Abstract
OBJECTIVE
We sought to define 24-h glycemia in normal-weight and obese pregnant women using continuous glucose monitoring (CGM) while they consumed a habitual and controlled diet both early and late in pregnancy.
RESEARCH DESIGN AND METHODS
Glycemia was prospectively measured in early (15.7 ± 2.0 weeks’ gestation) and late (27.7 ± 1.7 weeks’ gestation) pregnancy in normal-weight (n = 22) and obese (n = 16) pregnant women on an ad libitum and controlled diet. Fasting glucose, triglycerides (early pregnancy only), nonesterified fatty acids (FFAs), and insulin also were measured.
RESULTS
The 24-h glucose area under the curve was higher in obese women than in normal-weight women both early and late in pregnancy despite controlled diets. Nearly all fasting and postprandial glycemic parameters were higher in the obese women later in pregnancy, as were fasting insulin, triglycerides, and FFAs. Infants born to obese mothers had greater adiposity. Maternal BMI (r = 0.54, P = 0.01), late average daytime glucose (r = 0.48, P < 0.05), and late fasting insulin (r = 0.49, P < 0.05) correlated with infant percentage body fat. However, early fasting triglycerides (r = 0.67, P < 0.001) and late fasting FFAs (r = 0.54, P < 0.01) were even stronger correlates.
CONCLUSIONS
This is the first study to demonstrate that obese women without diabetes have higher daytime and nocturnal glucose profiles than normal-weight women despite a controlled diet both early and late in gestation. Body fat in infants, not birth weight, was related to maternal BMI, glucose, insulin, and FFAs, but triglycerides were the strongest predictor. These metabolic findings may explain higher rates of infant macrosomia in obese women, which might be targeted in trials to prevent excess fetal growth.
It is increasingly recognized that obese women, with presumably normal glucose tolerance (NGT), exhibit similar adverse perinatal complications and fetal overgrowth to women with gestational diabetes. Obesity has been identified by the American College of Obstetricians and Gynecologists as the leading health problem in pregnant women (1–3). In fact, obesity in pregnancies of women with NGT likely contributes to a larger percentage of adverse outcomes and infant macrosomia (birth weight >4,000 g) than does gestational diabetes (3). Although the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) trial demonstrated that lower glucose values than those currently adopted to diagnose gestational diabetes result in adverse pregnancy outcomes (4), debate persists over what constitutes normoglycemia in pregnancy and whether normal-weight and obese women have similar glycemic patterns if diet is controlled. In addition, the relative importance of other metabolic factors promoting adiposity in infants born to obese mothers requires clarification.
We prospectively used continuous glucose monitoring (CGM) to address whether obese women exhibit higher glycemic profiles both early and late in gestation, whether these glycemic profiles are influenced by dietary habits, and which specific glucose parameters correlate with infant percentage body fat. We hypothesized that obese pregnant women who do not fulfill criteria for gestational diabetes might exhibit occult hyperglycemia but that controlling macronutrient composition and energy intake would blunt these differences. We also postulated that 24-h glucose measures (24-h area under the curve [AUC]) would correlate best with infant percentage body fat.
RESEARCH DESIGN AND METHODS
Subjects and screening measures
Normal-weight (BMI 20–25 kg/m2) and obese (BMI 30–38 kg/m2) women with NGT were enrolled from the University of Colorado Hospital vicinity at <15 weeks’ gestation. Inclusion criteria included having singleton pregnancies, being aged 18–35 years, being English speaking, and having a fasting blood glucose (FBG) <95 mg/dL. Exclusion criteria included having a history of diabetes, hypertension, triglycerides >400 mg/dL, chronic diseases; tobacco or alcohol use; or treatment with steroids/β-blockers. A 1-h 50-g oral glucose challenge (50 g) was performed prior to 16 weeks, and if the glucose was ≥135 mg/dL, a 100-g 3-h oral glucose tolerance test was performed (3-h oral glucose tolerance test). Women who tested positive for gestational diabetes at baseline or 24–28 weeks’ gestation were excluded (5). Both early (14–16 weeks) and late (26–28 weeks) in gestation, all women had FBG, insulin, C-peptide, glycosylated hemoglobin (HbA1c), and nonesterified free fatty acids (FFAs) measured. Triglycerides were measured in early gestation only. Resting metabolic rate was measured both early and late in gestation (True One 2400, ParvoMedics, Sandy, UT; or Vmax CareFusion, San Diego, CA).
CGM and dietary control
Interstitial glucose profiles were measuring using CGMS Gold (MiniMed, Symlar, CA) for 96 h (4 days) in early and late gestation. The CGM system (CGMS) was calibrated four times per day against finger-stick glucose determinations, as per the manufacturer’s guidelines. During the first 2 days of each 4-day study period, women were instructed to eat and drink whatever they typically consumed (ad libitum). On the morning of study day 3, subjects were given a defined 2-day eucaloric diet prepared by the metabolic kitchen of the Clinical Translational Research Center. Energy intake for the controlled diet was calculated as the measured resting metabolic rate multiplied by 1.3 (6). The macronutrient composition of the diet was 50% carbohydrate (≤20% simple carbohydrate), 35% fat, and 15% protein, and calories were distributed throughout the day, with 12.5% of energy at breakfast, 28% at lunch, 28% at dinner. The remaining calories (31.5%) were divided among three snacks (7). Subjects spoke with a dietitian at the end of the 2-day ad libitum diet period to complete a dietary recall, which was used to estimate energy, carbohydrate, fat, and protein intake of the ad libitum diet (Nutrition Data System for Research, University of Minnesota, Minneapolis, MN). To minimize any run-in effect, CGM data were analyzed using only the second day of each diet period (days 2 and 4).
Infant adiposity
Infant weight, length, and head circumference were measured within the first 24 h after birth. Tricep and subscapular skinfolds and abdominal circumference were measured in triplicate by a single observer within 48 h of birth. The sum of the two skinfold measurements and the sex of the child were used to estimate percentage body fat (8–10).
Calculation of power
Sample size was estimated based on published CGMS findings (11); a difference of 9 mg/dL was detected between 1-h postprandial glucose in 42 normal-weight women (103 ± 13 mg/dL) and 15 obese women (112 ± 13 mg/dL). Forty participants was predicted to result in 94% power with α = 0.025 for a two-sided, two-group t test.
Statistical analysis
Data were analyzed using Sigma Stat for Windows version 2.03 (Jandel Scientific Software, San Rafael, CA). Missing data from the 24-h CGMS profiles were replaced with data corresponding to the same time period from the adjacent day for the same diet. To examine the effects of the control versus ad libitum diets and early versus late pregnancy, a two-way repeated-measures ANOVA with one-factor repetition was used. A Tukey post hoc test was used for multiple comparisons. Percentage relative cumulative frequency curves were generated to comprehensively depict CGMS data. For plasma measurements, t tests were used to compare differences among groups. When data were nonnormally distributed, a Mann-Whitney rank sum was used. Pearson product moment correlations were used to correlate maternal metabolic parameters with neonatal size measurements. A forward stepwise regression was used to generate models between infant adiposity and maternal metabolic parameters. Data are presented as the mean ± SEM.
RESULTS
Study measurements occurred at 15.7 ± 0.3 weeks’ (early) and 27.7 ± 0.3 weeks’ (late) gestation. Table 1 depicts maternal demographics, fasting plasma measures, and size-related infant outcomes. Of 50 subjects initially enrolled, 12 did not complete the study because of the demands of the study (n = 4), an early (n = 1) or late (n = 2) diagnosis of gestational diabetes, lack of completion of both study phases (n = 3), incomplete newborn data (n = 1), and the development of systemic lupus erythematosus (n = 1). There were no differences between groups in maternal weight gain or FBG. Fasting insulin, C-peptide, and FFAs were higher in obese compared with normal-weight women, both early and late in pregnancy. Triglycerides were not a primary metabolic variable in the study, as designed, and only were measured early to ensure that subjects were not at risk for triglyceride-induced pancreatitis. However, triglycerides were significantly higher in the obese mothers (P < 0.001). Birth weight, gestational age, and infant macrosomia were not statistically different between the offspring of both groups. However, percentage body fat was significantly higher in the offspring of obese compared with normal-weight subjects, as were measurements of the triceps, subscapular, and the sum of both skinfolds.
Table 1.
Maternal and infant characteristics
| Normal weight | Obese | |
|---|---|---|
| n | 22 | 16 |
| Maternal | ||
| BMI (kg/m2) | 22.4 ± 1.9 | 33.1 ± 3.4* |
| Age (years) | 31.2 ± 2.3 | 26.5 ± 4.2* |
| Gravity | 2.0 ± 1.0 | 3.3 ± 1.7† |
| Parity | 0.4 ± 0.6 | 1.2 ± 0.9† |
| Weight gain (kg) | 15.2 ± 2.9 | 17.3 ± 7.7 |
| FBG (mg/dL) | ||
| Early | 73 ± 2 | 76 ± 3 |
| Late | 74 ± 2 | 78 ± 3 |
| Insulin (ng/mL) | ||
| Early | 4.3 ± 0.4 | 15.9 ± 3.3* |
| Late | 5.0 ± 0.6 | 14.9 ± 2.0* |
| C-peptide (ng/mL) | ||
| Early | 1.1 ± 0.05 | 2.6 ± 0.3* |
| Late | 1.3 ± 0.06 | 2.8 ± 0.2* |
| Triglycerides (mg/dL) | ||
| Early | 85 ± 5.6 | 152 ± 14.3* |
| Late | — | — |
| FFAs (µEq/L) | ||
| Early | 366 ± 52 | 535 ± 55‡ |
| Late | 326 ± 29 | 547 ± 58* |
| HbA1c (%) | ||
| Early | 5.2 ± 0.1 | 5.1 ± 0.1 |
| Late | 4.8 ± 0.1 | 5.0 ± 0.1 |
| Infants | ||
| Gestational age (weeks) | 39.4 ± 0.3 | 39.6 ± 0.3 |
| Macrosomia (%) | 4.8 | 12.5 |
| Birth weight (g) | 3,248 ± 105 | 3,471 ± 117 |
| Body fat (%) | 7.3 ± 0.4 | 9.2 ± 0.5† |
| Tricep skinfold (mm) | 4.0 ± 0.2 | 4.9 ± 0.3* |
| Subscapular skinfold (mm) | 3.9 ± 0.2 | 4.8 ± 0.3‡ |
| Sum skinfold (mm) | 7.9 ± 0.4 | 9.7 ± 0.5† |
| Abdominal circumference (cm) | 28.9 ± 0.7 | 29.9 ± 0.6 |
Data are means ± SEM.
*P < 0.001; †P < 0.01; ‡P < 0.05 normal weight vs. obese.
The diets consumed by subjects and the effects of diet on glycemia are shown in Table 2. Most glucose parameters were not different between the ad libitum and control diets in the normal-weight and obese women both early (not shown) and later in pregnancy. Of interest, there were no significant differences in either the total calories ingested or the percentage of fat or carbohydrate between the ad libitum and control diets both early and late in pregnancy. Given the lack of appreciable effect of the ad libitum versus control diet on glycemic parameters, all comparisons between the groups were subsequently analyzed on the control diet.
Table 2.
CGMS data and macronutrient intake in normal-weight and obese subjects late in pregnancy on control and ad libitum diets
| Control |
Ad libitum |
Control |
Ad libitum |
|
|---|---|---|---|---|
| Normal weight | Normal weight | Obese | Obese | |
| Glucose (mg/dL) | ||||
| Fasting glucose | 87 ± 2 | 82 ± 2* | 94 ± 3 | 90 ± 5 |
| 1-h postprandial breakfast | 104 ± 3 | 109 ± 4 | 116 ± 4‡ | 115 ± 6 |
| 1-h postprandial lunch | 102 ± 2 | 107 ± 2 | 114 ± 3‡ | 110 ± 5 |
| 1-h postprandial dinner | 99 ± 3 | 104 ± 5 | 115 ± 5‡ | 110 ± 4 |
| Mean glucose | 88 ± 3 | 94 ± 2* | 105 ± 3§ | 103 ± 3 |
| Daytime glucose | 96 ± 1.7 | 99 ± 1.5 | 105 ± 2.8‡ | 104 ± 3.3 |
| AUC (mg/min/dL) | ||||
| Day | 100,018 ± 1,534 | 97,010 ± 1,733 | 107,521 ± 2,817‖ | 105,448 ± 3,387 |
| Night | 39,044 ± 916 | 36,593 ± 1,126* | 43,198 ± 1,413‡ | 42,121 ± 1,102 |
| 24-h | 139,607 ± 2,171 | 134,054 ± 2,429† | 151,241 ± 3,844§ | 148,085 ± 3,935 |
| Intake | ||||
| Total (kcal/day) | 2,138 ± 40 | 2,132 ± 70 | 2,459 ± 26 | 2,269 ± 106 |
| Carbohydrate (%) | 51.3 ± 0.1 | 51.8 ± 1.4 | 51.1 ± 0.1 | 53.1 ± 1.6 |
| Fat (%) | 36 ± 0.03 | 33.7 ± 1.2 | 35.8 ± 0.1 | 31.3 ± 1.3 |
| Protein (%) | 15.3 ± 0.07 | 16.8 ± 0.6* | 15.1 ± 0.04 | 16.9 ± 0.8* |
Data are means ± SEM. Fasting glucose is the mean of six consecutive values before the meal. 1-h postprandial breakfast, lunch, and dinner are the mean of three values 1 h after the meal. Daytime glucose is the mean glucose between 6:30 a.m. and 11:30 p.m., day AUC is between 6:30 a.m. and 11:30 p.m., and night AUC is between 11:30 p.m and 6:30 a.m.
*P < 0.05 and †P < 0.01 (control vs. ad libitum diets, paired t test).
‡P < 0.05, §P < 0.01, and ‖P < 0.001 (normal weight vs. obese, control diet only, t test).
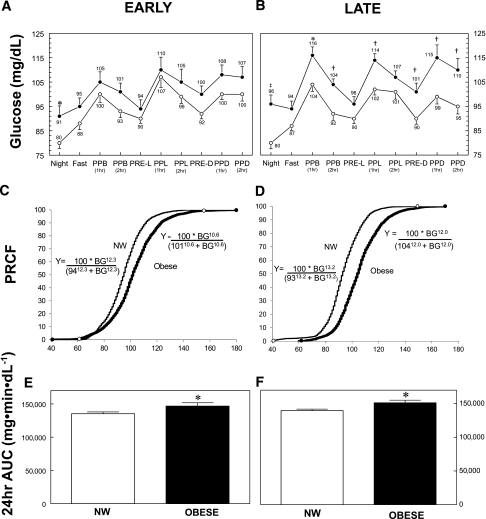
Figure 1 depicts CGMS parameters in normal-weight and obese women during the control diet early (Fig. 1A) and late (Fig. 1B) pregnancy. The 24-h AUC glucose, daytime AUC, nocturnal AUC, mean glucose, and mean daytime glucose were higher in the obese subjects compared with normal-weight subjects in both early and late (Table 2) pregnancy. Late in pregnancy, nearly all glycemic indices were statistically higher in the obese group compared with the normal-weight group (Fig. 1B). Both 1- and 2-h mean postprandial glucoses were significantly higher in the obese women late in pregnancy (P < 0.001) on the controlled diet (115 ± 2 and 107 ± 2 mg/dL, respectively) compared with normal-weight women (102 ± 2 and 96 ± 2 mg/dL), and mean nocturnal glucose was higher in the obese women (96 ± 4 vs. 80 ± 2 mg/dL; P < 0.01). Although the 1- and 2-h postprandial glucose concentrations in the obese group late in pregnancy were ~10 mg/dL higher than in the normal-weight group, the highest mean 1-h postprandial value in the obese group was 116 ± 4 mg/dL after breakfast, and the highest mean 2-h postprandial value was 110 ± 5 mg/dL after dinner, compared with 104 ± 3 and 95 ± 3 mg/dL, respectively, in normal-weight women (Table 2).
Figure 1.
Blood glucose measurements recorded by CGMS in lean and obese women early (A, C, and E) and late (B, D, and F) in pregnancy. Mean values ± SEM are listed by each data point for lean (○) and obese (●) women throughout the day and night, as defined. Night (mean of values between 11:30 p.m. and 6:30 a.m.); Fast (mean of six consecutive values before breakfast); PRE-L (mean of three consecutive values directly before lunch); PRE-D (mean of three consecutive values directly before dinner); PPB (1hr), PPL (1hr), and PPD (1hr) (mean of three values, 1 h after each meal); PPB (2hr), PPL (2hr), and PPD (2hr) (mean of three values, 2 h after each meal). Significant differences are denoted (*P < 0.05; †P < 0.01; ‡P < 0.001). C and D: CGMS data (~6,000 and ~4,000 for lean [thin line] and obese [thick line], respectively) were sorted, and frequency, cumulative frequency, and percentage relative cumulative frequency were determined for lean and obese women early and late in pregnancy over the range of glucose values. Equations for the resulting curves were fitted (r > 0.99) to the following equation: Y = 100 × H/(EC50H + XH), where Y = percentage relative cumulative frequency curves, X = blood glucose, H = slope, and EC50 = 50th percentile value for glucose-utilizing curve-fitting software (NCSS 2007; Kaysville, UT, www.ncss.com), and are given for each curve. The mean glucose values at the 50% percentile were significantly different between lean and obese subjects, both early and late in pregnancy (P < 0.001). The 24-h AUC for glucose values were significantly greater for obese women than lean women in early (P = 0.037) (E) and late (P = 0.007) (F) in pregnancy. NW, normal weight.
To portray all available data, CGMS data from normal-weight and obese subjects are depicted in Fig. 1C and D as the percentage of relative cumulative frequency curves for both early (Fig. 1C) and late (Fig. 1D) pregnancy, using an approach described by Raichi et al. (12). The curve for the obese mothers was shifted to the right, confirming that obese subjects exhibited more glucose values at higher levels compared with normal-weight subjects. In late pregnancy (Fig. 1D), 95% of all of the glucose values in normal-weight subjects were ≤116 mg/dL compared with <133 mg/dL in obese women. In normal-weight subjects late in pregnancy, 80% of glucose values were <103 mg/dL compared with 117 mg/dL in the obese subjects. The 24-h glucose AUC was significantly higher in obese compared with normal-weight women in both early (P < 0.05; Fig. 1E) and late (P < 0.01; Fig. 1F) pregnancy. Finally, the time spent with glucose >120 mg/dL during 24 h was significantly longer in obese compared with normal-weight women (209 ± 62 vs. 33 ± 12 min, P = 0.001) during late pregnancy.
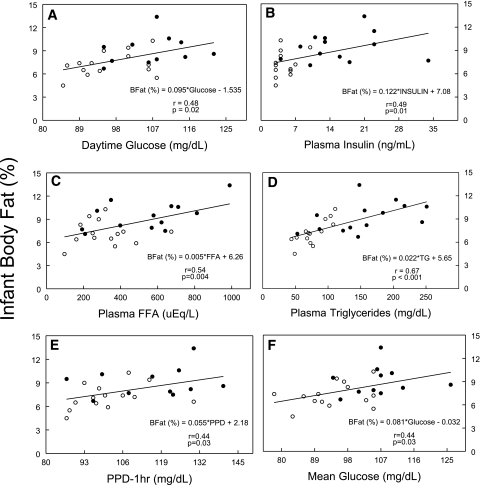
Figure 2 depicts the statistically significant correlates between the metabolic measures and infant percentage body fat independent of maternal BMI. Infants born to obese mothers had significantly higher percentage body fat compared with those born to normal-weight mothers (9.2 ± 0.5 vs. 7.3 ± 0.4%, P < 0.01). Although birth weight was correlated with infant percentage body fat (r = 0.63, P < 0.001), none of the metabolic measures correlated with birth weight (data not shown). It was a surprise to find that maternal fasting triglycerides, measured only early in pregnancy, demonstrated the highest correlation with infant percentage body fat (r = 0.67, P < 0.001). Late in pregnancy, FFAs showed the highest correlation (r = 0.54, P < 0.01). Fasting insulin (r = 0.49, P < 0.05), mean daytime glucose (r = 0.48, P < 0.05), 24-h mean glucose (r = 0.44, P < 0.05), and 1-h postprandial dinner glucose (r = 0.44, P < 0.05) also were correlated with infant percentage body fat. Early maternal BMI was correlated with infant percentage body fat (r = 0.54, P < 0.001). However, in a stepwise regression analysis that included maternal BMI, early triglycerides, fasting FFAs late, fasting insulin late, and daytime mean glucose late, early triglycerides explained 40% of the variance in infant percentage body fat, and the other variables did not add statistically to the predictive power.
Figure 2.
Relationships between maternal glucose, other plasma measurements, and infant adiposity. Triceps, subscapular skinfolds, and abdominal circumference were measured in triplicate by a single trained observer within 48 h of birth. The sum of the two skinfold measurements and the sex of the child were used to estimate percentage body fat [BFat (%)] (8–10) using the equations as follows: BFat (%) = 1.21 × (triceps + subscapular) – 0.008 × (triceps + subscapular)2 – 1.7 (for male subjects); BFat (%) = 1.33 × (triceps + subscapular) – 0.013 × (triceps + subscapular)2 – 2.5 (for female subjects). Linear regression analysis revealed significant relationships for daytime glucose (mean of all values between 6:30 a.m. and 11:30 p.m.) (A), plasma insulin (B), plasma FFA (C), plasma triglycerides (D), PPD-1hr (mean of three values, 1 h after dinner) (E), mean glucose (mean glucose of all values in a 24-h period) (F), and infant adiposity (percentage body fat). All maternal glucose and plasma measurements were measured late in pregnancy except for plasma triglycerides, which was only measured early in pregnancy. Equations, correlation coefficients, and P values are given for each relationship. ○, lean subjects; ●, obese subjects.
CONCLUSIONS
The novel findings of this study are that obese pregnant women with NGT have higher glycemic profiles in pregnancy compared with normal-weight women when diet and gestational age are controlled. The strongest correlation with infant percentage body fat was early fasting triglycerides in pregnancy, although late fasting FFAs, fasting insulin, and the mean daytime glucose also were highly correlated independent of maternal BMI. This is the first CGMS study to carefully control diet in addition to gestational age and to use the percentage relative cumulative frequency curves to portray all of the glycemic data. The higher glycemic patterns in the obese women were unrelated to diet.
Previous studies using CGM in pregnancy have not controlled for diet or examined the relationship between glycemic patterns and neonatal adiposity. The only CGM study comparing glycemic patterns in normal-weight versus obese women did not control for gestational age; women were studied between 23 and 36 weeks’ (29.7 ± 6.2 weeks) gestation (11). In that study, normal-weight women exhibited a mean 1-h postprandial glucose of 103 mg/dL compared with 112 mg/dL in obese women, similar to our mean of 102 mg/dL and 115 mg/dL, respectively. All values were much lower than current standards that recommend a 1-h postprandial glucose ≤140 mg/dL (5). It was a surprise that unlike our study, obese women in the first study (11) demonstrated lower nocturnal glucose compared with normal-weight women, but dietary intake was uncontrolled. The only CGM study in pregnancy in which gestational age was controlled for included normal-weight healthy pregnant women (n = 32) (13). A mean 2-h postprandial glucose of 100 mg/dL was reported at 30 weeks’ gestation and similar to our study, with a mean of 96 mg/dL at 26–28 weeks’ gestation in our normal-weight women. One-hour postprandial values were not reported in this study, and diet was not controlled, but diet records showed consistent caloric intake over the course of pregnancy. Those data, with our data, bring current postprandial therapeutic targets into question.
In addition, we graphically present the percentage of relative cumulative frequency curves of all glucose values in Fig. 2C and D. Glucose concentrations are shifted to the right in obese women, indicating a higher glucose exposure to the fetus over a 24-h period. This was true even when diet was controlled, suggesting the potential for greater fetal hyperinsulinemia. Of interest, only glycemic profiles later in pregnancy were associated with infant percentage body fat, and the strongest predictor was the mean daytime glucose, a value representing the fed state between 6:30 a.m. and 11:30 p.m.
An unexpected finding was that the strongest correlations with infant percentage body fat were not indices of glucose availability but triglycerides (r = 0.67, P < 0.001), measured early, and FFAs (r = 0.54, P < 0.01) measured late in pregnancy. Other investigators have shown that fasting maternal triglycerides and FFAs in the third trimester are correlated with birth weight (14–16), and, more recently, these metabolites were found to correlate with estimates of neonatal adiposity by skinfold measures in well-controlled patients with gestational diabetes (17). Furthermore, investigators from this study have previously reported that the increase in triglycerides from early to late pregnancy was the most predictive of neonatal adiposity (18) in both normal-weight and obese women. Although early maternal BMI correlated with neonatal adiposity (r = 0.54, P < 0.001), it did not add to the predictive value of triglycerides in a stepwise regression model. Our data show that in addition to lipids, fasting maternal insulin, an estimate of maternal insulin resistance, and mean daytime glucose, which reflects the postprandial state, also are significant correlates of infant percentage body fat in glucose-tolerant women late in pregnancy (Fig. 2).
A number of limitations warrant additional consideration. It is possible that the ad libitum diet was not significantly different from the control diet in both populations because the women were being actively monitored by CGM, affecting their behavior. In our study, the mean glucose, mean daytime glucose, and 1-h postprandial dinner glucose late in pregnancy were correlated with infant percentage body fat but not 1-h postprandial breakfast. This may be a result of only 12.5% of the total calories being given at breakfast, as recommended by Peterson and Jovanovic-Peterson (7) compared with 28% at lunch and dinner. Differences between the postprandial measures may have been blunted because the remaining calories were distributed throughout the day as snacks. Therefore, the measure that is likely to best represent the fed state is the mean daytime glucose, which was the glycemic measure most correlated with infant percentage body fat. An incongruity in the data are that the plasma FBG levels are lower in normal-weight and obese women late in pregnancy (74 ± 2 and 78 ± 3 mg/dL, respectively) compared with the fasting CGMS values on controlled diets. This may be attributed to the technical aspects of the CGMS-derived glucose values and variable times between bedtime snack consumption and arising in the morning during the CGM period, compared with a consistent 9- to 10-h fast for the plasma samples.
In conclusion, this study is the first to demonstrate that obese pregnant women with NGT manifest higher glycemic profiles than normal-weight women when both diet and gestational age are tightly controlled. Thus, “relative hyperglycemia” in obese women with NGT may contribute to excess fetal fat accretion and partially explain the observed increased macrosomic rate in the literature. However, obese women also manifest higher fasting triglyceride and FFA levels than normal-weight women, which correlate most strongly with infant adiposity. Such findings have direct implications for future interventional studies targeting these metabolic parameters in glucose-tolerant women to prevent excess fetal growth.
Acknowledgments
D.H.B. received support from the National Institutes of Health (NIH) Grant K24-DK-002935. K.A.H. and L.G. received support from T32-DK-007446. The study was additionally supported by NIH/National Center for Research Resources Colorado CTSI Grant UL 1-RR-025780 and a pilot project grant from the Colorado Nutrition Obesity Research Center (P30DK048520). The contents of this article are the authors’ sole responsibility and do not necessarily represent official NIH views.
No potential conflicts of interest relevant to this article were reported.
K.A.H., L.G., E.H.K., and M.S.R. researched data. T.L.H. contributed to the discussion and reviewed and edited the manuscript. D.R.J. performed the analyses and reviewed and edited the manuscript. L.A.B. and D.H.B. wrote the manuscript.
Footnotes
See accompanying editorial, p. 2331.
References
- 1.American College of Obstetricians and Gynecologists ACOG Committee opinion number 315, September 2005: obesity in pregnancy. Obstet Gynecol 2005;106:671–675 [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA 2006;295:1549–1555 [DOI] [PubMed] [Google Scholar]
- 3.Catalano PM. Management of obesity in pregnancy. Obstet Gynecol 2007;109:419–433 [DOI] [PubMed] [Google Scholar]
- 4.Metzger BE, Lowe LP, Dyer AR, et al. ; HAPO Study Cooperative Research Group Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 5.Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007;30(Suppl. 2):S251–S260 [DOI] [PubMed] [Google Scholar]
- 6.Kopp-Hoolihan LE, van Loan MD, Wong WW, King JC. Longitudinal assessment of energy balance in well-nourished, pregnant women. Am J Clin Nutr 1999;69:697–704 [DOI] [PubMed] [Google Scholar]
- 7.Peterson CM, Jovanovic-Peterson L. Percentage of carbohydrate and glycemic response to breakfast, lunch, and dinner in women with gestational diabetes. Diabetes 1991;40(Suppl. 2):172–174 [DOI] [PubMed] [Google Scholar]
- 8.Slaughter MH, Lohman TG, Boileau RA, et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol 1988;60:709–723 [PubMed] [Google Scholar]
- 9.Schmelzle HR, Fusch C. Body fat in neonates and young infants: validation of skinfold thickness versus dual-energy X-ray absorptiometry. Am J Clin Nutr 2002;76:1096–1100 [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez G, Moreno LA, Blay MG, et al. ; AVENA-Zaragoza Study Group Body fat measurement in adolescents: comparison of skinfold thickness equations with dual-energy X-ray absorptiometry. Eur J Clin Nutr 2005;59:1158–1166 [DOI] [PubMed] [Google Scholar]
- 11.Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol 2004;191:949–953 [DOI] [PubMed] [Google Scholar]
- 12.Riachi M, Himms-Hagen J, Harper ME. Percent relative cumulative frequency analysis in indirect calorimetry: application to studies of transgenic mice. Can J Physiol Pharmacol 2004;82:1075–1083 [DOI] [PubMed] [Google Scholar]
- 13.Siegmund T, Rad NT, Ritterath C, Siebert G, Henrich W, Buhling KJ. Longitudinal changes in the continuous glucose profile measured by the CGMS in healthy pregnant women and determination of cut-off values. Eur J Obstet Gynecol Reprod Biol 2008;139:46–52 [DOI] [PubMed] [Google Scholar]
- 14.Kitajima M, Oka S, Yasuhi I, Fukuda M, Rii Y, Ishimaru T. Maternal serum triglyceride at 24-32 weeks’ gestation and newborn weight in nondiabetic women with positive diabetic screens. Obstet Gynecol 2001;97:776–780 [DOI] [PubMed] [Google Scholar]
- 15.Son GH, Kwon JY, Kim YH, Park YW. Maternal serum triglycerides as predictive factors for large-for-gestational age newborns in women with gestational diabetes mellitus. Acta Obstet Gynecol Scand 2010;89:700–704 [DOI] [PubMed] [Google Scholar]
- 16.Di Cianni G, Miccoli R, Volpe L, et al. Maternal triglyceride levels and newborn weight in pregnant women with normal glucose tolerance. Diabet Med 2005;22:21–25 [DOI] [PubMed] [Google Scholar]
- 17.Schaefer-Graf UM, Graf K, Kulbacka I, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 2008;31:1858–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbour LA, Hernandez TL, Reece MS, et al. Change in fasting triglycerides from early to late gestation are highly predictive of neonatal adiposity independent of maternal BMI (Abstract). Diabetes 2009;58:A84 [Google Scholar]