Abstract
OBJECTIVE
Determine the efficacy of a home-based walking intervention to improve walking ability and quality of life in people with diabetes and peripheral arterial disease (PAD).
RESEARCH DESIGN AND METHODS
We conducted a randomized, controlled, single-blind trial within university-affiliated clinics in our local community. We randomized 145 participants (45 women) with diabetes and PAD to our intervention—a 6-month behavioral intervention targeting levels of readiness to engage in routine walking for exercise—versus attention control. Our primary outcome was 6-month change in maximal treadmill walking distance. Secondary outcomes included 3-month change in maximal walking distance, lower limb function (i.e., walking impairment scores), quality of life (Medical Outcomes Short Form Survey), exercise behaviors, depressive symptoms, and self-efficacy at 3 and 6 months.
RESULTS
The mean age of participants was 66.5 (SD 10.1) years. Intervention and control groups did not differ significantly in 6-month change in maximal treadmill walking distance (average [SE] 24.5 [19.6] meters vs. 39.2 [19.6] meters; P = 0.60). Among secondary outcomes, for the intervention and control groups, respectively, average walking speed scores increased by 5.7 [2.2] units and decreased by 1.9 [2.8] units (P = 0.03); the mental health quality of life subscale score increased by 3.2 [1.5] and decreased by 2.4 [1.5] units (P = 0.01).
CONCLUSIONS
A home-based walking intervention did not improve walking distance but did improve walking speed and quality of life in people with diabetes and PAD. Clinicians should consider recommending home-based walking therapy for such patients.
Peripheral arterial disease (PAD) affects 20–30% of adults aged 50 years and older (1). Individuals with PAD have slower walking speed, reduced walking distance, and lower physical activity levels (2,3). These functional deficits can become severe, hindering the ability to live independently.
Modifiable risk factors for PAD include smoking, diabetes (DM), hypertension, and dyslipidemia (1). In diabetic patients, PAD risk increases by 26% for every 1 percentage point increase in glycosylated hemoglobin (4). Controlling risk factors (e.g., lowering hyperglycemia) is an important component of care for patients with PAD (5); however, improvement in lower limb function from risk factor control does not approach that of walking therapy. Supervised walking therapy for PAD reduces impairment by increasing walking distance, speed, and/or stair climbing (6).
Although the benefits of supervised walking therapy in PAD have been documented (4,7), these findings have limited generalizability. First, almost all trials of exercise therapy for patients with PAD have been efficacy studies of one type of walking intervention: supervised, hospital-based, treadmill exercise therapy. Walking sessions of up to 55 min are supervised by an exercise technician three times per week for 3 to 6 months. This involves high patient burden (scheduling, transportation) and substantial resources.
Another limit to generalizability is subject selection. Approximately 55% of people with PAD have DM (8), but patients with coexisting DM are typically underrepresented in PAD walking trials. One possible reason is the variety of leg symptom subtypes in this group. Among patients with DM and PAD, over 50% had atypical leg symptoms, whereas only 5% had intermittent claudication (2,8). However, people with intermittent claudication are commonly targeted for PAD walking trials.
Another complication in studying these patients is disease detection using the standard ankle-brachial index: with increased prevalence of calcified arteries, people with diabetes commonly warrant additional testing in screening for PAD (e.g., toe-brachial index) (9).
To fill these knowledge gaps, we developed a home-based walking therapy intervention composed of a counseling-for-exercise intervention (delivered biweekly), two walking training sessions with an instructor (delivered within 2 weeks of randomization), and individual and group walking in the community. We evaluated the intervention in a two-arm, 6-month, prospective, single-blind, randomized controlled trial enrolling patients with DM and symptomatic PAD, including those with atypical leg symptoms. We hypothesized patients with symptomatic PAD and DM randomized to a home-based walking intervention would have better maximal treadmill walking distance at 6 months than patients randomized to an attention-control group. Secondary outcomes were maximal treadmill walking distance at 3 months and 3- and 6-month time to onset of leg pain, quality of life, lower limb function (i.e., distance, speed, and stair climbing), depressive symptoms, and self-efficacy.
RESEARCH DESIGN AND METHODS
The study was funded by the American Diabetes Association and approved by the University of Minnesota Institutional Review Board. All participants provided informed consent.
Recruitment, eligibility, and screening
We recruited participants between January 2007 and March 2009 from clinics and communities in the Twin Cities metro area of Minnesota. Patients were either referred to the study by their physicians or self-referred from flyers distributed at health fairs, community centers, and churches; media advertisements; word of mouth; or postcards.
The study included men and women aged 40 years and older with a diagnosis of PAD (resting or postexercise ankle-brachial index [ABI] of <0.90, toe-brachial index ≤0.7 (9), or prior surgery for PAD with continued exertional leg symptoms not including joint pain); a diagnosis of DM type I or II (medical history of medication use or diet control for hyperglycemia); and leg symptoms at enrollment (as captured by the San Diego Claudication Questionnaire) (10). Additionally, we excluded anyone who indicated “no intention to start exercising in the next 6 months.” By excluding these individuals, we focused on determining the effectiveness of our intervention at improving functional outcomes versus motivating very sedentary patients.
We excluded participants with no available phone, foot or lower leg amputation, critical leg ischemia, or lower extremity revascularization within 6 months before enrollment. Because of possible adverse consequences of exercise, we excluded patients with a myocardial infarction within the preceding 3 months; evidence of significant coronary ischemia at a low workload as determined by exercise treadmill testing; systolic blood pressure greater than 180 mmHg or diastolic pressure greater than 110 mmHg; diagnosis of a life-threatening malignancy within the past year; or exercise tolerance limited by leg pain of nonvascular origin or other factors such as arthritis, dyspnea, dizziness, angina class 2 or higher, and chronic obstructive pulmonary disease.
Potential participants were screened through telephone interviews. Research staff assessed medical history and administered the San Diego Claudication Questionnaire. Additionally, staff administered the Physical Activity Readiness Questionnaire (PAR-Q), a 7-item questionnaire that detects signs and symptoms that contraindicate exercise (11). Eligibility was confirmed with treadmill testing (described below) and ABI measurements as described previously (8). For people with an ABI >1.3, indicating arterial calcification, we obtained toe-brachial indexes (9). Arm pressures were obtained using the same protocol for the ABI. Great toe pressures were obtained with a toe cuff and photoplethysmography (Summit Doppler Vista AVS 2007) to detect the systolic pressure at which blood flow returned.
Randomization
Eligible individuals were randomized to intervention or control using permuted blocks with randomized block sizes 2, 4, 6, or 8 to ensure equal numbers in both groups. Outcomes were analyzed according to the randomized allocation (intention-to-treat).
Standard care
All participants viewed a 7-min educational video about PAD and its clinical leg symptoms, life-threatening consequences of PAD (heart attack and stroke), other adverse outcomes (walking disability), and strategies for disease and risk factor management (smoking cessation, weight control, aerobic activity). After the video, each participant met face-to-face with the research coordinator. Participants were encouraged to ask questions about the video material. The coordinator queried participants regarding self-management behaviors (i.e., glucose monitoring, blood pressure monitoring) and gave them a calendar in which to document their daily glucose results, weekly blood pressures, and any routine lipid results provided by their primary care physician. Participants received parking vouchers for each visit and $30 for each of the 3- and 6-month assessment visits.
Intervention group procedures
Intervention group subjects participated in a home-based walking program with three components: 1) a one-on-one interaction with the research coordinator at baseline; 2) walking training and weekly group walking classes with an instructor; and 3) biweekly telephone calls for 6 months.
For the baseline interaction, discussion focused on the participant’s current stage of change (12), as determined from his or her responses to Part 1 of the Patient-centered Assessment and Counseling for Exercise (PACE) protocol (e.g., “I have been thinking of starting to exercise in the next 6 months” = 2; “I’ve been doing vigorous exercise 3 or more days per week for the last 6 months or more” = 8). For this study, we modified the original PACE assessment and counseling treatment manual (13) to address the specific walking recommendations for patients with PAD, rather than any form of exercise. The participant then completed Part 2 of the PACE protocol (one of three possible instruments depending on the score in Part 1) to better define specific factors that facilitate adherence to routine exercise (walking). For example, individuals with a PACE score of 2–4 were asked to write down the two main benefits they hoped to gain from being active and were asked to make a physical activity plan and check off roadblocks to routine exercise via walking (e.g., “I do not have time”). The coordinator reviewed each participant’s plans for walking routinely and discussed how to overcome roadblocks.
After the baseline visit, participants were scheduled to complete two 1-h walking training sessions, led by an experienced exercise instructor. These sessions served as reinforcement and facilitated treatment adherence (14). Sessions were held at the University of Minnesota or another location suitable for walking (e.g., a park). Session one was designed to facilitate interaction among participants. The exercise instructor asked participants to describe what they hoped to gain from walking for exercise. The group then discussed strategies for staying in the walking program. Session two was a practice walking session with the exercise instructor and one or more participants. For this session, participants listened to an audiotaped instructional aid developed by the American Heart Association.
Participants were then encouraged to walk 1 day per week with the study exercise instructor and other participants, as available, and to continue walking on their own at least 3 days per week for a minimum of 4 days of walking each week; participants were advised to walk 50 min total for each session and, using their pedometers, to increase the number of steps by 50 each session.
The first call contact was 2 weeks after randomization. During each biweekly call, intervention participants completed the PACE assessment and the Exercise Behaviors Questionnaire (described below) (15) and discussed their strategies for atherosclerotic risk factor control and adherence to walking during the past 2 weeks.
Attention control group procedures
Individuals randomized to the attention control group participated in twice-monthly phone calls with the research coordinator. During these 10- to 15-min calls, control group participants shared and discussed the information documented in their calendars on blood glucose, blood pressure, and cholesterol levels (if available) and their smoking habits, if applicable. The Exercise Behaviors Questionnaire was also administered.
Measures
At baseline only, the Lifestyle and Clinical Survey was administered to ascertain sociodemographics and comorbidities. It has a summary κ-statistic for reliability of 0.81 (95% confidence interval [CI] 0.78, 0.84) and a summary κ-statistic for validity of 0.58 (95% CI 0.52, 0.64) (16).
The primary outcome was change from baseline to 6 months in mean maximal treadmill walking distance, determined from using the Gardner-Skinner graded exercise treadmill test with electrocardiographic monitoring (17).
Secondary outcomes were change from baseline to 3 months in mean maximal treadmill walking distance and changes from baseline to 3 and 6 months in the measures listed below.
Walking Impairment Questionnaire.
We captured the participant’s ability to walk in the community (i.e., lower limb function) using the validated interviewer-administered Walking Impairment Questionnaire (18). This survey captures three domains: walking distance, walking speed, and stair-climbing capacity. The domains are scored on 0 to 100 scales, 0 representing complete inability to perform the task and 100 representing no limitations in walking short and long distances, walking at a fast pace, and climbing three flights of stairs.
Medical Outcomes Short Form Survey.
Health-related quality of life was measured using the Medical Outcomes Study (MOS) Short Form Survey (SF-36) (19). Each subscale is scored from 0 to 100; higher scores indicate more positive quality of life.
Geriatric Depression Score Short Form.
Depressive symptoms, which are associated with walking impairment in patients with PAD (20), were measured using the Geriatric Depression Score, a 15-item screening instrument. Scores of 10 or higher are considered positive for depression.
Self-efficacy.
We measured self-efficacy—self-confidence to perform certain activities to manage their disease—using the six-item Self-Efficacy for Managing Chronic Disease scale (21). The association of self-efficacy with walking ability, measured in these patients at baseline, is reported elsewhere (22).
Exercise Behaviors Questionnaire.
We administered the Stanford Patient Education Research Center Exercise Behavior Survey during each follow-up phone call. The exercise behaviors survey is a six-item instrument with questions regarding the type of activity and the length of time during which the patient engaged in that activity during the past week (23).
Adverse events
During each biweekly phone call, study staff asked whether the patient had developed chest pain, shortness of breath, or any symptoms requiring hospitalization in the past 2 weeks. If the participant responded yes, he/she was asked about diagnostic testing, final diagnosis, and whether the hospital physician stated the participant could continue in the study. The study staff also ascertained leg symptoms, testing for PAD (e.g., angiography), or invasive therapy for PAD.
Statistical analysis
Intervention and control groups were compared according to baseline characteristics using Fisher’s exact tests for categorical variables and t tests (two-sided) for continuous variables. P values less than 0.05 were deemed statistically significant. Analyses were done using SAS (v. 9.1.3; SAS Institute, Cary, NC). The treatment groups were compared according to change from baseline to 6- and 3-month follow-up using the same tests.
As a check for selection bias from dropouts, we performed longitudinal analyses of four outcomes, the primary outcome and three secondary outcomes of particular interest: treadmill walking distance, walking speed from the Walking Impairment Questionnaire, and the physical and mental health aggregates of SF-36. The analysis used a mixed linear model, including up to 3 cases per participant (baseline, 3 months, 6 months), using all available data. Treatment groups were compared according to change from baseline to the average of the 3- and 6-month visits, an average treatment effect over the two follow-ups. The longitudinal results were very similar to the simpler comparisons of changes by the 3- and 6-month visits and are reported briefly.
A priori, 64 patients per arm gave 80% power to detect a moderate standardized effect size of 0.50 (a difference between groups of 0.06 miles in average change in treadmill walking distance). This was increased to accommodate 20% attrition. Analyses of secondary outcomes were not adjusted for multiple comparisons.
RESULTS
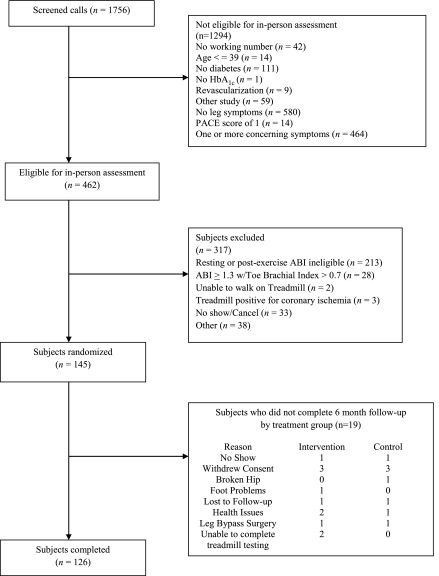
We telephone-screened 1,756 people and excluded 1,294 (Fig. 1). Of the 462 eligible for an in-person visit, we excluded 371 for lack of objective evidence of PAD or inability to complete treadmill testing. We enrolled 145 participants; 19 (13.1%) provided no treadmill outcome measures at the 6-month follow-up visit. Table 1 gives baseline characteristics of randomized subjects. Mean age was 66.5 (SD 10.1) years. Atherosclerotic risk factors were common: 106 (73%) participants were former or current smokers and 119 (82%) had hypertension. At baseline, 19 (26%) control participants used claudication medication as compared with six (8%) intervention participants. Table 1 also describes the study groups using baseline values of outcome measures.
Figure 1.
Study recruitment and retention.
Table 1.
Baseline characteristics by randomized group
| Control | Intervention | P value* | |
|---|---|---|---|
| n | 73 | 72 | |
| Age, mean (SD) | 66.8 (10.1) | 66.2 (10.2) | 0.735 |
| Female (%) | 20 (27) | 25 (35) | 0.373 |
| White (%) | 68 (94) | 63 (88) | 0.491 |
| African American (%) | 3 (4) | 5 (7) | |
| American Indian** | 1 (1) | 4 (6) | |
| Unknown | 1 (1) | 0 | |
| Education ≥ high school (%) | 65 (89) | 69 (96) | 0.208 |
| BMI, mean (SD) | 33.7 (7.0) | 35.0 (9.3) | 0.324 |
| HbA1c, mean (SD) | 7.2 (1.1) | 7.0 (1.3) | 0.388 |
| PACE score, mean (SD) | 3.7 (1.5) | 3.8 (1.4) | 0.533 |
| Prior myocardial infarction (%) | 16 (22) | 17 (24) | 0.845 |
| Current smoker (%) | 13 (18) | 7 (10) | 0.228 |
| Smoked at least 100 cigarettes during lifetime (%) | 52 (71) | 54 (75) | 0.281 |
| 0–4 cigarettes per day | 2 (4) | 6 (11) | |
| 5–15 cigarettes per day | 11 (21) | 14 (27) | |
| One pack per day | 17 (33) | 14 (27) | |
| More than 1 pack per day | 22 (42) | 17 (33) | |
| Resting ABI, mean (SD) | 0.94 (0.45) | 0.96 (0.38) | 0.807 |
| Renal insufficiency (%) | 5 (7) | 9 (13) | 0.275 |
| Hypertension (%) | 57 (78) | 62 (86) | 0.279 |
| High blood cholesterol (%) | 54 (74) | 54 (75) | 1.000 |
| Prior cerebrovascular event or transient ischemic event (%) | 5 (7) | 12 (17) | 0.076 |
| Medication use for claudication | 19 (26) | 6 (8) | 0.008 |
| Treadmill walk, maximum pain distance meters | 472.6 (238.9) | 422.7 (234.2) | 0.208 |
| Treadmill walk, onset of pain distance meters | 166.1 (169.6) | 149.1 (147.0) | 0.522 |
| Exercise behavior score | 78.3 (90.6) | 87.3 (100.1) | 0.401 |
| Walking distance | 43.9 (30.5) | 43.1 (33.5) | 0.873 |
| Walking speed | 43.7 (26.1) | 37.8 (23.5) | 0.159 |
| Stair climbing | 44.5 (30.4) | 43.5 (30.4) | 0.856 |
| Physical functioning§ | 54.2 (20.9) | 51.8 (21.7) | 0.501 |
| Role-physical | 53.8 (36.0) | 47.5 (38.9) | 0.315 |
| Bodily pain | 54.2 (28.6) | 49.0 (24.0) | 0.245 |
| General health | 53.0 (21.7) | 57.6 (20.3) | 0.187 |
| Vitality | 53.1 (20.5) | 54.1 (21.6) | 0.770 |
| Social functioning | 86.6 (20.0) | 85.4 (20.6) | 0.711 |
| Role-emotional | 80.1 (30.5) | 82.6 (31.1) | 0.628 |
| Mental health | 80.1 (16.3) | 79.7 (16.4) | 0.902 |
| Geriatric depression scale | 2.6 (2.9) | 2.5 (2.5) | 0.811 |
For the baseline outcome measures, data are mean (SD).
*Fisher’s exact test for categorical variables and t tests for continuous variables;
**two answered more than one category; t tests comparing means of the two randomized groups;
§physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health are captured by the MOS SF-36.
Overall, subjects walked a mean distance of 448 m (SD 237.1) on the treadmill at baseline. Considering changes from baseline to 6 months (Table 2), intervention and control groups did not differ significantly in the primary outcome, change in maximal treadmill walking distance (average [SE] change: control 39.2 [19.6] meters, intervention 24.5 [19.6]; P = 0.60), or change in treadmill walking distance until onset of pain (average [SE] change: control 52.3 [23.6] meters, intervention 66.7 [21.0]; P = 0.65). The intervention group had greater improvement than control subjects in two secondary outcomes, walking speed and mental health. The intervention group's average walking speed improved by 5.7 (2.2) percentage points, whereas the control group's average score decreased by 1.9 (2.8) percentage points (P = 0.034); and the intervention group's mental-health average score improved by 3.2 (1.5) units, compared with a decrease of 2.4 (1.5) units for the control group (P = 0.01). There was a nonsignificant trend toward greater improvement in quality of life (i.e., physical functioning and role-emotional) for the intervention group compared with the control group.
Table 2.
Comparison of intervention and control groups and change from baseline to 6 months
| Control | Intervention | P value* | |
|---|---|---|---|
| Treadmill walk, maximum pain distance meters | 39.2 (19.6) | 24.5 (19.6) | 0.598 |
| Treadmill walk, onset of pain distance meters | 52.3 (23.6) | 66.7 (21.0) | 0.651 |
| Exercise behavior score | 35.0 (13.3) | 55.3 (17.1) | 0.349 |
| Walking distance | 1.4 (3.3) | 5.6 (3.5) | 0.383 |
| Walking speed | −1.9 (2.8) | 5.7 (2.2) | 0.034 |
| Stair climbing | 2.9 (2.8) | 6.0 (3.5) | 0.487 |
| Physical functioning§ | −0.5 (1.9) | 4.5 (1.9) | 0.063 |
| Role-physical | −7.4 (5.6) | 4.0 (4.6) | 0.120 |
| Bodily pain | 2.2 (4.2) | 5.5 (3.7) | 0.568 |
| General health | 0.9 (1.6) | 1.6 (2.0) | 0.769 |
| Vitality | 1.2 (2.2) | 1.1 (2.2) | 0.985 |
| Social functioning | −4.2 (2.6) | −2.9 (2.5) | 0.717 |
| Role-emotional | −7.8 (4.3) | 2.9 (4.2) | 0.080 |
| Mental health | −2.4 (1.5) | 3.2 (1.5) | 0.010 |
| Geriatric depression scale | 0.0 (0.2) | −0.3 (0.2) | 0.380 |
Data are mean (SE).
*P value from a t test;
§physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health are captured by the MOS SF-36.
Results from the longitudinal analyses differed little from those in the simple analyses of change to 6- and 3-month follow-up. For example, in the longitudinal analysis of walking speed, the intervention group improved by 7.6 units more than the control group (SE 3.0, P = 0.013; compare with Table 2).
For our primary outcome, the effect of study treatment differed depending on whether the subjects were taking claudication medication at baseline (P = 0.0025 in a test of the interaction); overall, the study groups did not differ significantly in the primary outcome of maximal treadmill walking distance after adjusting for baseline use of claudication medication (P = 0.074). Additionally, there were no significant differences between study groups in 3 or 6-month changes in walking speed, physical functioning, mental health, physical component summary score, or mental component summary score after adjusting for baseline use of claudication medication.
No unanticipated adverse events were reported among randomized participants.
CONCLUSIONS
We have presented results from the first large-scale trial of a home-based walking intervention for people with DM and PAD. A 6-month home-based walking program did not improve the primary outcome of 6-month change in maximal treadmill walking distance compared with control. However, the 6-month home-based walking program did improve some secondary outcomes, specifically walking speed and 6-month physical functioning and mental health (i.e., role-emotional). These results suggest that home-based walking holds promise for improving walking speed and quality of life in people with DM and PAD.
Walking speed influences a patient’s ability to perform activities of daily living. Earlier studies of walking interventions in PAD demonstrated improvement in walking speed, but they were based on supervised treadmill walking interventions (24,25). Our intervention was home-based, with one weekly group walking class, and can be easily implemented in clinical practice.
An additional benefit of our intervention was improvement in mental health per the SF-36 quality of life measure. Prior trials (24,25) showed improved quality of life for people with PAD who completed a supervised treadmill walking program. We add to these prior studies by showing improved quality of life for people with PAD randomized to a 6-month home-based walking intervention.
Both physical and role-emotional functioning were improved for people randomized to the intervention. Although these improvements were not statistically significant, the changes highlight the need for development of more robust home-based walking interventions in future trials targeting people with PAD. When comparing intervention to control, there were trends toward benefit for several outcome measures including time to onset of pain, walking impairment parameters (i.e., distance, stair climbing), all quality of life subscale scores, and exercise behaviors. We found no differences between the intervention and control groups on changes from baseline to 6 months in the outcomes of self-efficacy or depressive symptoms. We did find an association of self-efficacy with walking ability at baseline (22), but the final results from the trial suggest that our intervention did not affect self-efficacy.
Strengths of this study include a 6-month intervention focused on home-based walking and patients with diabetes with PAD and either intermittent claudication or atypical leg symptoms. This latter group, people with DM and PAD with atypical leg symptoms, is often underrepresented in exercise trials for PAD. An additional strength of this study was the translation of an intervention initially developed for a primary-care clinical setting (i.e., PACE) into a community-based intervention to promote home-based walking in a high-risk population.
Limitations of the study include possible contamination of the attention control group, since the counselors were the same for both groups. Additionally, over 90% of participants enrolled in the study because they hoped to increase their walking. This was discovered in a close-out survey in which participants were asked about their perceptions of the study and whether enrolling in the study motivated them to walk. This finding highlights the need to carefully develop recruitment materials so the focus of intervention is not so apparent as to lead to participation bias. An additional limitation was our low enrollment of minority participants. We used various methods to increase minority participation (e.g., announcements on radio stations in which listening audiences were largely minorities, attendance at churches in which members were minorities) but, given that most study assessments required visiting the university and people in the community were not as willing to do that, less than 10% of our participants were ethnic minorities. Finally, at baseline, control participants were more likely to use claudication medication than intervention participants. This could have influenced the trend toward a greater change in the primary outcome for control as compared with intervention participants.
Our study is the first large-scale walking intervention trial in PAD to use home-based walking versus an attention control. We demonstrated that a home-based walking program can be used in patients with DM and PAD. Such a program may improve walking speed and quality of life in this high-risk population.
Acknowledgments
This work was funded by the American Diabetes Association (Clinical Research Project # 7-06-CR-10).
No potential conflicts of interest relevant to this article were reported.
T.C.C. wrote the manuscript and researched data. S.L. conducted most data analyses and reviewed and edited the manuscript. T.C. assisted with data collection. K.H. and M.L. assisted with data collection and reviewed and edited the manuscript. B.N. assisted with data collection. J.S.H. supervised data analysis, conducted data analyses, and reviewed and edited the manuscript.
Parts of this work were presented at the 33rd Annual Society of General Internal Medicine Meeting, Minneapolis, Minnesota, 28 April–1 May 2010.
The authors acknowledge the editorial assistance of Anne Marie Weber-Main (Department of Medicine, University of Minnesota).
Footnotes
Clinical trial reg. no. NCT00611988, clinicaltrials.gov.
References
- 1.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001;286:1317–1324 [DOI] [PubMed] [Google Scholar]
- 2.Collins TC, Petersen NJ, Suarez-Almazor M. Peripheral arterial disease symptom subtype and walking impairment. Vasc Med 2005;10:177–183 [DOI] [PubMed] [Google Scholar]
- 3.McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA 2004;292:453–461 [DOI] [PubMed] [Google Scholar]
- 4.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007;45(Suppl. S):S5-S67 [DOI] [PubMed]
- 5.Mohler ER, 3rd, Hiatt WR, Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation 2003;108:1481–1486 [DOI] [PubMed] [Google Scholar]
- 6.Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise training for claudication. N Engl J Med 2002;347:1941–1951 [DOI] [PubMed] [Google Scholar]
- 7.Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA 1995;274:975–980 [PubMed] [Google Scholar]
- 8.Collins TC, Petersen NJ, Suarez-Almazor M, Ashton CM. The prevalence of peripheral arterial disease in a racially diverse population. Arch Intern Med 2003;163:1469–1474 [DOI] [PubMed] [Google Scholar]
- 9.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ; American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary of a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol 2006;47:1239–1312 [DOI] [PubMed] [Google Scholar]
- 10.Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med 1996;1:65–71 [DOI] [PubMed] [Google Scholar]
- 11.Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can J Sport Sci 1992;17:338–345 [PubMed] [Google Scholar]
- 12.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 1983;51:390–395 [DOI] [PubMed] [Google Scholar]
- 13.Calfas KJ, Long BJ, Sallis JF, Wooten WJ, Pratt M, Patrick K. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med 1996;25:225–233 [DOI] [PubMed] [Google Scholar]
- 14.Friedman L, Furberg C, DeMets D. Participant Adherence. In Fundamentals of Clinical Trials. 3rd ed. New York, Springer-Verlag, 1999, p. 204–220 [Google Scholar]
- 15.Lorig K, Stewart A, Ritter P, Gonzalez V, Laurent D, Lynch J. Outcome Measures for Health Education and other Health Care Interventions. Thousand Oaks, Calif., Sage Publications, 1996 [Google Scholar]
- 16.Collins T, O'Malley K, Petersen N, Suarez-Almazor M. The Lifestyle and Clinical Survey: A pilot study to validate a medical history questionnaire. Fed Pract 2005;22:25–26, 29–32, 38–46 [Google Scholar]
- 17.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs. single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc 1991;23:402–408 [PubMed] [Google Scholar]
- 18.Regensteiner J, Steiner J, Panzer R, Hiatt W. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. Vasc Med 1990;2:142–152 [Google Scholar]
- 19.Ware JE, Jr, Gandek B. Methods for testing data quality, scaling assumptions, and reliability: the IQOLA Project approach. International Quality of Life Assessment. J Clin Epidemiol 1998;51:945–952 [DOI] [PubMed] [Google Scholar]
- 20.McDermott MM, Greenland P, Guralnik JM, et al. Depressive symptoms and lower extremity functioning in men and women with peripheral arterial disease. J Gen Intern Med 2003;18:461–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract 2001;4:256–262 [PubMed] [Google Scholar]
- 22.Collins TC, Lunos S, Ahluwalia JS. Self-efficacy is associated with walking ability in persons with diabetes mellitus and peripheral arterial disease. Vasc Med 2010;15:189–195 [DOI] [PubMed] [Google Scholar]
- 23.Lorig K, Stewart A, Ritter P, Gonzalez V, Laurent D, Lynch J. Outcome Measures for Health Education and other Health Care Interventions. In Standford Chronic Disease Self-Management Study Thousand Oaks, Calif., Sage Publications, 1996, p. 24–25 [Google Scholar]
- 24.McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA 2009;301:165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regensteiner JG, Steiner JF, Hiatt WR. Exercise training improves functional status in patients with peripheral arterial disease. J Vasc Surg 1996;23:104–115 [DOI] [PubMed] [Google Scholar]