Abstract
OBJECTIVE
To assess, in the general diabetic population, 1) the prevalence of painful neuropathic symptoms; 2) the relationship between symptoms and clinical severity of neuropathy; and 3) the role of diabetes type, sex, and ethnicity in painful neuropathy.
RESEARCH DESIGN AND METHODS
Observational study of a large cohort of diabetic patients receiving community-based health care in northwest England (n = 15,692). Painful diabetic neuropathy (PDN) was assessed using neuropathy symptom score (NSS) and neuropathy disability score (NDS).
RESULTS
Prevalence of painful symptoms (NSS ≥5) and PDN (NSS ≥5 and NDS ≥3) was 34 and 21%, respectively. Painful symptoms occurred in 26% of patients without neuropathy (NDS ≤2) and 60% of patients with severe neuropathy (NDS >8). Adjusted risk of painful neuropathic symptoms in type 2 diabetes was double that of type 1 diabetes (odds ratio [OR] = 2.1 [95% CI 1.7–2.4], P < 0.001) and not affected by severity of neuropathy, insulin use, foot deformities, smoking, or alcohol. Women had 50% increased adjusted risk of painful symptoms compared with men (OR = 1.5 [1.4–1.6], P < 0.0001). Despite less neuropathy in South Asians (14%) than Europeans (22%) and African Caribbeans (21%) (P < 0.0001), painful symptoms were greater in South Asians (38 vs. 34 vs. 32%, P < 0.0001). South Asians without neuropathy maintained a 50% increased risk of painful neuropathy symptoms compared with other ethnic groups (P < 0.0001).
CONCLUSIONS
One-third of all community-based diabetic patients have painful neuropathy symptoms, regardless of their neuropathic deficit. PDN was more prevalent in patients with type 2 diabetes, women, and people of South Asian origin. This highlights a significant morbidity due to painful neuropathy and identifies key groups who warrant screening for PDN.
Neuropathy is one of the most common long-term complications of diabetes and is the main initiating factor for foot ulceration, Charcot neuroarthropathy, and lower-extremity amputation (1). However, the quality and even quantity of epidemiological data on symptomatic diabetic neuropathy remain poor due to inconsistent definitions, poor ascertainment, and a lack of population-based studies. Of three large, clinic-based studies from Europe, the prevalence of diabetic polyneuropathy varied from 23 to 29% (2–4). In the Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes (BARI 2D) cohort of 2,368 type 2 diabetic patients with coronary artery disease, the prevalence of diabetic peripheral neuropathy was 51% (5). However, in all of these studies, although neuropathy symptoms were assessed as part of the diagnostic definition of diabetic neuropathy, the prevalence of painful diabetic neuropathy (PDN) per se was not established. In our large, community-based survey of 9,710 predominantly type 2 diabetic patients derived from general practice in northwest England, the prevalence of at least moderate neuropathic deficits as defined by a neuropathy disability score (NDS ≥6) was 22% and at least moderate neuropathy symptoms as defined by the neuropathy symptom score (NSS ≥5) was 34% (6).
PDN is considered to be the cause of considerable morbidity and, under the auspices of the American Academy of Neurology, evidence-based guidelines have been published for the management of this difficult condition (7). However, there is a distinct paucity of robust, population-based epidemiological data on the prevalence and natural history of this condition, limited to a few small studies. Thus, in a small population-based study of 269 diabetic patients from Wales, whereas 64% reported pain, only 26% were confirmed to have PDN, but interestingly those with PDN had a significantly poorer quality of life compared with those with nonneuropathic pain (8). In a study of 350 diabetic patients from Liverpool, 13% of patients with PDN had never reported their symptoms to their treating physician and a further 39% had not received any treatment for PDN (9). In a recent study of 1,113 diabetic patients attending secondary care clinics across Turkey, whereas 62% had neuropathy based on abnormal nerve conduction and clinical examination, only 16% had neuropathic pain according to the Leeds Assessment of Neuropathic Symptoms and Signs score (10).
The natural history of PDN remains unclear, although in a small longitudinal study, 77% of 56 diabetic patients with painful neuropathy were found to continue with nonabating pain after 5 years (11). Although it has been suggested that painful symptoms abate with progressive worsening of neuropathy, this has not been supported by a study that has demonstrated equal prevalence of painful symptoms in those with mild compared with more advanced neuropathy (12).
Therefore there is a significant lack of large, population-based data defining the size of the neuropathic pain problem and attempting to provide some explanations toward pain etiology. We have had the unique opportunity to assess the following in a large, community-based diabetic population: 1) the prevalence of painful neuropathic symptoms; 2) the relationship between neuropathic symptoms and severity of clinical neuropathy; 3) the differences in neuropathic symptoms between patients with type 1 and type 2 diabetes; and 4) the role of sex and ethnicity.
RESEARCH DESIGN AND METHODS
The North-West Diabetes Foot Care Study (NWDFCS), a population-based investigation of diabetes-related foot problems in the community health care setting, provided the study population (6). The study was approved by the local research ethics committees, general practitioner (GP)–based diabetes teams, and hospital-based diabetes teams in each district and was funded by the Department of Health. One full-time research podiatrist or research nurse was appointed to screen diabetic patients in the GP practices, diabetes centers, and hospital outpatient clinics for each district. At GP practices, the vast majority of patients were screened while attending for their annual review; others were screened while attending podiatry clinics. Remaining patients were invited to attend a special clinic at the practice, or the patient was visited residentially. Each patient was assessed once for symptoms and signs of peripheral neuropathy, peripheral vascular disease (less than or equal to two palpable pedal pulses), demographic data, and medical history during a short (20–30 min) screening session.
Assessment of neuropathy
Peripheral neuropathy was assessed as previously described (6). Neuropathic deficits in the feet were determined using the NDS, derived from inability to detect pin-prick sensation (using Neurotip), vibration (using 128-Hz tuning fork), and differences in temperature sensation (using warm and cool rods) plus Achilles reflex (using tendon hammer) (6).
NSS
Patients were asked about their experience of pain or discomfort in the legs. If the patient described burning, numbness, or tingling, a score of 2 was assigned; fatigue, cramping, or aching scored 1. The presence of symptoms in the feet was assigned a score of 2, the calves 1, and elsewhere a score of 0. Nocturnal exacerbation of symptoms scored 2 vs. 1 for both day and night and 0 for daytime alone. A score of 1 was added if the symptoms had ever woken the patient from sleep. The patients were asked if any maneuver could reduce the symptoms; walking was assigned a score of 2, standing 1, and sitting or lying down 0. The maximum symptom score was 9. The severity of symptoms was graded according to the NSS as follows: none (0–2), mild (3–4), moderate (5–6), and severe (7–9) (2). The NSS has been used as part of the assessment of PDN in several previous studies (2,9,11,13). We defined PDN as at least moderate symptoms with mild neurologic signs (NSS score ≥5 and NDS score ≥3) (9,11).
Statistical analysis
Variables were stratified into normal and abnormal categories, and χ2 tests were performed for categorical data. Normally distributed, continuous data were tested using Student t test, whereas nonnormally distributed data were first analyzed using Kruskal-Wallis, followed by a Mann-Whitney U test. After obtaining 95% CIs, age-adjusted prevalence rate differences were evaluated between the diabetes type, sex, and ethnic groups. Logistic regression was used to obtain odds ratios (ORs) for neuropathy symptoms between the comparison groups. Modifiers of the ORs were entered into the final logistic regression models to determine which risk factors may account for symptom differences. Statistical package SPSS 16.0 was used to analyze data.
RESULTS
Over 4 years, our community-based screening program assessed 15,692 patients with diabetes within six health care districts of northwest England, representing ∼60% of involved GPs’ diabetic patients (6,14). The majority (70%) of patients were screened while attending their diabetes annual review in primary care, with the remainder (30%) screened at referral sites in diabetes centers and hospital outpatient clinics.
Demographic and medical characteristics of the entire community-based diabetic cohort and type 1 and 2 diabetic subcohorts are given in Table 1. The patients with type 2 diabetes were substantially older than those with type 1 diabetes (63.6 ± 11.8 vs. 37.6 ± 12.9 years, respectively) and had a greater proportion of South Asian/African Caribbean patients (15.1 vs. 3.9%, respectively). Duration of type 2 diabetes was one-quarter that of the type 1 group (P < 0.0001). Type 2 diabetic patients were less likely to be current smokers (22 vs. 33%, P < 0.0001). Despite substantially greater levels of clinical neuropathy, peripheral arterial disease (PAD), and foot deformities in the type 2 diabetic patients, foot ulcer rates (past or present) were similar between the two groups (4.9 vs. 6.0%, respectively, P = 0.07). Paradoxically, lower-limb amputation rate was significantly lower in type 2 compared with type 1 diabetic patients (1.2 vs. 1.8%, P < 0.05).
Table 1.
Demographic and medical characteristics of patient cohorts
| Characteristic | Total population | Type 1 diabetes | Type 2 diabetes | P |
|---|---|---|---|---|
| N | 15,692 | 1,338/15,544 (8.6%) | 14,206/15,544 (91.4%) | — |
| Male | 8,448/15,684 (53.9%) | 750/1,338 (56.1%) | 7,631/14,203 (53.3%) | 0.10 |
| Age (years) | 61.4 ± 14.0 | 37.6 ± 12.9 | 63.6 ± 11.8 | <0.0001 |
| Duration of diabetes (years) | 5 (2–10) | 17 (10–26) | 4 (2–10) | <0.0001 |
| Ethnicity | ||||
| White European | 13,409/15,692 (85.5%) | 1,283/1,338 (96.0%) | 12,015/14,206 (84.6%) | |
| South Asian | 1,866/15,692 (11.9%) | 42/1,338 (3.1%) | 1,791/14,206 (12.6%) | |
| African Caribbean | 371/15,692 (2.4%) | 11/1,338 (0.8%) | 357/14,206 (2.5%) | |
| Other | 46/15,692 (0.2%) | 2/1,338 (0.1%) | 43/14,206 (0.3%) | <0.0001 |
| Diabetes treatment | ||||
| Diet only | 4,643/15,622 (29.7%) | — | 4,601/14,163 (32.5%) | |
| OHA + diet | 7,696/15,622 (49.3%) | — | 7,637/14,163 (53.9%) | |
| Insulin (±OHA) | 3,283/15,622 (21.0%) | 1,337/1,337 (100.0%) | 1,925/14,163 (13.6%) | <0.0001 |
| Overt nephropathy | 440/15,274 (2.9%) | 58/1,308 (4.4%) | 379/13,841 (2.7%) | <0.001 |
| Impaired vision | 1,700/15,455 (11.0%) | 108/1,319 (8.2%) | 1,574/14,006 (11.2%) | <0.001 |
| Smoking history | ||||
| Never smoked | 6,568/15,632 (42.0%) | 626/1,335 (46.8%) | 5,859/14,156 (41.4%) | |
| Current smoker | 3,581/15,632 (22.9%) | 445/1,335 (33.3%) | 3,111/14,156 (22.0%) | |
| Ex-smoker | 5,483/15,632 (35.1%) | 264/1,335 (19.7%) | 5,186/14,156 (36.6%) | <0.0001 |
| Alcohol (≥7 units/week) | 6,998/15,474 (45.2%) | 877/1,323 (66.3%) | 6,074/14,020 (43.3%) | <0.0001 |
| Clinical neuropathy | 3,333/15,659 (21.3%) | 217/1,337 (16.2%) | 3,077/14,183 (21.7%) | <0.0001 |
| Foot deformities | 4,699/15,600 (30.1%) | 204/1,335 (15.3%) | 4,444/14,126 (31.5%) | <0.0001 |
| Peripheral arterial disease | 3,139/15,664 (20.0%) | 139/1,337 (10.4%) | 2,957/14,186 (20.8%) | <0.0001 |
| Foot ulcer history | 774/15,484 (5.0%) | 80/1,331 (6.0%) | 684/14,015 (4.9%) | 0.070 |
| Lower-limb amputation history | 191/15,422 (1.2%) | 24/1,327 (1.8%) | 164/13,955 (1.2%) | 0.045 |
Data are mean ± SD, n (%), or median (25th–75th percentiles); P values for type 1 vs. type 2.
Prevalence of painful neuropathy
The distribution of neuropathy symptom severity within the entire cohort was as follows: no symptoms (NSS 0–2) = 52% (8,073/15,638), mild symptoms (NSS 3–4) = 14% (2,254/15,638), moderate symptoms (NSS 5–6) = 18% (2,780/15,638), and severe symptoms (NSS 7–9) = 16% (2,531/15,638). The overall prevalence of painful neuropathy symptoms (i.e., NSS ≥5) in this cohort was 34% (5,311/15,638).
Relationship between neuropathy symptoms and clinical severity of neuropathy
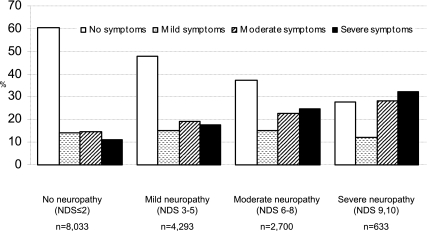
The prevalence of painful neuropathy symptoms in the presence of clinical neuropathy (PDN) (i.e., NSS score ≥5 and NDS score ≥3) for all patients was 21% (3,242/15,614). The distribution of increasing neuropathy symptoms in patient groups stratified by the severity of clinical neuropathy is given in Fig. 1. Sixty percent (379/629) of diabetic patients with severe clinical neuropathy (NDS >8) had painful neuropathic symptoms (NSS ≥5), whereas only 26% (2,060/8,016) of patients without clinical neuropathy (NDS ≤2) had painful symptoms. There was an emerging pattern of worsening clinical neuropathy scores associated with an increasing proportion of patients with more severe painful neuropathic symptoms (P < 0.0001), and there was a significant, positive correlation between NSS and NDS (r = 0.24, P < 0.0001). This relationship between signs and symptoms was stronger in type 1 (r = 0.37, P < 0.0001) than type 2 diabetic subjects (r = 0.22, P < 0.0001).
Figure 1.
Percentage prevalence of neuropathic symptoms in 15,659 diabetic patients characterized by their level of clinical neuropathy.
Type 1 versus type 2 diabetes
Painful symptoms (NSS ≥5) were more prevalent in type 2 (35.0% [4,962/14,166]) versus type 1 (22.7% [303/1,334], P < 0.0001) diabetic patients, as was PDN (21.5% [3,039/14,144] vs. 13.4% [178/1,333], respectively, P < 0.0001). The risk of painful neuropathy symptoms in type 2 diabetic patients was 83% higher than in type 1 patients (OR = 1.8 [95% CI 1.6–2.1], P < 0.0001); this risk doubled after adjusting for differences in age and diabetes duration (OR = 2.1 [1.7–2.4], P < 0.0001). When examining patients with moderate to severe clinical neuropathy (i.e., NDS ≥6) only, the age- and diabetes duration–adjusted risk of painful symptoms in type 2 versus type 1 diabetic patients was still significantly greater (OR = 1.8 [1.2–2.5], P < 0.0001). Adjustment for type 2 versus type 1 diabetes and differences in severity of neuropathy, insulin use, foot deformities, smoking status, and alcohol intake had no impact on these differences in painful symptoms (data not shown); i.e., these variables could not account for the disparity in symptoms between type 1 and type 2 diabetes.
Age effect
Increasing age was very weakly associated with NSS severity in the entire population (r = 0.083, P < 0.0001); however, this relationship was stronger in type 1 (r = 0.20, P < 0.0001) than type 2 diabetes (r = 0.022, P = 0.008). Indeed, increasing age categories in type 1 diabetic patients showed an almost doubling in prevalence of painful symptoms (NSS ≥5) (aged <35 years, 17.2%; 35–54 years, 26.4%; 55+ years, 33.1%; P < 0.0001), with a similar, but less marked, association in type 2 diabetic patients (aged <35 years, 30.6%; 35–54 years, 32.7%; 55+ years, 35.7%; P < 0.01).
Effect of diabetes treatment
Insulin use versus oral hypoglycemic agents (OHAs) and/or diet had no effect on painful neuropathy symptoms, i.e., 33% (1,085/3,272) of patients using insulin had NSS ≥5 compared with 34% (4,206/12,303) of patients treated with diet and OHA (P = 0.27). However, when treatments were examined individually, symptoms were most prevalent in patients treated with OHA (37.3%) compared with insulin (33.2%) or diet alone (29.1%) (P < 0.0001). Restricting the analysis to patients with clinical neuropathy, painful symptoms were most prevalent in the insulin-treated group > OHA group > diet-only group (54.7, 50.6, and 42.1%, respectively; P < 0.0001).
Effect of sex
A significantly greater proportion of females (38% [2,732/7,212]) than males (31% [2,578/8,423]) reported painful neuropathy symptoms (P < 0.0001), despite fewer females than males having clinical neuropathy (NDS ≥6) (19 vs. 23%, P < 0.0001). PDN (NSS ≥5 and NDS ≥3) was, similarly, more prevalent in females than males (23 vs. 19%, respectively, P < 0.0001). After adjustments for age, diabetes duration, and differences in clinical neuropathy, women still had a 50% increased risk of painful symptoms compared with men (OR = 1.5 [95% CI 1.4–1.6], P < 0.0001).
Effect of ethnicity
Despite a lower unadjusted prevalence of clinical neuropathy (NDS ≥6) in South Asians (14%) compared with Europeans (22%) and African Caribbeans (21%) (P < 0.0001), painful neuropathy symptoms (NSS ≥5) were significantly and, conversely, greater in South Asians (38%) compared with Europeans (34%) and African Caribbeans (32%) (P < 0.0001). Greater neuropathy symptoms in South Asians, however, were only evident in patients without clinical neuropathy (i.e., NSS ≥5 and NDS ≤2: South Asians 19% [352/1,845], Europeans 13% [1667/13,354], African Caribbeans 10% [36/370]; P < 0.0001), whereas PDN (NSS ≥5 and NDS ≥3) was similarly prevalent in all ethnic groups (21% [2,803/13,354], 19% [349/1,845], and 22% [80/370], respectively; P = 0.11). After adjustments for age and diabetes duration, South Asians without significant clinical neuropathy were still 50% more likely to have painful neuropathy symptoms compared with other ethnic groups (OR = 1.5 [95% CI 1.3–1.6], P < 0.0001).
CONCLUSIONS
We have shown that one-third of all patients with diabetes in the community have painful neuropathic symptomatology, regardless of whether they have clinical neuropathy. These data show a higher prevalence of painful neuropathic symptoms than previously reported in two small population-based studies (9,11). In a recent study using the validated DN4 (a clinician-administered neuropathic pain diagnostic questionnaire), the prevalence of PDN in 1,039 diabetic patients in secondary care was found to be 65% (15). Our current data indicate a large morbidity for neuropathic pain in a community-based diabetic population. They also challenge the dogma that painful neuropathic symptoms improve as the severity of neuropathy worsens and provide support for a previous study that actually demonstrated comparable prevalence of painful neuropathy in diabetic patients with mild and more severe neuropathy (12). Furthermore, approximately one-quarter of our patients without clinical neuropathy on examination had significant painful neuropathic symptoms, indicating the large disparity between signs and symptoms. But of course even patients with impaired glucose tolerance and no apparent neuropathy develop painful neuropathic symptoms and small nerve fiber damage (16). This emphasizes the need to ask all patients about the occurrence of painful neuropathic symptoms, not just those who have clinical neuropathy. Painful symptoms were twice as prevalent in type 2 versus type 1 diabetic patients, even after adjusting for differences in age, neuropathy, PAD, and other known risk factors for neuropathic pain (17). These data are consistent with a previous study that demonstrated a higher prevalence of clinical neuropathy in type 2 compared with type 1 diabetic patients, assessed using a combination of the NSS and NDS (2). Previously, the prevalence of PDN has not been found to differ between type 1 and type 2 diabetes, although the proportion of patients with type 1 diabetes was very small (9,15). Women had a 50% increased risk of painful symptoms compared with men. This has also been demonstrated recently in a study from Saudi Arabia (15). This latter study demonstrated no ethnic differences for the incidence of PDN (15). In the current study, however, we demonstrate a significantly higher prevalence of painful neuropathic symptoms in South Asians compared with Europeans and African Caribbeans, with a 50% increased risk of neuropathic pain in South Asians in the absence of clinical neuropathy. Paradoxically, we have previously demonstrated a lower prevalence of both large and small fiber neuropathy (18), as well as incidence of foot ulceration (14), in South Asians.
The major strength of this epidemiological study, compared with others, is that it is substantially larger than any previously published study on the prevalence of PDN. Furthermore, it is community based and therefore reflects the magnitude of this problem in a nonselected cohort of diabetic patients. As this study was designed to be totally inclusive for community-based patients with diabetes, we did not exclude patients with neuropathic pain from an etiology other than diabetes, or attempt to identify pain from a different origin. We did not assess the duration of pain, hence the prevalence of chronic pain (≥6 months) could not be established. The use of medications for the treatment of neuropathic pain was not recorded. We used the NSS as it is relatively quick to administer and is weighted toward positive neuropathic symptoms in the lower limbs, consistent with PDN. Indeed, three key lower-limb symptoms that characterize neuropathic from nonneuropathic pain are tingling pain, numbness, and increased pain due to touch (19) and are incorporated in the NSS. Numbness or “cotton wool-like feeling” is a positive, identifiable, painful symptom described by patients and thus different to loss of sensation, which patients may or may not be aware of. These symptoms were captured by the NSS along with a measure of the distribution, presence of nocturnal exacerbation, and relieving factors.
The demonstration that one-third of all diabetic patients have significant tingling/shooting, burning pain, with or without numbness, in the lower limbs indicates a larger morbidity than previously established in relatively small and selective studies in the U.K. (9,11) and in a recent larger, but selected, population-based study from Germany (17). Our finding that one-quarter of community patients without clinical neuropathy have painful neuropathic symptoms implies that a large proportion of the diabetic community are being neglected in the treatment of their symptoms, and that classic neuropathic, lower-limb symptoms may well be inappropriately considered “nonneuropathic” if there are no concomitant signs of clinical neuropathy. Davies et al. (8) also showed that a significant proportion (7.4%) of subjects with PDN using the Toronto Clinical Scoring System had no clinical signs of neuropathy. We have extended this observation in a large cohort of patients and shown that ∼40% of all patients without signs of neuropathy will have at least mild neuropathic symptoms.
To conclude, we have observed greater neuropathic pain levels in type 2 diabetes, in women, and in people of South Asian origin. These areas demand further investigation and also highlight key groups who may warrant screening for PDN.
Acknowledgments
Funding of the NWDFCS foot screening program was originally provided by the Department of Health.
No potential conflicts of interest relevant to this article were reported.
C.A.A. coordinated the study, monitored data collection, cleaned and analyzed data, and drafted and revised the manuscript. R.A.M. analyzed data and drafted and revised the manuscript. E.R.E.v.R. and J.K. designed the project and revised the manuscript. A.J.M.B. initiated and designed the project and drafted and revised the manuscript.
Parts of this study were presented orally at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
The authors would like to thank all original members of the NWDFCS who implemented the foot screening and data collection.
References
- 1.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–1724 [DOI] [PubMed] [Google Scholar]
- 2.Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993;36:150–154 [DOI] [PubMed] [Google Scholar]
- 3.Tesfaye S, Stevens LK, Stephenson JM, et al. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia 1996;39:1377–1384 [DOI] [PubMed] [Google Scholar]
- 4.Cabezas-Cerrato J; Neuropathy Spanish Study Group of the Spanish Diabetes Society (SDS) The prevalence of clinical diabetic polyneuropathy in Spain: a study in primary care and hospital clinic groups. Diabetologia 1998;41:1263–1269 [DOI] [PubMed] [Google Scholar]
- 5.Pop-Busui R, Lu J, Lopes N, Jones TL; BARI 2D Investigators Prevalence of diabetic peripheral neuropathy and relation to glycemic control therapies at baseline in the BARI 2D cohort. J Peripher Nerv Syst 2009;14:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbott CA, Carrington AL, Ashe H, et al. ; North-West Diabetes Foot Care Study The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med 2002;19:377–384 [DOI] [PubMed] [Google Scholar]
- 7.Bril V, England J, Franklin GM, et al. ; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2011;76:1758–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 2006;29:1518–1522 [DOI] [PubMed] [Google Scholar]
- 9.Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med 2004;21:976–982 [DOI] [PubMed] [Google Scholar]
- 10.Erbas T, Ertas M, Yucel A, Keskinaslan A, Senocak M; TURNEP Study Group Prevalence of peripheral neuropathy and painful peripheral neuropathy in Turkish diabetic patients. J Clin Neurophysiol 2011;28:51–55 [DOI] [PubMed] [Google Scholar]
- 11.Daousi C, Benbow SJ, Woodward A, MacFarlane IA. The natural history of chronic painful peripheral neuropathy in a community diabetes population. Diabet Med 2006;23:1021–1024 [DOI] [PubMed] [Google Scholar]
- 12.Veves A, Manes C, Murray HJ, Young MJ, Boulton AJ. Painful neuropathy and foot ulceration in diabetic patients. Diabetes Care 1993;16:1187–1189 [DOI] [PubMed] [Google Scholar]
- 13.Tapp RJ, Shaw JE, de Courten MP, Dunstan DW, Welborn TA, Zimmet PZ; AusDiab Study Group Foot complications in type 2 diabetes: an Australian population-based study. Diabet Med 2003;20:105–113 [DOI] [PubMed] [Google Scholar]
- 14.Abbott CA, Garrow AP, Carrington AL, Morris J, Van Ross ER, Boulton AJ; North-West Diabetes Foot Care Study Foot ulcer risk is lower in South-Asian and African-Caribbean compared with European diabetic patients in the U.K.: the North-West Diabetes Foot Care Study. Diabetes Care 2005;28:1869–1875 [DOI] [PubMed] [Google Scholar]
- 15.Halawa MR, Karawagh A, Zeidan A, Mahmoud AE, Sakr M, Hegazy A. Prevalence of painful diabetic peripheral neuropathy among patients suffering from diabetes mellitus in Saudi Arabia. Curr Med Res Opin 2010;26:337–343 [DOI] [PubMed] [Google Scholar]
- 16.Boulton AJ, Malik RA. Neuropathy of impaired glucose tolerance and its measurement. Diabetes Care 2010;33:207–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziegler D, Rathmann W, Meisinger C, Dickhaus T, Mielck A; KORA Study Group Prevalence and risk factors of neuropathic pain in survivors of myocardial infarction with pre-diabetes and diabetes. The KORA Myocardial Infarction Registry. Eur J Pain 2009;13:582–587 [DOI] [PubMed] [Google Scholar]
- 18.Abbott CA, Chaturvedi N, Malik RA, et al. Explanations for the lower rates of diabetic neuropathy in Indian Asians versus Europeans. Diabetes Care 2010;33:1325–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Backonja MM, Krause SJ. Neuropathic pain questionnaire—short form. Clin J Pain 2003;19:315–316 [DOI] [PubMed] [Google Scholar]