Abstract
OBJECTIVE
Both in vitro and in vivo studies indicate that metformin inhibits cancer cell growth and reduces cancer risk. Recent epidemiological studies suggest that metformin therapy may reduce the risks of cancer and overall cancer mortality among patients with type 2 diabetes. However, data on its effect on colorectal cancer are limited and inconsistent. We therefore pooled data currently available to examine the association between metformin therapy and colorectal cancer among patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
The PubMed and SciVerse Scopus databases were searched to identify studies that examined the effect of metformin therapy on colorectal cancer among patients with type 2 diabetes. Summary effect estimates were derived using a random-effects meta-analysis model.
RESULTS
The analysis included five studies comprising 108,161 patients with type 2 diabetes. Metformin treatment was associated with a significantly lower risk of colorectal neoplasm (relative risk [RR] 0.63 [95% CI 0.50–0.79]; P < 0.001). After exclusion of one study that investigated colorectal adenoma, the remaining four studies comprised 107,961 diabetic patients and 589 incident colorectal cancer cases during follow-up. Metformin treatment was associated with a significantly lower risk of colorectal cancer (0.63 [0.47–0.84]; P = 0.002). There was no evidence for the presence of significant heterogeneity between the five studies (Q = 4.86, P = 0.30; I2 = 18%).
CONCLUSIONS
From observational studies, metformin therapy appears to be associated with a significantly lower risk of colorectal cancer in patients with type 2 diabetes. Further investigation is warranted.
Colorectal cancer is one of the most frequent malignant tumors and a leading cause of cancer-related death worldwide (1). The incidence of colorectal cancer continues to increase in economically transitioning countries such as Asia, Eastern Europe, and selected countries in South America (2,3), whereas a declining trend has been noted in several developed countries in recent years (1).
Type 2 diabetes is also a common disease, and it is well established that type 2 diabetes is associated with a higher risk of colorectal cancer (4–8). Metformin is a relative of isoamylene guanidine and has been recommended as the initial glucose-lowering therapy for diabetes. Emerging evidence from both in vitro and in vivo studies indicates that metformin may inhibit cancer cell growth and reduce cancer risks. Previous research suggests that metformin may be involved in the tumor suppressor pathway by indirectly activating AMP-activated protein kinase (9)—a key sensor of cellular ATP and AMP balance—and plays a role on activating tumor suppressor genes, e.g., LKB1. Subsequent in vitro studies have shown that metformin inhibits cancer cell proliferation (10,11) and selectively kills cancer stem cells (12). Animal experiments concur with these findings. Rodent models have shown that metformin suppresses colonic epithelial proliferation and colorectal aberrant crypt foci formation (13,14). Similarly, animal models of colon cancer have shown that metformin inhibits colon carcinoma growth (11,15). Given these encouraging findings, interest has arisen that metformin could potentially serve as a new antineoplasm drug to prevent colorectal cancer.
Results from preliminary studies conducted in humans are encouraging. In a short-term randomized clinical trial among nondiabetic patients with rectal aberrant crypt foci, a significant decrease in the mean number of aberrant crypt foci was observed after metformin treatment for 1 month as compared with no significant changes in the control group (16). Findings from several epidemiological studies also support an antineoplastic role of metformin on cancer risks (17,18). If metformin therapy ultimately proves effective on reducing the risk of colorectal cancer, it would likely be recommended for the overwhelming majority of diabetes patients for both blood glucose control and cancer prevention. Nonetheless, despite accumulating evidence from population studies that indicate a lower risk of cancer at large with metformin therapy (17,19,20), data on its effect on colorectal cancer are limited and inconsistent. Accordingly, we performed a meta-analysis to pool studies currently available to examine the effect of metformin treatment on colorectal cancer risk among patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Study selection
A comprehensive literature search was performed using the PubMed (January 1966–March 2011) and SciVerse Scopus databases. Keywords for searching included metformin, biguanides, cancer, neoplasms, colorectal cancer, colon cancer, rectal cancer, and bowel cancer. We selected studies that investigated the effect of metformin on colorectal cancer or bowel cancer (ICD-9 153–154, ICD-10 C18–C20) in patients with type 2 diabetes. The search was restricted to studies conducted in humans. Because colorectal cancer develops from precursor lesions (21), we also included studies that investigated the association between metformin therapy and colorectal adenoma. We required either information on sample size, effect estimate, and corresponding 95% CI or published results in which these parameters could be derived. References from recent review articles were also checked for relevant articles. The most complete or more recent publications were given precedence if there were multiple publications from the same study.
Data extraction and statistical analyses
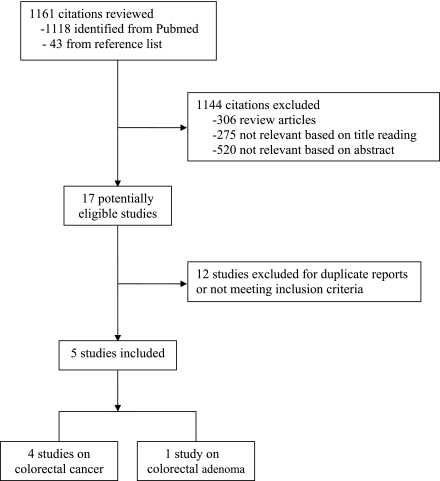
Two members independently reviewed titles and abstracts of all identified citations. The full text of any article that was deemed potentially eligible was evaluated for the decision on inclusion or exclusion. Figure 1 depicts the process of study selection. The results of the data extraction were summarized in a structured table to explore the variation in study design, region of study, source population, number of participants, length of follow-up (if applicable), and control for confounding. The relative risk (RR) was used as the common measure of association across studies. Odds ratios were assumed to be a reasonable estimate for relative risks for the present study. Forest plots were used to compare results across studies. For those studies consisting of multiple pairwise comparisons, we pooled the effect estimates with an inverse variance weight and used the combined estimate for that study. The resulting variance of the pooled estimate does not allow for the correlation between the multiple effect estimates in the same study and can be either too large or too small, depending on the correlation. Thus, to examine the influence of the correlation, we performed sensitivity analyses by including one pairwise comparison at a time when recalculating the summary RRs. The pooled RR was derived by averaging per-study natural logarithmic RRs weighted by the inverses of their variances. We used the DerSimonian and Laird random-effects models to incorporate between-study variability and to report pooled effect estimates (22).
Figure 1.
Flowchart of study selection process.
As part of sensitivity analyses, we excluded one small study (23) that investigated the association between metformin therapy and colorectal adenoma to examine its influence on the summary effect estimate. To assess for heterogeneity between studies, we calculated the Cochran Q statistic with significance level of P < 0.10 (24). P values were obtained by comparing the statistic with a χ2 distribution with k−1 degrees of freedom (where k is the number of studies). Because the power of the Q test is low to detect heterogeneity in circumstances of small number of studies, we also calculated the I2 statistic, which quantifies the percentage of total variation across studies that is due to heterogeneity rather than chance (25). An I2 value of <50% was considered low heterogeneity. Publication bias was assessed by visually examining a funnel plot and performing the Begg test (26). A two-tailed P value <0.05 was considered significant for statistical tests for metformin effects. All analyses were performed using Stata version 10.0 (StataCorp, College Station, Texas).
RESULTS
Five relevant studies were retrieved, including two case-control studies (6,23) and three retrospective cohort studies (17,18,27), comprising a total of 108,161 patients with type 2 diabetes. Information on region, source population, and confounding adjustment is presented in Table 1. One study was based in China (27), one study in Korea (23), and three studies in the U.K. (6,17,18). Four studies investigated the association between metformin therapy and colorectal cancer (6,17,18,27), and the other one investigated the association for colorectal adenoma (23).
Table 1.
Designs of studies included in the present meta-analysis
| 1st author, year | Study type | Region | Source population | Total participants (events) | Follow-up (years) | Confounding adjustment |
|---|---|---|---|---|---|---|
| Yang, 2004 (6) | Nested case-control study | U.K. | A cohort of patients with type 2 diabetes in the General Practice Research Database from 1987 to 2002 | 24,918 (125) | 5.6* | Sex, history of cholecystectomy, smoking, duration of type 2 diabetes, BMI, 3 or more years of sulfonylurea use prior to index date, 1 or more years of recent NSAID/aspirin use |
| Chung, 2008 (23) | Case-control study | Korea | A cohort of patients with type 2 diabetes without colorectal adenoma from 2003 to 2006 | 200 (100) | — | Matching on age (± 1 year) and sex, adjusting with age, sex, BMI, diabetes duration, HbA1c, lipids, insulin, aspirin |
| Libby, 2009 (17) | Retrospective cohort study | Scotland, U.K. | A cohort of patients with type 2 diabetes in Tayside, Scotland, U.K. from 1994 to 2003 | 8,000 (116) | Open cohort, Jan 1994–Dec 2003 | Sex, age, BMI, HbA1c, deprivation, smoking, other drug use |
| Currie, 2009 (18) | Retrospective cohort study | U.K. | A cohort of diabetes patients (diagnosed >40 years of age) in The Health Information Network, after 2002 | 59,609 (292) | 2.4 | Age, sex, smoking status, and a diagnosis of a prior cancer |
| Lee, 2011 (27) | Retrospective cohort study | Taiwan, China | A cohort of participants ≥20 years, diabetes-cancer-free in Jan 2000 | 15,434 (56) | 3.5 | Age, sex, other oral antihyperglycemic medication, CCI score |
*Mean follow-up for the 125 cases. CCI, Charlson Comorbidity Index; NSAID, nonsteroidal anti-inflammatory drug.
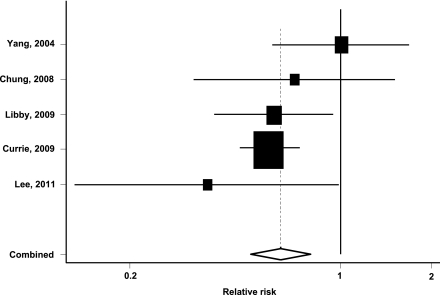
Compared with nonmetformin treatment, metformin was associated with a significantly lower risk of colorectal neoplasm among patients with type 2 diabetes (pooled RR 0.63 [95% CI 0.50–0.79]; P < 0.001). Figure 2 shows the estimated RR and 95% CI for each individual study comparing metformin with nonmetformin treatment. There was no evidence for the presence of significant heterogeneity between the five studies (Q = 4.86, P = 0.30; I2 = 18%). There was also no evidence of publication bias from the funnel plot examination or the Begg test (data not shown).
Figure 2.
Pooled estimate of relative risk and 95% CIs of colorectal neoplasm associated with metformin therapy based on five studies comprising 108,161 patients with type 2 diabetes. Squares indicate relative risk in each study. The square size is proportional to the weight of the corresponding study in the meta-analysis; the length of horizontal lines represents the 95% CI. The unshaded diamond indicates the pooled relative risk and 95% CI.
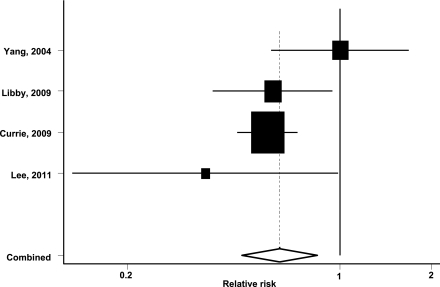
After exclusion of one small case-control study that investigated colorectal adenoma (23), the database consisted of three retrospective cohort studies (17,18,27) and one nested case-control study (6). Among a total of 107,961 patients with type 2 diabetes, 589 colorectal cancer events were documented over follow-up. Metformin therapy was associated with a lower risk of colorectal cancer (RR 0.63 [95% CI 0.47–0.84]; P = 0.002). Thus, exclusion of the small case-control study had no appreciable effects on the overall results. Figure 3 shows the estimated RR and 95% CI for each individual study comparing metformin with nonmetformin treatment. Again, there was no evidence for the presence of significant heterogeneity between the four studies (Q = 4.75, P = 0.19; I2 = 37%). Although the results showed a reduction of similar magnitude in the risk of colorectal cancer with metformin treatment when compared with two other treatment groups (18), we performed sensitivity analysis by including one of the two effect estimates at a time. The results did not change materially after excluding either comparison (data not shown).
Figure 3.
Pooled estimate of relative risk and 95% CIs of colorectal cancer associated with metformin therapy based on four studies comprising 107,961 diabetic patients and 589 incident colorectal cancer cases. Squares indicate relative risk in each study. The square size is proportional to the weight of the corresponding study in the meta-analysis; the length of horizontal lines represents the 95% CI. The unshaded diamond indicates the pooled relative risk and 95% CI.
CONCLUSIONS
In this study, we pooled all studies currently available and evaluated the association between metformin and colorectal cancer risk among patients with type 2 diabetes. The results indicate that metformin therapy was associated with an estimated reduction of 37% in the risk of colorectal cancer among patients with type 2 diabetes. The effect estimates were homogeneous across studies of different designs, including retrospective cohort and nested case-control studies. The multiple sensitivity analyses showed that the results were robust.
Other lines of evidence support the antineoplastic role of metformin on colorectal cancer. In vitro studies have shown that metformin inhibits the proliferation of colorectal cancer cells (28). In vivo studies have demonstrated that metformin delays tumor onset in mouse models for p53 mutant colon cancer (11). Another animal model of colon cancer has shown that metformin inhibits colon carcinoma growth stimulated by a high-energy diet (15). Two animal models of colorectal aberrant crypt foci showed that metformin significantly suppresses colonic epithelial proliferation by inhibiting the mammalian target of rapamycin pathway (13,14). A randomized clinical trial among nondiabetic patients with a follow-up of 1 month demonstrated that the number of aberrant crypt foci decreased significantly in the metformin group (8.78 before treatment vs. 5.11 at 1 month follow-up, P = 0.01) but not in the nonmetformin group (7.23 before treatment vs. 7.56 at 1 month follow-up, P = 0.61) (16). These findings are encouraging as metformin, in addition to its apparent anticancer properties, has the advantage of high tolerance, affordability, and good compatibility with other hypoglycemic agents.
The mechanisms underlying this antineoplastic potential of metformin for colorectal cancer are manifold and not yet completely understood. A previous study showed that metformin indirectly activates AMP-activated protein kinase (9), which induces glucose uptake in muscles (29). Because AMP-activated protein kinase is also activated by the product of the Peutz-Jegher tumor suppressor gene LKB1 (30), it may play a role in suppressing colorectal cancer development (31,32). This relationship between metformin and LKB1 represents one of the more plausible mechanisms for a potential protective role of metformin against colorectal cancer. Another possible mechanism is amelioration of endogenous hyperinsulinemia by use of metformin therapy (33,34). Insulin stimulates cellular proliferation, and multiple signaling pathways are activated after insulin receptors or insulin-like growth factor receptors interact with their ligands (35,36). Metformin therapy decreases levels of insulin-like growth factors and insulin in circulation which, in turn, may reduce the risk of colorectal cancer. Other possible mechanisms underlying this potential antitumor effect could be antagonizing obesity (37), anti-inflammatory effects (38), p-53 activation (11), downregulation of cyclin D1 (39), and killing of cancer stem cells (12). Further studies investigating potential underlying mechanisms are needed.
Our meta-analysis pooled all published data currently available and provided strong evidence of antineoplastic effect on colorectal cancer with metformin treatment. However, caution is needed when interpreting these results. Information on metformin use was collected retrospectively in all of the included observational (uncontrolled) studies. Most of the included studies were based on historical medical data or insurance data that were not specifically designed to assess the effect of metformin therapy on colorectal cancer. Details on dose, duration, variation over time for metformin treatment, and other adjunctive therapy were incomplete. The possibility of immortal bias was not completely ruled out in some included studies. Most of the included studies did not collect or analyze data on pathological type or site of colorectal cancer. In the present study, metformin therapy was compared with other hypoglycemic therapies, including insulin, sulfonylureas, thiazolidinediones, or other oral medicines. It should be noted that insulin and insulin analogs have been reported to be associated with a higher risk of colorectal cancer (6,7). The observed apparent beneficial effect of metformin could thus be an overestimate partly due to the potential hazardous effect associated with other hypoglycemic agents in the reference group. On the other hand, metformin receivers were reported to be more likely to receive lower endoscopy (40), which could lead to early detection of colorectal cancer in metformin receivers and bias the effect estimate toward the null (i.e., no anticancer effect). Finally, the possibility of publication bias cannot be ruled out although we found no evidence of this through the funnel plot examination and the Begg test.
In conclusion, our meta-analysis suggests that metformin treatment appears to be associated with a significantly lower risk of colorectal cancer in patients with type 2 diabetes. Further investigation is warranted.
Acknowledgments
This work was supported by the Shanghai Municipal Education Commission (Grant 11YZ51) and the Science and Technology Commission of Shanghai Municipality (Grant 11ZR1419500), and Shanghai Jiao Tong University (SMC Outstanding Young Teachers) to Z.-J.Zha.
No potential conflicts of interest relevant to this article were reported.
Z.-J.Zha. designed the study, researched data, and wrote and revised the manuscript. Z.-J.Zhe., H.K., Y.S., W.C., G.Z., and K.E.K. reviewed and edited the manuscript.
Footnotes
See accompanying editorial, p. 2336.
References
- 1.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:1893–1907 [DOI] [PubMed] [Google Scholar]
- 2.Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev 2009;18:1688–1694 [DOI] [PubMed] [Google Scholar]
- 3.Sung JJ, Lau JY, Young GP, et al. ; Asia Pacific Working Group on Colorectal Cancer Asia Pacific consensus recommendations for colorectal cancer screening. Gut 2008;57:1166–1176 [DOI] [PubMed] [Google Scholar]
- 4.Campbell PT, Deka A, Jacobs EJ, et al. Prospective study reveals associations between colorectal cancer and type 2 diabetes mellitus or insulin use in men. Gastroenterology 2010;139:1138–1146 [DOI] [PubMed] [Google Scholar]
- 5.Seow A, Yuan JM, Koh WP, Lee HP, Yu MC. Diabetes mellitus and risk of colorectal cancer in the Singapore Chinese Health Study. J Natl Cancer Inst 2006;98:135–138 [DOI] [PubMed] [Google Scholar]
- 6.Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology 2004;127:1044–1050 [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010;33:1674–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Balluz LS, Ford ES, et al. Association between diagnosed diabetes and self-reported cancer among U.S. adults: findings from the 2009 behavioral risk factor surveillance system. Diabetes Care 2011;34:1365–1368 [DOI] [PMC free article] [PubMed]
- 9.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu B, Fan Z, Edgerton SM, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle 2009;8:2031–2040 [DOI] [PubMed] [Google Scholar]
- 11.Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res 2007;67:6745–6752 [DOI] [PubMed] [Google Scholar]
- 12.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res 2009;69:7507–7511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosono K, Endo H, Takahashi H, et al. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Mol Carcinog 2010;49:662–671 [DOI] [PubMed] [Google Scholar]
- 14.Tomimoto A, Endo H, Sugiyama M, et al. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci 2008;99:2136–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Algire C, Amrein L, Zakikhani M, Panasci L, Pollak M. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr Relat Cancer 2010;17:351–360 [DOI] [PubMed] [Google Scholar]
- 16.Hosono K, Endo H, Takahashi H, et al. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev Res (Phila) 2010;3:1077-1083 [DOI] [PubMed]
- 17.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009;32:1620–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009;52:1766–1777 [DOI] [PubMed] [Google Scholar]
- 19.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005;330:1304–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monami M, Colombi C, Balzi D, et al. Metformin and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care 2011;34:129-131 [DOI] [PMC free article] [PubMed]
- 21.Mallinson EK, Newton KF, Bowen J, et al. The impact of screening and genetic registration on mortality and colorectal cancer incidence in familial adenomatous polyposis. Gut 2010;59:1378–1382 [DOI] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188 [DOI] [PubMed] [Google Scholar]
- 23.Chung YW, Han DS, Park KH, Eun CS, Yoo KS, Park CK. Insulin therapy and colorectal adenoma risk among patients with Type 2 diabetes mellitus: a case-control study in Korea. Dis Colon Rectum 2008;51:593–597 [DOI] [PubMed] [Google Scholar]
- 24.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820–826 [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558 [DOI] [PubMed] [Google Scholar]
- 26.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–1101 [PubMed] [Google Scholar]
- 27.Lee MS, Hsu CC, Wahlqvist ML, et al. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer 2011;11:20 [DOI] [PMC free article] [PubMed]
- 28.Zakikhani M, Dowling RJ, Sonenberg N, Pollak MN. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev Res (Phila) 2008;1:369–375 [DOI] [PubMed] [Google Scholar]
- 29.Koo SH, Flechner L, Qi L, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 2005;437:1109–1111 [DOI] [PubMed] [Google Scholar]
- 30.Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005;310:1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong SM, Kim KM, Kim SY, et al. Frequent somatic mutations in serine/threonine kinase 11/Peutz-Jeghers syndrome gene in left-sided colon cancer. Cancer Res 1998;58:3787–3790 [PubMed] [Google Scholar]
- 32.Park WS, Moon YW, Yang YM, et al. Mutations of the STK11 gene in sporadic gastric carcinoma. Int J Oncol 1998;13:601–604 [PubMed] [Google Scholar]
- 33.Yang YX. Do diabetes drugs modify the risk of pancreatic cancer? Gastroenterology 2009;137:412–415 [DOI] [PubMed] [Google Scholar]
- 34.Goodwin PJ, Ligibel JA, Stambolic V. Metformin in breast cancer: time for action. J Clin Oncol 2009;27:3271–3273 [DOI] [PubMed] [Google Scholar]
- 35.Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst 2002;94:972–980 [DOI] [PubMed] [Google Scholar]
- 36.LeRoith D, Baserga R, Helman L, Roberts CT., Jr Insulin-like growth factors and cancer. Ann Intern Med 1995;122:54–59 [DOI] [PubMed] [Google Scholar]
- 37.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–578 [DOI] [PubMed] [Google Scholar]
- 38.Grenader T, Goldberg A, Shavit L. Metformin as an addition to conventional chemotherapy in breast cancer. J Clin Oncol 2009;35:e259; author reply e260 [DOI] [PubMed]
- 39.Zhuang Y, Miskimins WK. Cell cycle arrest in Metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J Mol Signal 2008;3:18 [DOI] [PMC free article] [PubMed]
- 40.Lewis JD, Capra AM, Achacoso NS, et al. Medical therapy for diabetes is associated with increased use of lower endoscopy. Pharmacoepidemiol Drug Saf 2007;16:1195–1202 [DOI] [PubMed] [Google Scholar]