Abstract
Recent advances in the understanding of the metastatic phenomenon in cancer have led to the description of a metastatic niche. This concept describes a site prepared for the tumor cells in areas frequently associated with metastasis for the individual tumor studied. This niche is a “soil” that allows for the tumor cell or “seed” to lodge and grow. Certain aspects of the biology of infantile hemangioma cells suggest a relationship to the placenta as a possible site of origin for the hemangioma precursor cells. In this article, a relationship between the placenta, with or without a chorangioma and the hemangioma sites of localization, is hypothesized. The placenta is suggested as the site of humoral factors that prepare a niche similar to the function of malignant tumor cells. If the hypothesis proves to be valid, clues for possible treatment are outlined.
In 2000, North et al. published an article that identified the marker glucose transporter enzyme 1, so-called GLUT 1, as being characteristic of infantile hemangiomas.1 The authors also discovered that infantile hemangiomas expressed the Lewis Y antigen (LeY), FcγRII and merosin. In their investigation of other tissues, the authors found that the placenta showed an identical phenotype with regard to these markers.2 Further study has been carried out by Dr Carmen Barnes, working at Boston Children’s Hospital, who discovered that the placenta and the infantile hemangioma have high levels of transcriptome similarity when compared with normal skin and seven other normal structures and diseased tissue based on an analysis of more than 7800 genes by hierarchical and non-hierarchical clustering analysis. The similarity was even more marked when a study of several endothelial specific genes were analyzed. These findings supported the possibility of the placenta being the origin site of the infantile hemangioma.3 The natural progression of infantile hemangiomas is also similar to that of the placenta (rapid proliferation followed by subsequent stabilization). Taken together, these findings suggest the possibility that the hemangioma precursor cell comes from the placenta as a ‘benign metastasis’.
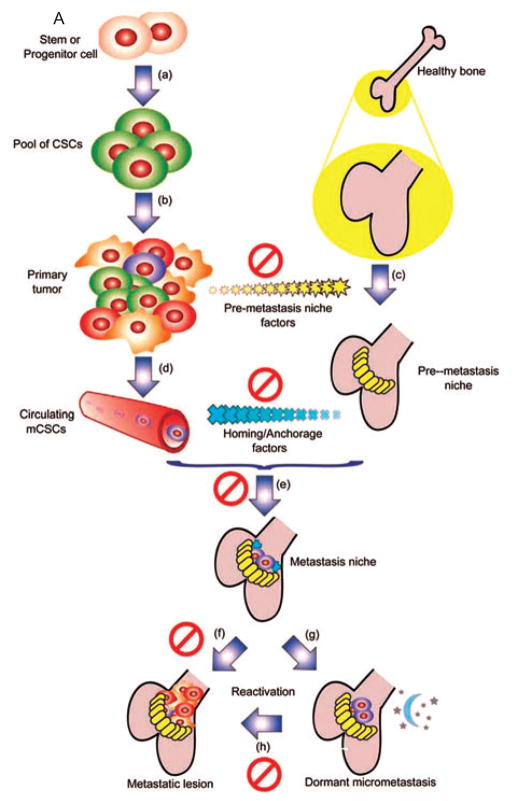
In 1989, Sir Stephen Paget4 proposed the seed and soil hypothesis, namely, ‘When a plant goes to seed, its seeds are carried in all directions; but they can only live and grow if they fall on congenial soil’. For many years, there have been questions as to what constitutes the congenial soil which determines, for example, that breast cancer and ocular melanoma will consistently metastasize to bone and liver, respectively. Over the last several years, several investigators, including Kaplan and Massague, have attempted to explain the metastatic hypothesis.5–11 They defined the metastatic niche (soil) as a friendly site for tumor cells (seed) to attach and grow. In Kaplan’s and Massague’s hypothesis the metastatic niche is prepared by substances secreted by the primary tumor. The metastatic niche contains precursor cells or bone marrow-derived stem cells that express vascular endothelial growth factor receptor 1 (VEGFR1) and the cell surface glycoproteins CD133 and CD34 and CD117. First, fibronectin is laid down as a result of the influence of tumor cells on stromal cells. Thereafter, VEGFR1-expressing cells arrive at the preferred sites.7 They also express VLA4, the integrin, Alpha4Beta1, the ligand of which is fibronectin.9–11 As well, inhibitor of differentiation 3 (Id3) is expressed and is necessary for formation of the niche and survival of the cells.8 The niches favor such sites as capsules and membranes; for example, lymph node, liver capsules or lung membranes. Once the site has been prepared, the tumor cells arrive as do precursor endothelial cells that express VEGFR2 that leads to angiogenesis, vasculogenesis and continued tumor growth. VEGFR1 expression is suppressed.7 Evidence to support this hypothesis has been offered by Kaplan et al., in which they showed that the supernatant derived from tissue cultures of breast cancer and B16 melanoma cells is sufficient to mobilize bone marrow-derived cells and form a niche at favored sites of tumor metastases for both cell types.7 Thus, the primary tumor prepares the sites to receive metastases so that there is an appropriate ‘soil’ primed for their adhesion and growth. A diagram of this process clearly outlines these steps (Fig. 1A,B). Why this hypothesis is so important is that it gives the possibility to block metastases by use of preventive agents that interact with the different critical steps in the process. In Massague’s diagram (Fig. 1A),10 he indicates possible sites of therapeutic intervention. Kaplan et al. have proven that the use of an antibody that specifically blocks VEGFR1 inhibits completely the formation of metastases in animals in which niche preparation had been accomplished by B16 melanoma cells and lung adenocarcinoma cells.7 In summary, metastases can no longer be considered as random events.
Fig 1.
Fig. 1A. A model for tissue-specific metastasis mediated by mCSCs irrespective of the cell of origin, the first step of this model is a transformation event (a) after which the self-renewal capacity leads to an expansion of the CSC pool. This pool of tumor-initiating cells has the capacity to expand into a fully heterogenous primary tumor mass (b). Secretion of pre-metastasis niche forming factors (c) plays a critical role in determining the tissue tropism of the future metastatic lesion. Once the mCSCs begin to migrate through the blood (d), they are guided by homing and anchorage factors produced by the niche (e). After seeding, the local microenviroment in the niche helps determine if the mCSCs will either proliferate into a metastatic lesion directly (f), or will enter a quiescent period (g), which can be cut short by reactivation signals (h) that promote expansion into a full blown metastatic lesion. These key steps of metastasis present several potential targets for therapeutic interventions. Ø = Potential therapeutic intervention.
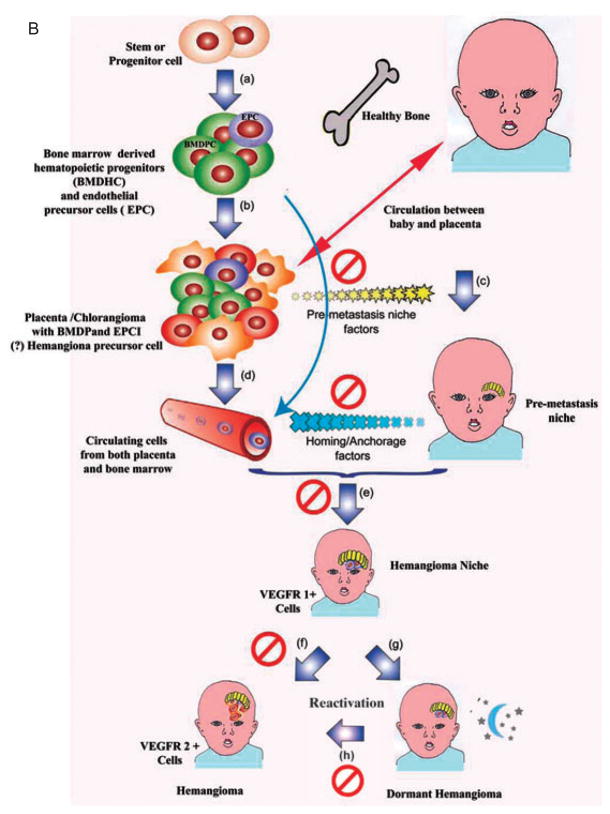
Fig. 1B. A model for tissue specific hemangioma growth mediated by hemangioma progenitor cells. Irrespective of origin or site of the cell of origin is the proliferation of bone marrow-derived progenitor cells (a). These cells can continue to the placenta or can go directly into the circulation (b). The factors are secreted by the placenta or chorangioma (c) that prepare the niche to which the hemangioma precursor cells are directed by homing and anchorage factors subsequently produced by the newly formed niche (d). At this niche hemangioma precursor cells arrive (e) and either form a hemangioma (f) or can become dormant (g). The possibility of dormancy deserves investigation. Dormancy can be cut short by reactivation signals (h). The symbol Ø indicates possible sites of therapeutic intervention. Figure 1A printed with permission of Nature. Reprinted by permission from Macmillan Publishers Ltd: Cell Res. 2007 Jan;17(1):3–14. Figure 1B adapted from Figure 1A. See also References 7, 10, 12, 13.
How does the forgoing information apply to infantile hemangiomas? We have already discussed the preliminary data that raises the possibility that infantile hemangiomas are metastases from the placenta, but now let us hypothesize further on this event. Other important background information includes the observation that when plotting the location of infantile hemangiomas on the head and neck the lesions tend to occur along lines of fusion of the facial placodes, which are of course covered by membranes (Fig. 2).12 Hemangiomas can no longer be considered to be random events.13 Furthermore, placodes have an end artery circulation or a vascular dead end. Another interesting fact is that 4 – 5% of the placentas associated with a single fetus, and up to 9% of those from twins, have chorangiomas (Fig. 3), which are benign tumors of the placenta. There has been a correlation with the presence of chorangiomas and the incidence of hemangiomatosis.13–15 Finally, the placenta has both a fetal and a maternal component which could then explain the differences in infant genotypes; the fetal component could be the source of the hemangioma.
Fig 2.
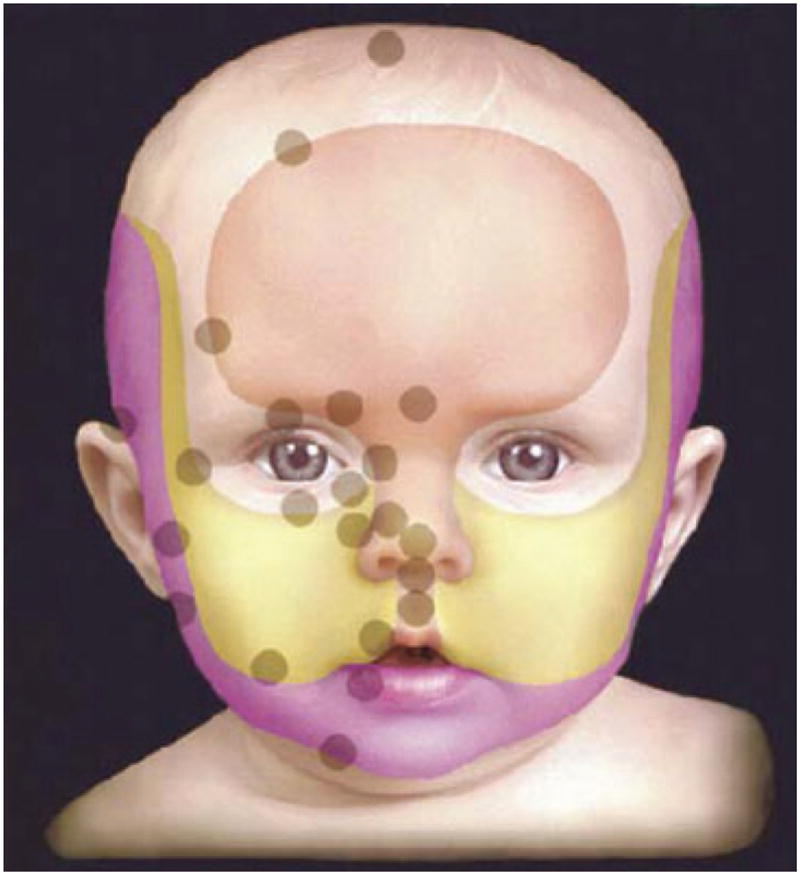
Note concentration of hemangiomas along lines of fusion. Arch Dermatol. 2003 Jul; 139(7): 869 – 875. Copyright American Medical Association. All rights reserved.
Fig 3.
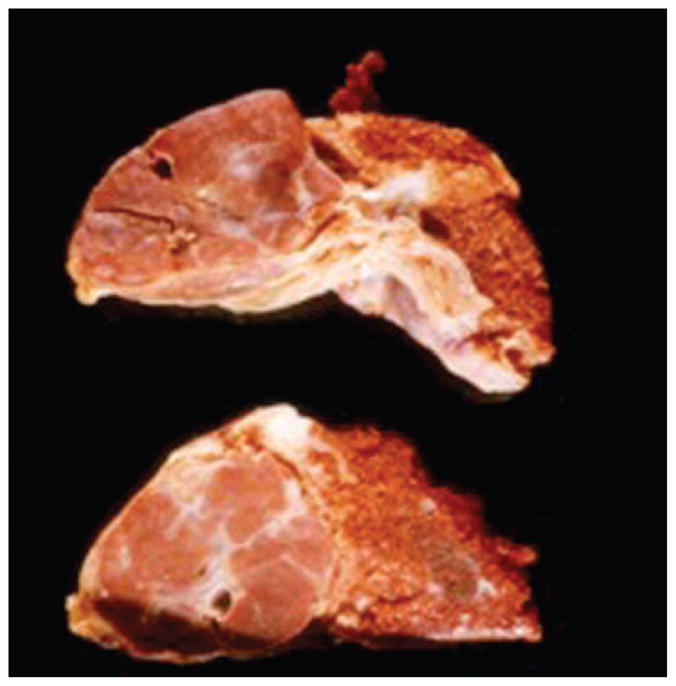
Large sharply demarcated nodular growth on left characteristic of chorangioma (Picture contributed by Dr Drucilla Roberts).
Further information of note is the presence of CD133 and CD34 positive cells, considered endothelial cell precursors, in the peripheral blood taken from five hemangioma patients compared with controls.13,16 A 15-fold increase was noted. Furthermore, in another study 11 of 12 proliferating hemangioma endothelial cells marked for both CD133 and CD34.13,17 Very importantly the finding of a myeloid specific marker on the hemangioma endothelial cells may be evidence that the hemangioma progenitor cell may derive from a myeloid lineage.18,19
In consideration of this collective information, we propose that the chorangioma, or possibly the placenta itself, secretes substances that prepare the sites where infantile hemangiomas occur.19 It is clear that hemangiomas have many bone marrow-derived precursor hemangioblasts/progenitor endothelial cells.20,21 Furthermore, we have already discussed that the niche in tumors favors areas below capsules or membranes, an observation relevant to the fact that there is a concentration of infantile hemangiomas along placode fusion lines, which involve membranes where the metastatic niche could be prepared. The basic hemangioma precursor cell could come from the fetal side of the placenta and, of course, would then show the karyotype consonant with the gender of the child. Whether the lesion represents metastases from the placenta or a site where bone marrow-derived precursor cells arrive at the prepared site or niche does not in anyway preclude the hypothesis that the niche or site where the hemangioma forms is prepared by the placental factors that determine the location of the infantile hemangioma, very similar to the site prepared by cancers for their metastasis. One final association worth mentioning is that in the hemangioma VEGFR1 is suppressed and there is expression of VEGFR2.22 Although the mechanisms resulting in this expression probably differ in cancer metastasis as opposed to hemangioma evolution, the similarity begs comparison.22
Why is this significant for infantile hemangiomas? Firstly, in mouse model experimentation by Kaplan,7 it was clearly shown that metastases could be blocked by using an antibody against the VEGF receptor.7,8 This evidence could also be applied to infantile hemangioma if indeed the VEGF receptor is involved at the site development of the niche formation. Furthermore, there are VEGF receptor 2 and 3 kinase inhibitors, such as sorafenib, that could possibly be applied topically to the site of infantile hemangioma once the first sign of the lesion appears. Also, antibodies against the VEGF receptor could theoretically be applied to the infant’s skin in an effort to prevent the growth of an infantile hemangioma. Secondly, through our understanding of niche formation and development, it might be possible to block infantile hemangioma proliferation by the topical application of substances such as rapamycin, which blocks VEGF transcription.23 Thus, being able to understand how an infantile hemangioma develops could lead to expanded and novel therapeutic options for treatment. In Fig. 1A, Massague has showed possible steps in the metastatic process that would lead to blocking the metastasis formation. One can replace, in the diagram, the tumor cells with the placenta/chorangioma (Fig. 1A). The same blockade of factors indicated in this diagram could apply to the placental/chorangioma hypothesis. These steps can be compared with the events that would take place if indeed the placenta were the source of the agents that recruit cells to form the niche. Furthermore, if indeed the chorangioma is at all related to the production of substances that form the niche, children born with a placenta containing a chorangioma could be carefully observed so that the early signs of infantile hemangioma are detected by increased physician and parental surveillance. The result would lead to very early treatment with possible prevention of the growth of a hemangioma.
Footnotes
Conflicts of interest
None.
References
- 1.North PE, Mizeracki A, Waner M, Mihm MC., Jr GLUT1: a newly discovered marker for juvenile hemangiomas. Human Pathol. 2000;31(1):11. doi: 10.1016/s0046-8177(00)80192-6. [DOI] [PubMed] [Google Scholar]
- 2.North PE, Waner M, Mizeracki A, et al. A unique microvascular phenotype shared by juvenile hemangiomas and human placenta. Arch Dermatol. 2001;137(5):559. [PubMed] [Google Scholar]
- 3.Barnés CM, Huang S, Kaipainen A, et al. Evidence by molecular profiling for a placental origin of infantile hemangioma. Proc Natl Acad Sci U S A. 2005;102(52):19097. doi: 10.1073/pnas.0509579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paget S. The distribution of secondary growths in cancer of the breast 1889. Cancer Metastasis Rev. 1989;8:98. [PubMed] [Google Scholar]
- 5.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Vol. 2. Nature Publishing Group; 2002. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Vol. 8. Nature Publishing Group; 2007. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan RN, Rafii S, Lyden D. Preparing the “Soil”: the premetastatic niche. Cancer Res. 2006;66(23):2006. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta GP, Massague J. Cell. Vol. 127. Elsevier Inc; 2006. Cancer Metastasis: Building a Framework; p. 679. [DOI] [PubMed] [Google Scholar]
- 10.Li F, Tiede B, Massagué J, Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 2007;17(1):3. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- 11.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nature Reviews/Cancer. 2009;9:285. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waner M, North PE, Scherer KA, Frieden IJ, Waner A, Mihm MC., Jr The nonrandom distribution of facial hemangiomas. Arch Dermatol. 2003;139(7):869. doi: 10.1001/archderm.139.7.869. [DOI] [PubMed] [Google Scholar]
- 13.Ritter MR, Butschek RA, Friedlander M, Friedlander SF. Pathogenesis of infantile hemangioma: new molecular and cellular insights. Expert Rev Mol Med. 2007;9(32):1. doi: 10.1017/S146239940700052X. Review. [DOI] [PubMed] [Google Scholar]
- 14.Bakaris S, Karabiber H, Yuksel M, Parmaksiz G, Kiran H. Case of large placental chorioangioma associated with diffuse neonatal hemangiomatosis. Pediatr Dev Pathol. 2004;7(3):258. doi: 10.1007/s10024-003-4042-1. [DOI] [PubMed] [Google Scholar]
- 15.Metry DW, Hebert AA. Benign cutaneous vascular tumors of infancy: when to worry, what to do. Arch Dermatol. 2000;136(7):905. doi: 10.1001/archderm.136.7.905. [DOI] [PubMed] [Google Scholar]
- 16.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34 (+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952. [PubMed] [Google Scholar]
- 17.Yu Y, Fuhr J, Boye E, et al. Mesenchymal stem cells and adipogenesis in hemangioma involution. Stem Cells. 2006;24(6):1605. doi: 10.1634/stemcells.2005-0298. [DOI] [PubMed] [Google Scholar]
- 18.Ritter MR, Reinisch J, Friedlander SF, Friedlander M. Myeloid cells in infantile hemangioma. Am J Pathol. 2006;168(2):621. doi: 10.2353/ajpath.2006.050618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo K, Mihm MC, Fay A. Current theories on the pathogenesis of infantile hemangioma. Semin Ophthalmol. 2009;24 (3):172. doi: 10.1080/08820530902805438. [DOI] [PubMed] [Google Scholar]
- 20.Bischoff J. Progenitor cells in infantile hemangioma. J Craniofac Surg. 2009;1(Suppl):695. doi: 10.1097/SCS.0b013e318193d6ac. Review. Erratum in: J Craniofac Surg 2009; 20(5): 1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boscolo E, Bischoff J. Vasculogenesis in infantile hemangioma. Angiogenesis. 2009;12(2):197. doi: 10.1007/s10456-009-9148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jinnin M, Medici D, Park L, et al. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med. 2008;14(11):1236. doi: 10.1038/nm.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phung TL, Oble DA, Jia W, Benjamin LE, Mihm MC, Jr, Nelson JS. Can the wound healing response of human skin be modulated after laser treatment and the effects of exposure extended? Implications on the combined use of the pulsed dye laser and a topical angiogenesis inhibitor for treatment of port wine stain birthmarks. Lasers Surg Med. 2008;40(1):1. doi: 10.1002/lsm.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]