stratify our results according to ethnicity or sex or to examine which components of MetS were most important in combination with CAC or CIMT. Our lower than expected CHD event rates in those with diabetes may be the result of recently increased rates of treatment seen in contemporary cohorts such as MESA (27% on lipid medication, 63% on hypertensive medication, and 28% on aspirin), so results may differ from those studies using older diabetes cohorts with lower treatment rates. Furthermore, our cohort may be healthier than or may be getting more regular medical care than the general U.S. population. Furthermore, we did not have measures of glycosylated hemoglobin (HbA1c) available and thus could not evaluate risk prediction according to HbA1c groups.While CIMT could be more predictive of stroke, it was beyond the scope of this article to compare CIMT and CAC in relation to individual CVD end points, and results may differ if other MetS definitions were used or in other populations where MetS prevalence or severity may vary. Finally, because diabetes duration could be related to CAC and event risk but was available in only 69% of our sample, we did a sensitivity analysis in that sample and found the results were unaffected by its inclusion.
Our data suggest CAC screening (with CIMT less useful) may improve CHD risk stratification in those with MetS and diabetes. While guidelines do not exist for treating subgroups of people with MetS or diabetes more aggressively than others (except in the presence of CHD), those with very high levels of CAC are at much higher risk than those without or with low levels of CAC and, thus, a more aggressive treatment approach in such individuals may be justified. This is still an open question, however, given the expense of such additional screening and limited availability of the technology at medical offices where most patient visits will occur. One can certainly debate that it is not clear whether more aggressive treatment in such subgroups will ultimately result in improved clinical outcomes; however, a large prospective trial recently demonstrated that those randomized to CAC scanning compared with no scanning have beneficial CHD RF effects without increased downstream medical procedures or costs (25). It is important to note that individuals with diabetes who have CAC scores <100 or who have CIMT that is not significantly increased have levels of risk similar to most individuals without diabetes, providing further evidence that many people with diabetes are not CHD risk equivalents. Further investigation should determine whether there is a true benefit on clinical outcomes if we are to change our approach by intensifying treatment in higher risk individuals with MetS or diabetes or on improving patient adherence to treatment based on the level of subclinical atherosclerosis detected.
Acknowledgments
This research was supported by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute.
M.J.B. has been a consultant for and serves on the speaker’s bureau for GE Healthcare. N.D.W. reports research funding through the University of California–Irvine from Bristol Myers-Squibb and Merck. No other potential conflicts of interest relevant to this article were reported.
S.M. wrote the manuscript and provided critical review and revision. M.J.B. conceived the project and provided critical review and revision. R.K., R.S.B., A.G.B., K.N., M.S., and R.G.B. provided critical review and revision to the manuscript. N.D.W. wrote the manuscript and provided critical review and revision.
Parts of this study were presented in abstract form at the American Heart Association Scientific Sessions, Orlando, Florida, 14–18 November 2009.
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
References
- 1.Butler J, Mooyaart EA, Dannemann N, et al. Relation of the metabolic syndrome to quantity of coronary atherosclerotic plaque. Am J Cardiol 2008;101:1127–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation 2004;110:1245–1250 [DOI] [PubMed] [Google Scholar]
- 3.Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971-1993. Diabetes Care 1998;21:1138–1145 [DOI] [PubMed] [Google Scholar]
- 4.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002;288:2709–2716 [DOI] [PubMed] [Google Scholar]
- 5.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234 [DOI] [PubMed] [Google Scholar]
- 6.Bulugahapitiya U, Siyambalapitiya S, Sithole J, Idris I. Is diabetes a coronary risk equivalent? Systematic review and meta-analysis. Diabet Med 2009;26:142–148 [DOI] [PubMed] [Google Scholar]
- 7.Wong ND, Sciammarella MG, Polk D, et al. The metabolic syndrome, diabetes, and subclinical atherosclerosis assessed by coronary calcium. J Am Coll Cardiol 2003;41:1547–1553 [DOI] [PubMed] [Google Scholar]
- 8.Kullo IJ, Cassidy AE, Peyser PA, Turner ST, Sheedy PF, 2nd, Bielak LF. Association between metabolic syndrome and subclinical coronary atherosclerosis in asymptomatic adults. Am J Cardiol 2004;94:1554–1558 [DOI] [PubMed] [Google Scholar]
- 9.McNeill AM, Rosamond WD, Girman CJ, et al. Prevalence of coronary heart disease and carotid arterial thickening in patients with the metabolic syndrome (The ARIC Study). Am J Cardiol 2004;94:1249–1254 [DOI] [PubMed] [Google Scholar]
- 10.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336–1345 [DOI] [PubMed] [Google Scholar]
- 11.Nambi V, Chambless L, Folsom AR, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol 2010;55:1600–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA 2010;303:1610–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qu W, Le TT, Azen SP, et al. Value of coronary artery calcium scanning by computed tomography for predicting coronary heart disease in diabetic subjects. Diabetes Care 2003;26:905–910 [DOI] [PubMed] [Google Scholar]
- 14.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol 2004;43:1663–1669 [DOI] [PubMed] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881 [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Daniels SR, et al. ; American Heart Association; National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 17.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology 2005;234:35–43 [DOI] [PubMed] [Google Scholar]
- 18.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–1847 [DOI] [PubMed] [Google Scholar]
- 19.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–753 [DOI] [PubMed] [Google Scholar]
- 20.Folsom AR, Kronmal RA, Detrano RC, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med 2008;168:1333–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anand DV, Lim E, Hopkins D, et al. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J 2006;27:713–721 [DOI] [PubMed] [Google Scholar]
- 22.Elkeles RS, Godsland IF, Feher MD, et al. ; PREDICT Study Group Coronary calcium measurement improves prediction of cardiovascular events in asymptomatic patients with type 2 diabetes: the PREDICT study. Eur Heart J 2008;29:2244–2251 [DOI] [PubMed] [Google Scholar]
- 23.Elkeles RS. Coronary artery calcium and cardiovascular risk in diabetes. Atherosclerosis 2010;210:331–336 [DOI] [PubMed] [Google Scholar]
- 24.Greenland P, Alpert JS, Beller GA, et al. ; American College of Cardiology Foundation; American Heart Association 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010;56:e50–e103 [DOI] [PubMed] [Google Scholar]
- 25.Rozanski A, Gransar H, Shaw LJ, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol 2011;57:1622–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]