Abstract
OBJECTIVE
There is limited evidence on how intensive multifactorial treatment (IT) improves outcomes of diabetes when initiated in the lead time between detection by screening and diagnosis in routine clinical practice. We examined the effects of early detection and IT of type 2 diabetes in primary care on the prevalence of diabetic peripheral neuropathy (DPN) and peripheral arterial disease (PAD) 6 years later in a pragmatic, cluster-randomized parallel group trial.
RESEARCH DESIGN AND METHODS
A stepwise screening program in 190 general practices in Denmark was used to identify 1,533 people with type 2 diabetes. General practices were randomized to deliver either IT or routine care (RC) as recommended through national guidelines. Participants were followed for 6 years and measures of DPN and PAD were applied.
RESULTS
We found no statistically significant effect of IT on the prevalence of DPN and PAD compared with RC. The prevalence of an ankle brachial index ≤0.9 was 9.1% (95% CI 6.0–12.2) in the RC arm and 7.3% (5.0–9.6) in the IT arm. In participants tested for vibration detection threshold and light touch sensation, the prevalence of a least one abnormal test was 34.8% (26.7–43.0) in the RC arm and 30.1% (24.1–36.1) in the IT arm.
CONCLUSIONS
In a population with screen-detected type 2 diabetes, we did not find that screening followed by IT led to a statistically significant difference in the prevalence of DPN and PAD 6 years after diagnosis. However, treatment levels were high in both groups.
Diabetes is increasingly considered suitable for screening (1). However, even though modeling studies suggest that screening may be cost effective, there are several critical uncertainties (2). In particular, there is limited evidence that benefit estimates obtained from studies of clinically detected type 2 diabetes also apply to screen-detected populations. The multicenter Anglo-Danish-Dutch Study of Intensive Treatment in People With Screen-Detected Diabetes in Primary Care (ADDITION) was set to obtain this evidence base. The ADDITION study showed that an intervention to promote target-driven, intensive management of patients with screen-detected type 2 diabetes was associated with a nonstatistically significant 17% relative reduction in the incidence of a composite cardiovascular event end point over 5 years (3). There is limited trial evidence regarding prevention of diabetic peripheral neuropathy (DPN) and peripheral arterial disease (PAD) in people with diabetes. As current knowledge on PAD and DPN in diabetes has been gained in patients with clinically diagnosed and sometimes longstanding diabetes, and as the prevalence of PAD and DPN in patients with screen-detected diabetes is unknown, our aim was to describe the effect of early detection and intensive multifactorial treatment (IT) on the prevalence of DPN and PAD in patients with screen-detected type 2 diabetes in the Danish arm of the ADDITION study.
RESEARCH DESIGN AND METHODS
Design
The design and rationale of the ADDITION study have been reported (4). In brief, ADDITION-Denmark consists of two phases: 1) a screening phase and 2) a pragmatic, cluster-randomized parallel group trial. In five regions of Denmark, 744 general practices were invited to participate and 190 agreed and were randomized to screening plus routine care (RC) of diabetes or screening followed by IT. Randomization was stratified by region and the number of full-time general practitioners per practice.
A population-based stepwise screening program among people aged 40–69 years without known diabetes was undertaken, and individuals were diagnosed with diabetes according to World Health Organization (WHO) criteria, as previously described (5). Overall 1,533 (RC, 623; IT, 910) eligible participants with screen-detected diabetes agreed to take part in the trial. After an average of 6 years of follow-up, 1,278 participants were re-examined. One hundred eight people were only seen by their own general practitioner and nine people were not examined with the tests included in the present analysis and did not answer the questionnaires. These participants were excluded from our analysis, yielding a study sample of 1,161 participants for the analysis presented in this paper. Supplementary Fig. A1 displays the practice and participant flow.
Intervention
The specific characteristics of the interventions to promote IT have been described previously in detail (4). The purpose of the IT was to provide the best possible evidence-based treatment in primary care. We aimed to educate and support general practitioners and practice nurses in target-driven management (using medication and promotion of healthy lifestyle) of hyperglycemia, blood pressure, and cholesterol, based on the stepwise regimen used in the Steno-2 study (6). Intensive treatment was promoted through the addition of several features to existing diabetes care. Practice staff was provided with educational materials for patients, and patients were sent reminders if annual check-up appointments were overdue. Practices received additional funding to support the delivery of extra care added to the usual care and consultations. All treatment targets and algorithms were based on evidence from randomized controlled trials demonstrating the benefits of IT on cardiovascular risk factors in people with type 2 diabetes (3) and are displayed in Supplementary Table A2. Although targets for treatment were specified and classes of medication recommended, decisions on prescriptions, including choice of individual drugs, were made by practitioners and patients.
In the RC group, general practitioners were only provided with diagnostic test results. Patients with screen-detected diabetes received the standard pattern of diabetes care according to the Danish national recommendations (7,8).
Measurements
Health assessments at baseline and follow-up included biochemical, anthropometric, and questionnaire measures and were undertaken by centrally trained staff unaware of study group allocation, according to standard operating procedures. Blood pressure was calculated as the mean of three measurements performed after at least 10-min rest, while participants were seated with the cuff on the right arm resting at the level of the heart, using Omron blood pressure recorders. Height and weight were measured in light indoor clothing, without shoes, using a fixed rigid stadiometer and a Tanita scale, respectively.
All biochemical measures were analyzed at baseline and follow-up at the Aarhus University Hospital and Steno Diabetes Center. The specific analyses have been described previously. Microalbumiuria was defined as an albumin/creatinin ratio on spot urine: men, 2.5–25 mg/mmol; women, 3.5–25 mg/mmol; macroalbuminuria, >25 mg/mmol (9).
Light touch sensory testing was performed after a standardized protocol at four test sites on each foot (plantar aspect of the great toe and first, third, and fifth metatarsal heads) using a Semmes-Weinstein 10 g/5.07 monofilament (Bailey Instruments, Manchester, U.K.). Inability to feel one or more of the test sites was considered to be abnormal (10). This is a single set level used to detect unequivocal insensitivity. Vibration detection threshold (VDT) was determined on the dorsal side of the great toe proximal to the nail on both feet using a CASE IV and the 4-2-1 stepping algorithm (11), following a standard protocol. If the patient failed to comply with the null stimuli twice, the test was rerun once after reinstruction of the patient. If the patient again did not comply with the null value twice, the test on that foot was aborted. The CASE IV system uses a set of 25 standardized vibratory levels, and for each test the computer calculates the “just noticeable difference” (JND) from the subject’s responses. If the participant was unable to detect the highest vibration level, VDT was set at 26 JND. The CASE IV system also expresses the VDT values in normal deviates and percentiles as compared with a normal population based on age, sex, height, and weight. Values ≥95th percentile were considered abnormal (12). Ankle brachial index (ABI) was measured by trained technicians using a Doppler MiniD900 med Huntleigh Transducer EZ8 Widebeam. After a 10-min rest in supine position, brachial systolic blood pressure was measured twice on both arms. Then the distal systolic blood pressure was determined twice (a third time if there was more than 10 mmHg difference) in the arteria dorsalis pedis and the arteria tibialis posterior, alternating on each foot. ABI for each foot was calculated from the highest distal blood pressure divided by the highest brachial blood pressure from either side (13). Low ABI was defined as 0.9 or less (13).
Standardized self-report questionnaires were used to collect information on socio-demographic characteristics (education, employment, and ethnicity), lifestyle habits (smoking status and alcohol consumption), and self-reported cardiovascular disease (previous myocardial infarction, stroke, or operation/instrumentation on the heart). The patients were asked to answer the questionnaire part of the Michigan Neuropathy Screening Instrument (MNSI) and scored accordingly. A score ≥7 was considered abnormal (14). Patients also completed the Brief Pain Inventory short form (15). Patients were defined as having painful diabetic neuropathy if they indicated having pain in both legs (from knees down) and/or both arms (from elbow down).
ABI was done on all patients in three centers (Holstebro, Aarhus, and Copenhagen), and it was possible to calculate ABI in both legs in 836 out of 863 patients. Monofilament was done on all patients in two centers (Aarhus and Steno, 635 participants); 618 had data from all eight test sites. CASE IV was introduced in the follow-up examination from 8 September 2009 in Aarhus and from 22 September 2009 at Steno Diabetes Center. Three hundred ninety-nine participants were examined with CASE IV, in 28 of whom data were not obtained from both feet (20 aborted tests due to incompliance with null stimuli, and 8 had different disabilities, making CASE IV impossible to test in both legs). Forty-six participants were not measured with CASE IV even though the machine was available. One thousand one hundred forty eight participants returned the supplementary questionnaire containing the Michigan neuropathy questionnaire and Brief Pain Inventory short form.
The study was approved by local ethics committees in each center and was conducted in accordance with the principles of the 1996 Helsinki Declaration. All participants provided informed consent.
Statistical analysis
Patient characteristics at baseline and at follow-up are presented as unadjusted means or, in case of skewed distributions, as medians. Patient groups that were examined with different measures (CASE IV, monofilament, or ABI) were compared with all examined patients with the use of Student t test or the Mann-Whitney U test where appropriate. In case of unequal variances, means were compared by approximate inference based on the t distribution. Participants with missing values (CASE IV, monofilament, or ABI) were compared with persons without missing values. Participants with an aborted test on CASE IV were compared with participants without an aborted test. The prevalence for each treatment group of DPN and PAD was calculated as the number of participants with abnormal tests divided by the number who participated in that specific test and did not have a missing value. Data are presented with 95% CI. Odds ratios of an effect of IT as compared with RC were calculated using logistic regression taking cluster effect (general practitioner practice) into account. The mean of the normal deviates of VDT in each intervention group was compared using Student t test. Statistical analyses were performed with Stata software (version 11, StataCorp LP, College Station, TX).
RESULTS
Baseline characteristics are shown in Table 1. The average follow-up time was 5.8 years and 5.9 years for RC and IT, respectively. Median HbA1c did not change over the follow-up period in either group, but there was a similar significant decline in systolic blood pressure and total cholesterol in both groups. There was an overall increase in the proportion of participants using medication during the follow-up time. In the RC group, the proportion taking antihypertensive drugs increased from 44 to 77% at follow-up, whereas lipid-lowering drugs increased from 19 to 73%. In the IT group, the corresponding proportions increased from 47 to 80% and from 23 to 81%. At follow-up, antiglycemic drugs were taken by 52% in the RC group and 63% in the IT group. Numbers of smokers significantly declined in both groups with no difference between groups, and the total alcohol consumption also declined. There was no change in BMI from baseline. Patient characteristics of participants at follow-up are summarized in Supplementary Table A3.
Table 1.
Patient characteristics at baseline
| Patient characteristics | RC | IT |
|---|---|---|
| No. of patients | 459 | 702 |
| Male, N (%) | 269 (59) | 421 (60) |
| Age (years) | 59.9 (6.8) | 59.6 (6.9) |
| Glycosylated hemoglobin (% of hemoglobin)* | 6.4 (6.0; 7.0) | 6.4 (6.0; 7.0) |
| Systolic blood pressure (mmHg) | 149.8 (19.3) | 147.0 (19.1) |
| Diastolic blood pressure (mmHg) | 88.3 (11.3) | 87.3 (10.6) |
| Weight (kg) | F: 82.8 (17.0); M: 93.7 (15.9) | F: 84.6 (18.3); M: 94.2 (15.9) |
| Height (cm) | F: 162.6 (5.9); M: 175.6 (6.7) | F: 163.7; M: 175.9 (6.7) |
| BMI | F: 31.2 (6.0); M: 30.4 (4.4) | F: 31.5 (6.5); M: 30.4 (3.4) |
| Total cholesterol (mmol/L) | 5.77 (1.14) | 5.58 (1.08) |
| HDL (mmol/L) | 1.40 (0.34) | 1.36 (0.36) |
| Triglycerides (mmol/L)* | 1.6 (1.1; 2.4) | 1.6 (1.1; 2.4) |
| Smoking daily, N (%) | 134 (30) | 215 (31) |
| Smoking less than daily, N (%) | 169 (37) | 271 (39) |
| Alcohol (units per week)* | 6 (2; 14) | 6 (2; 14) |
| Microalbuminuria, N (%) | 52 (13) | 78 (13) |
| Macroalbuminuria, N (%) | 6 (2) | 14 (2) |
| Antihypertensive drugs (%) | 200 (44) | 333 (47) |
| Lipid-lowering drugs (%) | 87 (19) | 162 (23) |
Data are mean (SD), unless otherwise indicated. Patient characteristics at baseline of participants in the Danish arm of ADDITION, who completed a neuropathy questionnaire and/or were tested for DPN or PAD at follow-up.
*Median (25th; 75th percentile). F, female; M, male.
We found no substantial differences between subgroups and the total patient population, and no differences between participants with missing values compared with participants with no missing values, except for a few small differences in the CASE IV subgroup. The CASE IV–examined group was slightly younger (RC, 58.5 years; IT, 58.3 years at baseline) and had a shorter diabetes duration (RC, 5.1 years; IT, 5.4 years). The 46 patients not tested with CASE IV, even though the test was available at that time, had a significantly higher BMI and larger waist circumference. The 31 tested patients with missing data on CASE IV were significantly older but otherwise did not differ from the other patients tested with CASE IV (data not shown).
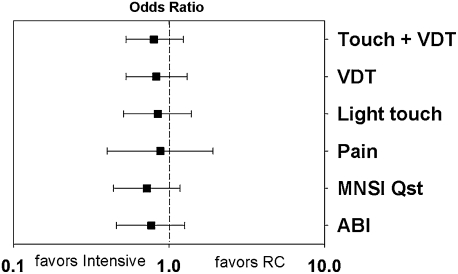
The prevalence of low ABI and different measures of DPN are presented in Table 2. The effect of intervention is expressed as an odds ratio and presented in Fig. 1. The means of the normal deviates of VDT in each foot were higher in the RC group compared with the IT group, but the difference was not statistically significant.
Table 2.
Prevalence (% of examined population) of different measures of DPN and low ABI in RC or IT group
| Variable | N | RC (95% CI) | IT (95% CI) |
|---|---|---|---|
| ABI ≤0.9 (%) | 329/507 | 9.1 (6.0; 12.2) | 7.3 (5.0; 9.6) |
| Light touch, 1/8 (%) | 231/387 | 20.3 (15.3; 26.1) | 17.8 (14.1; 22.0) |
| VDT, >95th percentile (%) | 136/235 | 25.7 (18.3; 33.2) | 22.6 (17.2; 28.1) |
| Light touch + VDT (%) | 135/229 | 34.8 (26.7; 43.0) | 30.1 (24.1; 36.1) |
| MNSI Qst, cut ≥7 | 430/656 | 9.3 (6.5; 12.1) | 8.7 (6.5; 10.9) |
| Pain (%) | 400/581 | 4.5 (2.5; 6.5) | 4.6 (2.9; 6.4) |
Light touch sensation tested with 10 g monofilament. MNSI Qst, MNSI questionnaire; pain, distal peripheral diabetic pain defined by self-reported bilateral pain distal from knees or elbows.
Figure 1.
Effect of IT in general practice as compared with RC expressed in odds ratios taking cluster effect into account. Light touch sensation tested with 10 g monofilament. MNSI Qst, MNSI questionnaire ≥7; pain, distal peripheral diabetic pain defined by self-reported bilateral pain distal from knees or elbows.
CONCLUSIONS
We found no statistically significant effect of IT in general practice, delivered through education and support of general practitioners and practice nurses in evidence-based, target-driven management and strict treatment targets/algorithms, on the prevalence of DPN and PAD in people with screen-detected type 2 diabetes as compared with RC delivered according to the national recommendations.
Intervention studies in people with type 2 diabetes have so far only found modest effects on the prevention of DPN. The UK Prospective Diabetes Study indicated a benefit of tighter glycemic control on microvascular complications, but there was no effect on surrogate end points of DPN (16). In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, intensive glycemic control only had a modest positive impact on the development of neuropathy (17), with a significant difference between treatment groups in light touch measured with monofilament but no differences in MNSI >2 or loss of vibration sense measured with a 128-Hz tuning fork alone. In the Steno-2 study, there was no difference between the treatment groups in the development of peripheral neuropathy measured with biothesiometry (6). With regard to treatment, the inference from the UK Prospective Diabetes Study was that dyslipidemia and blood pressure were potentially modifiable risk factors for the development of peripheral vascular disease in people with type 2 diabetes (18). Prospective data from the Fremantle Diabetes study suggested that treatment with statins or fibrates may protect against the development of DPN (19).
In our study of patients with screen-detected diabetes in primary care, we did not find that IT had a significant impact on the prevalence of DPN or PAD. However, all observed risk estimates favored multifactorial intervention at a nonstatistically significant level. This finding might be due to the relatively early stage in the disease process at which patients with previously undiagnosed diabetes are picked up by screening. It is conceivable that at this stage where the progression of the disease process underlying PAD and DPN is also at its initial stages, a longer duration of IT is necessary to observe an effect. A future repeated examination of our participants after a longer follow-up period will show whether this is the case. It should also be remarked that in the ADDITION study, which was pragmatic in design, both treatment groups saw a marked improvement in treatment between baseline and follow-up as general population treatment guidelines developed in the same time period (7,8). Consequently, the treatment levels did not differ as much between RC and IT groups as was expected at study inception.
Current knowledge on PAD and DPN in diabetes has been gained in patients with traditionally diagnosed and sometimes longstanding diabetes. Reported prevalence of DPN and PAD estimates vary widely, most likely depending on differences in patient selection, definition, and methods of assessment. In Germany, the MONICA/KORA Augsburg surveys found the prevalence of DPN to be 28% using the MNSI score >2, and the prevalence of ABI <0.9 to be 16% in 195 patients with diabetes (20). At baseline in the Australian Fremantle Diabetes study, the prevalence of PAD (ABI <0.9) was 13.6% in 1,181 patients assessed for PAD, but the authors state that this is a conservative estimate, as 113 unclassified patients had higher levels of risk factors (21). In the same cohort, the prevalence of DPN using MNSI score >2 was 30.9% (19). A recent Swedish population–based study including 156 patients found a prevalence of DPN of 34% based on inability to feel three out of four test sites with the monofilament or feel a neurothesiometer at a cut point of >25 V (22).
A prevalence study in a U.K. general practice reported the prevalence of painful diabetic neuropathy to be 26.4% (23), but estimates on the prevalence of painful neuropathy are sparse and vary from 8 to 26% in diabetic populations (24).
It is challenging to compare prevalences, especially DPN. But having all objections in mind, our prevalences of PAD and different measures of DPN in our RC group seem slightly lower than previously reported in other studies. This could be a reflection of screening identifying patients earlier in the trajectory of diabetes and/or that the overall diabetes treatment has improved. However, it is more important to stress that our findings show that even in this group of patients at an early stage of type 2 diabetes, DPN and PAD are present in a sufficiently large proportion of patients to warrant close clinical attention.
ADDITION-Europe is the first international study on the effects of early detection and IT of type 2 diabetes. Only the ADDITION-Denmark population was examined for PAD and DPN, and this study, presenting prevalence in a screen-detected population, is therefore unique. All patients were identified and treated in primary care, and the trained personnel used broadly accepted and standardized methods for diagnosis. This study therefore provides a solid overview of the size of the problem of PAD and DPN in this highly relevant population. At baseline there was a very low level of retinopathy (25), but we did not measure our outcome characteristics during the baseline assessment and can therefore not calculate incidence rates. However, we can assume that the prevalence of abnormal DPN and PAD at baseline was equally distributed among the randomization groups. The reported prevalence differences can therefore be interpreted as differences in cumulative incidence during the follow-up period.
Our study has some selection problems. More patients were included in the intensive care groups, even though there was no difference in number and types of practices in the randomization groups. Randomization of practices was performed before the screening and inclusion of patients started. But it seems that the intervention, which included training doctors and nurses in diabetes treatment, enhanced the focus in the IT group on screening and including patients. Not all patients were tested with all of our measures, but, except for the CASE IV subgroup, we found no substantial difference between those with and without measurement. We are therefore confident that the findings in these groups can be considered representative for all included patients. The patients tested with CASE IV were slightly younger with shorter diabetes duration. As older age and longer diabetes duration are associated with higher levels of DPN, it is possible that our results on VDT slightly underestimate the prevalence in both treatment groups. It is also possible that the small number of aborted VDT tests could be associated with decreased or eradicated sensibility and therefore caused a slight underestimation of the prevalence of VDT. Ideally, summed nerve conduction measures would have been the most objective criterion of DPN.
In a population of people with screen-detected type 2 diabetes, education and support of general practitioners and practice nurses in evidence-based, target-driven management and strict treatment targets/algorithms did not cause a statistically significant difference in the prevalence of DPN and PAD 6 years after diagnosis, as compared with RC delivered according to the national recommendations. The high prevalence of PAD and different measures of DPN in our RC group seem to be only slightly lower than previously reported values among people with clinically diagnosed diabetes. As both DPN and PAD are associated with the development of foot ulcers and cardiovascular disease, clinicians should be aware of these high prevalences when dealing with patients with screen-detected diabetes.
Supplementary Material
Acknowledgments
ADDITION-Denmark was supported by the National Health Services in the counties of Copenhagen, Aarhus, Ringkøbing, Ribe, and South Jutland in Denmark; the Danish Council for Strategic Research; the Danish Research Foundation for General Practice; Novo Nordisk Foundation; the Danish Center for Evaluation and Health Technology Assessment; the Diabetes Fund of the National Board of Health; the Danish Medical Research Council; and the Aarhus University Research Foundation. The trial has been given unrestricted grants from Novo Nordisk AS, Novo Nordisk Scandinavia AB, Novo Nordisk UK, ASTRA Denmark, Pfizer Denmark, GlaxoSmithKline Pharma Denmark, Servier Denmark A/S, and HemoCue Denmark A/S. Parts of the grants from Novo Nordisk Foundation, Danish Council for Strategic Research, and Novo Nordisk were transferred to the other centers. K.B.-J. holds stock in Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
M.C. wrote the manuscript and collected and researched data. N.E. and D.R.W. collected data and reviewed and edited the manuscript. K.B.-J. and T.L. designed the study, reviewed and edited the manuscript, and obtained the grants. A.S. designed the study, collected data, reviewed and edited the manuscript, and obtained the grants.
Parts of this study were presented in oral form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
Footnotes
Clinical trial reg. no. NCT00237549, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0903/-/DC1.
References
- 1.Wareham NJ, Griffin SJ. Should we screen for type 2 diabetes? Evaluation against National Screening Committee criteria. BMJ 2001;322:986–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glümer C, Yuyun M, Griffin S, et al. What determines the cost-effectiveness of diabetes screening? Diabetologia 2006;49:1536–1544 [DOI] [PubMed] [Google Scholar]
- 3.Griffin SJ, Borch-Johnsen K, Davies MJ, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet 2011;378:156–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauritzen T, Griffin S, Borch-Johnsen K, Wareham NJ, Wolffenbuttel BH, Rutten G; Anglo-Danish-Dutch Study of Intensive Treatment in People with Screen-Detected Diabetes in Primary Care The ADDITION study: proposed trial of the cost-effectiveness of an intensive multifactorial intervention on morbidity and mortality among people with type 2 diabetes detected by screening. Int J Obes Relat Metab Disord 2000;24(Suppl. 3):S6–S11 [DOI] [PubMed] [Google Scholar]
- 5.Sandbaek A, Griffin SJ, Rutten G, et al. Stepwise screening for diabetes identifies people with high but modifiable coronary heart disease risk. The ADDITION study. Diabetologia 2008;51:1127–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–393 [DOI] [PubMed] [Google Scholar]
- 7.Royal College of General Practitioners in Denmark. Type 2 Diabetes in General Practice—Diagnosis and Treatment Copenhagen, Denmark, Royal College of General Practitioners, 1999
- 8.Royal College of General Practitioners in Denmark. Type 2 Diabetes in General Practice—An Evidence Based Guideline Copenhagen, Denmark, Royal College of General Practitioners, 2004
- 9.Mogensen CE. Microalbuminuria in perspectives. In Diabetic Renal-Retinal Syndrome: Pathogenesis and Management, Update 2002. Friedman EA, L'Esperance FA, Eds. London, Klüwer, 2002, p. 105–119 [Google Scholar]
- 10.Feng Y, Schlosser FJ, Sumpio BE. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg 2009;50:675–682 [DOI] [PubMed]
- 11.Dyck PJ, O’Brien PC, Kosanke JL, Gillen DA, Karnes JL. A 4, 2, and 1 stepping algorithm for quick and accurate estimation of cutaneous sensation threshold. Neurology 1993;43:1508–1512 [DOI] [PubMed] [Google Scholar]
- 12.Dyck PJ, O’Brien PC, Litchy WJ, Harper CM, Daube JR, Dyck PJ. Use of percentiles and normal deviates to express nerve conduction and other test abnormalities. Muscle Nerve 2001;24:307–310 [DOI] [PubMed] [Google Scholar]
- 13.Hirsch AT, Haskal ZJ, Hertzer NR, et al. , American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease); American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463–e465 [DOI] [PubMed] [Google Scholar]
- 14.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–1289 [DOI] [PubMed] [Google Scholar]
- 15.Cleeland C. Pain assessment in cancer. In Effect of Cancer on Quality of Life. Osobo D, Ed. Boca Raton, FL, CRC Press, 1991, p. 293–305 [Google Scholar]
- 16.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 17.Ismail-Beigi F, Craven T, Banerji MA, et al. ; ACCORD trial group Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adler AI, Stevens RJ, Neil A, Stratton IM, Boulton AJ, Holman RR. UKPDS 59: hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes Care 2002;25:894–899 [DOI] [PubMed] [Google Scholar]
- 19.Davis TM, Yeap BB, Davis WA, Bruce DG. Lipid-lowering therapy and peripheral sensory neuropathy in type 2 diabetes: the Fremantle Diabetes Study. Diabetologia 2008;51:562–566 [DOI] [PubMed] [Google Scholar]
- 20.Ziegler D, Rathmann W, Meisinger C, Dickhaus T, Mielck A; KORA Study Group Prevalence and risk factors of neuropathic pain in survivors of myocardial infarction with pre-diabetes and diabetes: the KORA Myocardial Infarction Registry. Eur J Pain 2009;13:582–587 [DOI] [PubMed] [Google Scholar]
- 21.Norman PE, Davis WA, Bruce DG, Davis TM. Peripheral arterial disease and risk of cardiac death in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care 2006;29:575–580 [DOI] [PubMed] [Google Scholar]
- 22.Kärvestedt L, Mårtensson E, Grill V, et al. Peripheral sensory neuropathy associates with micro- or macroangiopathy: results from a population-based study of type 2 diabetic patients in Sweden. Diabetes Care 2009;32:317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 2006;29:1518–1522 [DOI] [PubMed] [Google Scholar]
- 24.Ziegler D. Painful diabetic neuropathy: treatment and future aspects. Diabetes Metab Res Rev 2008;24(Suppl. 1):S52–S57 [DOI] [PubMed] [Google Scholar]
- 25.Bek T, Lund-Andersen H, Hansen AB, Johnsen KB, Sandbaek A, Lauritzen T. The prevalence of diabetic retinopathy in patients with screen-detected type 2 diabetes in Denmark: the ADDITION study. Acta Ophthalmol 2009;87:270–274 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.