Abstract
We report on a 63-year-old woman, previously in good health, who had undergone nephrectomy for clear cell renal cell carcinoma in 2002. Because of systemic relapse with multiple lung metastases in 2006, the patient was treated with sunitinib 50 mg daily on a 4-weeks on-/2-weeks off-schedule. After 3 years of treatment, she developed a purpuric rash on her feet and trunk. Biopsy revealed leukocytoclastic vasculitis. No other organ involvement was diagnosed. She was started on oral prednisone 30 mg daily with rapid resolution of the vasculitic skin lesions. Sunitinib was temporally discontinued and reintroduced at the same dose level. Reappearance of a less serious vasculitis after 2 cycles of re-treatment was resolved in the weeks off-treatment and by reducing the dose of sunitinib along with 5 mg of prednisone daily. One year after the diagnosis, the patient is still on this therapy. Oncology providers should be aware of this rare but potentially serious, possible adverse effect of sunitinib.
Key words: Renal cell cancer, Sunitinib, Leukocytoclastic vasculitis
Introduction
Renal cell carcinoma (RCC) is the third most common malignancy of the urinary tract and accounts for 3% of all adult malignancies. Although cure can be achieved by surgery in patients with localized disease, more than a quarter of patients present with advanced disease, and one-third of patients who undergo resection of localized disease will experience a recurrence [1]. Vascular endothelial growth factor (VEGF) is now recognized as a target in the treatment of metastatic RCC (mRCC), and several inhibitors of the tyrosine kinases of its receptors have been established in the treatment of mRCC [1, 2]. Sunitinib malate (SUTENT®; Pfizer Inc., New York, N.Y., USA) is an oral, multitargeted tyrosine kinase inhibitor (TKI) of VEGF receptors (VEGFRs), platelet-derived growth factor receptors (PDGFRs), stem cell factor receptor, FMS-like tyrosine kinase 3 receptor, colony-stimulating factor 1 receptor, and glial cell line-derived neurotrophic factor receptor (rearranged during transfection). In RCC, sunitinib's mechanism of action is thought to occur through inhibition both of the endothelial cell VEGF pathway and PDGFR-β expression in pericytes. Sunitinib is approved for the treatment of mRCC in the first-line setting following the results of a randomized phase III trial demonstrating its superiority compared with interferon-a [1]. Sunitinib, like other multikinase inhibitors, is responsible for a high incidence of a variety of skin disorders [3]. In phase II and III studies cutaneous side effects were reported in more than 20% of patients. These side effects are usually mild with only 2-5% of grade 3 or 4 toxicities reported. The most common are hand-foot syndrome, periorbital edema, hair depigmentation, skin discoloration, subungual splinter hemorrhages, and genital rash [3, 4]. The pathophysiology of skin changes is largely unknown and all cutaneous manifestations cannot be attributed to the same mechanism. For example, hair depigmentation may be due to the inhibition of the stem cell factor or cKIT signaling, while periorbital edema is probably secondary to increased vascular permeability due to PDGFR inhibition [4]. Systemic manifestations, such as vasculitis, as a consequence of sunitinib treatment have been rarely reported. We hereby report a case of leukocytoclastic vasculitis, successfully treated with oral prednisone, not requiring permanent interruption of sunitinib.
Case Presentation
A 63-year-old woman underwent a right nephrectomy for a stage II clear cell RCC in 2002. She had no previous medical history and was a nonsmoker. In 2006, she presented with cough, low fever and anemia. The chest computed tomography (CT) scan showed multiple lung metastases, and abdominal CT scan showed a tumor of the left kidney, suggestive of a second primary malignancy. CT-guided biopsy set the histological confirmation of the systemic relapse of RCC. The patient was initially treated with interferon-a, which was soon discontinued due to intolerable toxicity, mainly pyrexia and fatigue. She was started on sunitinib 50 mg daily on a 4-weeks on-/2-weeks off-schedule. Six months later, she achieved a partial response in the lungs. After 3 years of treatment, pulmonary metastases remain in remission, while the renal mass remains stable. During that period, she presented with several toxicities, the most serious being hypertension, skin toxicity and hypothyroidism. All toxicities were lower than grade 3 and were appropriately managed without dose reduction or treatment delays. In September 2009 and after having completed almost 3 years on sunitinib treatment, the patient developed a gangrene eruption on her feet (fig. 1). There was no hematological or biochemical abnormality and urinalysis was normal (table 1). She was examined by a dermatologist; skin biopsy was obtained and set the diagnosis of leukocytoclastic vasculitis (fig. 2). Anti-dsDNA, ANA, AMA, ASMA, p-ANCA, and c-ANCA were negative, while rheumatoid factor and C3 and C4 levels were within normal limits (table 1). CT scans of the thorax and abdomen showed no evidence of disease progression. Concomitant medication included hydrochlorothiazide for the last 3 years and levothyroxine sodium for the last 2 years (T4). The skin lesions were attributed to drug toxicity. Concomitant medication has been described in the literature as a possible cause of vasculitis. We suppose that sunitinib was the most likely cause, since this manifestation has been only rarely described with the other medication the patient was receiving. The patient discontinued sunitinib treatment and was started on oral prednisone 30 mg daily with significant improvement of the vasculitic lesions. Sunitinib was reintroduced at full dose but a less serious vasculitis reappeared after the first 2 cycles. The sunitinib dose was reduced to 37.5 mg. The dose of prednisone had been gradually reduced and maintained at 5 mg b.i.d. because of the reappearance of a few lesions at lower doses. After lowering the sunitinib dose to 35 mg, along with the low dose of prednisone, no clinical sign of reappearance of vasculitis has been observed 1 year after the diagnosis.
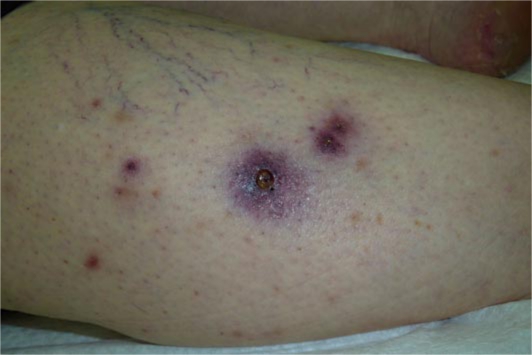
Fig. 1.
Scattered purpuric nodules and plaques on the right tibia. The central hemorrhagic plaque has a necrotic center.
Table 1.
Laboratory examinations
| Value | Normal range | |
|---|---|---|
| White blood cells | 2.4 × 103/μl | 4,000–10,000 |
| Neutrophils | 1.3 × 103/μl | 00.40–74% |
| Lymphocytes | 0.7 × 103/μl | 00.19–48% |
| Hemoglobin | 13.7 g/dl | 00.14–17.5 |
| Platelet | 147 × 103/μl | 0.140–400 |
| Glucose | 106 mg/dl | 00.75–115 |
| Urea | 42 mg/dl | 00.18–50 |
| Creatinine | 1.15 mg/dl | 000.5–1.3 |
| ALT | 30 U/l | 000.5–40 |
| AST | 40 U/l | 000.5–40 |
| Calcium | 9.3 mg/dl | 008.8–10.2 |
| Phosphorus | 3.5 mg/dl | 002.7–4.5 |
| LDH | 186 U/l | 0.135–225 |
| ALP | 58 U/l | 00.35–104 |
| γ-GT | 11 U/l | 000.5–36 |
| Potassium | 4 mmol/l | 003.5–5.1 |
| Sodium | 137 mmol/l | 0.136–148 |
| ANA | negative | |
| Anti-dsDNA | negative | |
| AMA | negative | |
| ASMA | negative | |
| p-ANCA | negative | |
| c-ANCA | negative | |
| C3 | 107 mg/dl | 0.075–180 |
| C4 | 24.8 mg/dl | 0. 10–40 |
| Rheumatoid factor | <9.8 U/ml | 000.0–15 |
| Urinalysis | protein | negative |
| hemoglobin | negative | |
| blood cells | 0–1 |
ALT = Alanine transferase; AST = aspartate transferase; LDH = lactate dehydrogenase; ALP = alkaline phosphatase; -GT = glutamate transferase; ANA = antinuclear antibodies; Anti-dsDNA = anti-double-stranded DNA antibodies; AMA = antimitochondrial antibodies; ASMA = anti-smooth muscle cell antibodies; p-ANCA = perinuclear antineutrophil cytoplasmic antibodies; c-ANCA = cytoplasmic antineutrophil cytoplasmic antibodies.
Fig. 2.
The epidermis exhibits acanthosis, hyperkeratosis and focal hyperkeratosis. The small vessels of the upper dermis show occasional transmural fibrinoid necrosis in association with minimal nuclear debris and some foci of neutrophils into the vessel wall as well as into the vascular lumen (HE, power ×200).
Discussion
Leukocytoclastic vasculitis is an immune complex mediated disease predominantly involving small vessels of the skin (also known as cutaneous leukocytoclastic angiitis), with occasional involvement of other organ systems. Its severity can range from benign, self-limiting, cutaneous eruption to a life-threatening disease with multiple organ failure. Cutaneous vasculitis manifests as palpable purpura of the lower extremities. It can be associated with drugs (propylthiouracil, hydralazine, hydrochlorothiazide and levothyroxine sodium colony-stimulating factors, interleukin-2, allopurinol, cefaclor, minocycline, D-pencillamine, phenytoin, isotretinoin and methotrexate) or it can be a component of other disease such as infections, (subacute bacterial endocarditis, Epstein-Bar virus, HIV, chronic active hepatitis, etc.), connective tissue diseases, ulcerative colitis, congenital deficiencies of various complement components, retroperitoneal fibrosis and malignancies (hematopoietic neoplasias and solid tumors) [5]. Paraneoplastic [lymphoproliferative-, myeloproliferative- (more frequently) or carcinoma-induced] vasculitis represents less than 5% of all cases of cutaneous leukocytoclastic angiitis and improves with treatment of the tumor. Leukocytoclastic vasculitis has been rarely reported in association with RCC as the initial manifestation of the disease [6]. It is supposed that RCC patients have an impairment of their immune system which manifests in a shift from type 1 to type 2 immune response [7]. In contrast to this immunosuppressive theory of RCC patients, autoimmune diseases and specifically vasculitic diseases, such as Wegener's granulomatosis [8], Henoch-Schönlein purpura [9], and leukocytoclastic vasculitis [6, 8, 9, 10, 11], have been rarely described as the first clinical manifestation of the disease. Our literature review yielded few cases of leukocytoclastic vasculitis associated with antineoplastic therapy with TKIs. Two cases have been described in patients receiving erlotinib, a TKI of EGFR [12], while 2 cases of small vessel vasculitis have also been reported in association with the multikinase inhibitors imatinib and sorafenib [13]. There is only 1 report of leukocytoclastic vasculitis with sunitinib during a phase I study [14]. It was described in a biopsy from genital rash. No information about the treatment and the outcome was reported. In our case, we consider it to be a drug adverse event, specifically of sunitinib, since all other possible causes have been excluded. More importantly, vasculitis resolved after the discontinuation of sunitinib, while all other medication was continued after its discontinuation. The causality is further supported by the reappearance of vasculitis following the reintroduction of sunitinib. This could be a manifestation of paraneoplastic syndrome. Nevertheless, vasculitis was not present at the initial diagnosis of RCC, and the disease was in remission at the onset of vasculitis. The fact that vasculitis improved with sunitinib discontinuation and prednisone and not with antitumor therapy is also against the possibility of a paraneoplastic manifestation. Clinical and laboratory examinations revealed no association of vasculitis with an infection or an underlying connective tissue disease. The mechanism by which sunitinib induces vasculitis is not clear. Sunitinib is supposed to reverse immunosuppression, enhancing the antitumor role of the immune system. Review of the literature reveals a correlation of sunitinib treatment with autoimmune diseases, and several preclinical and clinical studies suggest an immunotropic effect of the TKI molecules. The main mechanism is thought to be the inhibition of the VEGFR pathway which is expressed in dendritic cells, in T-regulatory cells, myeloid-derived suppressor cells and other cells of the immune system [15]. The high rate of hypothyroidism, a common adverse event in sunitinib-treated patients, is also supportive of its immunotropic role. Treatment of leukocytoclastic vasculitis is based on the control of the underlying disease or the discontinuation of the triggering factor. Nonsteroidal anti-inflammatory drugs can be used in mild cases, while corticosteroids and immunosuppressive agents, such as cyclophosphamide, are reserved for systemic disease. Regression of skin lesions by removing the suspected agent offers an indirect proof of causality. In our case, we administered prednisone in addition to the discontinuation of sunitinib due to the potential systemic involvement by vasculitis. Indeed, the patient presented with deep ulcers, which are uncommon in leukocytoclastic vasculitis and suggest arterial involvement and possible presence of systemic disease. The persistent remission of the skin lesions in spite of the reintroduction of sunitinib is in concert with the observation that many side effects of the new targeting molecules can be effectively treated without permanent discontinuation of anticancer therapy. Furthermore, dose modification of sunitinib and concomitant treatment with low doses of prednisone were the necessary manipulations that maintain the good result.
However, the experience of such a long period of treatment with sunitinib as in our case is limited due to the relatively short overall survival of patients with mRCC. Consequently, such long-term adverse events of targeted therapies are largely unknown. The fact that this type of vasculitis is rarely associated with renal involvement is of great importance as most of these patients have a single kidney and frequently a degree of preexisting renal dysfunction. In our case, management of vasculitis consisted of small doses of prednisone. Sunitinib can be combined with prednisone but when long periods of therapy are required, an escalation of the sunitinib dose even to 62.5 mg can be considered due to the induction of the CYP3A4 enzyme by prednisone, which results in a faster catabolism of the drug. In our case, we did not attempt such dose escalation to avoid triggering of vasculitis.
Conclusion
In conclusion, sunitinib is responsible for various skin-related adverse events such as hand-foot syndrome and rash. Discrimination of these side effects from vasculitis is not always straightforward when based purely on clinical criteria, and the involvement of a dermatologist may be necessary. Knowing that a skin lesion can be the clinical manifestation of a systemic disorder such as a vasculitis, oncology providers should be aware of this rare but potentially serious, possible adverse effect of sunitinib.
Disclosure Statement
The authors have nothing to disclose.
References
- 1.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 2.Lainakis G, Bamias A. Targeting angiogenesis in renal cell carcinoma. Curr Cancer Drug Targets. 2008;8:349–358. doi: 10.2174/156800908785133132. [DOI] [PubMed] [Google Scholar]
- 3.Bhojani N, Jeldres C, Patard JJ, Perrotte P, Suardi N, Hutterer G, Patenaude F, Oudard S, Karakiewicz PI. Toxicities associated with the administration of sorafenib, sunitinib, and temsirolimus and their management in patients with metastatic renal cell carcinoma. Eur Urol. 2008;53:917–930. doi: 10.1016/j.eururo.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Soria JC, Spatz A, Le Cesne A, Malka D, Pautier P, Weschler J, Lhomme C, Escudier B, Boige V, Armand JP, Le Chevalier T. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol. 2005;6:491–500. doi: 10.1016/S1470-2045(05)70243-6. [DOI] [PubMed] [Google Scholar]
- 5.Carlson JA, Chen KR. Cutaneous vasculitis update: Small vessel neutrophilic vasculitis syndromes. Am J Dermatopathol. 2006;28:486–506. doi: 10.1097/01.dad.0000246646.45651.a2. [DOI] [PubMed] [Google Scholar]
- 6.Poggio ED, Mazzone PJ, Horvath J, Mandell BF. Systemic vasculitis and polyarthritis as the presenting feature of renal cell carcinoma. J Clin Rheumatol. 1998;4:266–269. doi: 10.1097/00124743-199810000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, Wood L, Elson P, Garcia J, Dreicer R, Bukowski R. Sunitinib reverses type 1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14:6674–6682. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 8.Tatsis E, Reinhold-Keller E, Steindorf K, Feller AC, Gross WL. Wegener's granulomatosis associated with renal cell carcinoma. Arthritis Rheum. 1999;42:751–756. doi: 10.1002/1529-0131(199904)42:4<751::AID-ANR19>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Hong YH. Renal cell carcinoma presenting as Henoch-Schönlein purpura with leukocytoclastic vasculitis, hematuria, proteinuria and abdominal pain. Rheumatol Int. 2009 doi: 10.1007/s00296-009-1063-8. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Curgunlu A, Karter Y, Uyanik O, Tunçkale A, Curgunlu S. Leukocytoclastic vasculitis and renal cell carcinoma. Intern Med. 2004;43:256–257. doi: 10.2169/internalmedicine.43.256. [DOI] [PubMed] [Google Scholar]
- 11.Lacour JP, Castanet J, Perrin C, Vitetta A, Ortonne JP. Cutaneous leukocytoclastic vasculitis and renal cancer: two cases. Am J Med. 1993;94:104–108. doi: 10.1016/0002-9343(93)90128-c. [DOI] [PubMed] [Google Scholar]
- 12.Boeck S, Wollenberg A, Heinemann V. Leukocytoclastic vasculitis during treatment with the oral EGFR tyrosine kinase inhibitor erlotinib. Ann Oncol. 2007;18:1582–1583. doi: 10.1093/annonc/mdm420. [DOI] [PubMed] [Google Scholar]
- 13.Heidary N, Naik H, Burgin S. Chemotherapeutic agents and the skin: an update. J Am Acad Dermatol. 2008;58:545–570. doi: 10.1016/j.jaad.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand JP, Scigalla P, Raymond E. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 15.Karadimou A, Sereti E, Lainakis G, Tsiatas M, Gyftaki R, Gavalas N, Dimopoulos A, Bamias A: Changes in lymphocytic populations and autoantibodies resulting from sunitinib treatment of metastatic renal cell carcinoma (mRCC) 15 ECCO-34 ESMO Multidisciplinary Congress 20-24 September 2009, Berlin. E.J.C. supplements 2009, vol 7/2, p 435.