Abstract
Splenic marginal zone lymphomas (SMZL) are an uncommon type of B-cell non-Hodgkin's lymphoma (NHL-B) in which no specific chromosomal translocations have been described. In contrast, the most frequent cytogenetic abnormality is the loss of the long arm of chromosome 7 (7q). Previous reports have located this loss in the 7q32 region. In order to better characterize the genomic imbalances in SMZL, molecular studies were carried out in 73 patients with SMZL. To gain insight into the mapping at 7q a tiling array was also used. The results confirmed the loss of 7q as the most frequent change. In addition, several abnormalities, including 4q22.1, 1q21.3–q22, 6q25.3, 20q13.33, 3q28, 2q23.3–q24.1 and 17p13, were also present. A loss of 7q22.1 at 99925039–101348479 bp was observed in half of the cases. The region of 7q22.1 has not previously been characterised in SMZL. Our results confirmed the presence of a new region of loss on chromosome 7 in these NHL.
Introduction
Splenic marginal zone lymphomas (SMZL) are low-grade B-cell lymphomas with a micronodular pattern of spleen involvement, occupying the marginal zone [1]. In the Revised European-American Classification of Lymphoid Neoplasm (REAL), SMZL is considered as a provisional entity, and is included with marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) type and nodal marginal zone lymphoma in the class of marginal zone lymphomas [2]. However, in the World Health Organization classification, SMZL is regarded as a separate entity [3]. SMZL accounts for fewer than 1% of the non-Hodgkin's B-cell lymphomas (NHL-B).
Cytogenetic abnormalities are commonly present in SMZLs. The most frequent of these are deletions on 7q (30–40%) and gains of 3q (20–30%) and 12q (15–20%). Complex chromosomal imbalances are also common [4]. Losses on 7q mainly involve band 7q32, although distinct regions of loss located either centromerically or telomerically to this region have also been identified [5]–[12]. Upon analyzing SMZL by means of chromosome-based comparative genomic hybridization (CGH) the most frequent chromosomal numerical imbalances proved to be gains of 3q (25%), 5q (28%), 9q (21%), 12q and 20q (22%), and losses of 7q (25%), 6q (20%), 14q (10%), and 17p (10%) [5], [13]–[16]. Using interphase fluorescence in situ hybridization (FISH), microsatellite LOH analysis, and chromosome-based CGH analysis, several studies have mapped the common region of the 7q deletion in SMZL to 11.4 Mb at 7q31.3–7q33 [9], [17]. By contrast, the information about the presence of gains and losses of chromosomes obtained from array-based comparative genomic hybridization (CGH arrays) is scarce and comprised only of small series. These have not contributed to the further delineation of the minimal common region of the 7q deletion [18]–[21].
In the present study, a large series of SMZL was analyzed by CGH arrays, followed by a high-resolution chromosome 7 tiling array. The results were confirmed by molecular studies, to characterize the minimal common region of the 7q deletion. Our results identify new regions involved in this disease, and characterize the losses on 7q22.1 as a common molecular abnormality in SMZL.
Results
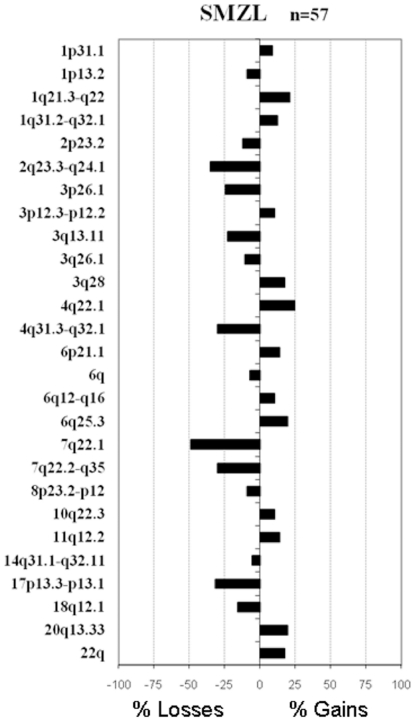
BAC CGH array
A total of 73 samples of SMZL were analyzed. Sixty-eight samples were assayed by BAC CGH array and those regions affected by genomic imbalances were annotated for each case. Most of the patients (84%) showed genomic changes. The median number of changes per patient was four (with a range from 0 to 12). The most frequent changes were chromosomal gains involving 4q22.1 (14/57; 25% of patients), 1q21.3–q22 (12/57; 21%), 6q25.3 (11/57; 19%), 20q13.33 (11/57; 19%), 3q28 (10/57; 18%), 22q (10/57; 18%), 6p21.1 (8/57; 14%), and 11q12.2 (8/57; 14%) while the genomic losses were located on 7q22.1 (28/57; 49%), 2q23.3–q24.1 (20/57; 35%), 17p13.3–p13.1 (18/57; 32%), 4q31.3–q32.1 (17/57; 30%), 7q31–q35 (17/57; 30%), 3p26.1 (14/57; 25%), 3q13.11 (13/57; 23%) and 18q12.1 (9/57; 16%) (Fig. 1). The analysis performed by BAC CGH array in SMZL did not identify any homozygous loss in the 7q22.1 region.
Figure 1. The commonest regions of genomic imbalances as revealed by CGH arrays in splenic marginal zone lymphoma (SMZL).
The tree shows the chromosomal regions that exhibited gains (right) or losses (left). For each region, a corresponding cytogenetic location and the respective frequency of change within the cohort are provided.
Oligonucleotide CGH array
In addition to the FISH studies, an oligonucleotide CGH arrays analysis was carried out in a group of 19 SMZLs from the global series. Overall, the results provided by the two platforms used confirmed the BAC CGH array results. Thus, alterations such as gains on chromosomes 3, 5q13.2, 6p22.1–p21.1, 8q, 17q and 18, and losses in 4q28.3–q31.23, 10q24.33–q25.3, 15q15.1–q21.1 and 17p13.3–p13.1 were observed with the three CGH array methods.
FISH validation of losses identified by BAC CGH array
To confirm the genetic imbalances on 7q revealed by BAC CGH array, FISH experiments were carried out in a total of 20 patients. In all cases FISH analysis confirmed the BAC CGH array results. For this purpose, FISH studies in twelve patients, seven of whom had losses in 7q revealed by BAC CGH array and five who had no genetic imbalances in 7q, were performed. FISH confirmed the losses on 7q22.1 previously assessed by BAC CGH array (Table 1). FISH analysis of the 7q33.1 region was performed in eight cases, six of which showed loss of this region with BAC CGH array and FISH studies confirmed the presence of one hybridization signal in these cases. The remaining two cases did not show 7q33.1 abnormalities with either the BAC CGH array or FISH.
Table 1. BAC Clones located in the 7q22.1 region.
| BAC clone | Position (bp) | FISH Alteration | BAC CGH array | |
| Start | End | |||
| bA506M12 | 99507535 | 99508053 | No data | Normal |
| bA44M6 | 99873610 | 100038722 | Loss | Loss |
| dJ1059M17 | 100976355 | 101150307 | No data | Loss |
| bA333G13 | 101175494 | 101390682 | Loss | Loss |
| bA401L13 | 102514284 | 102705988 | No data | Normal |
High-resolution analysis of chromosome 7q
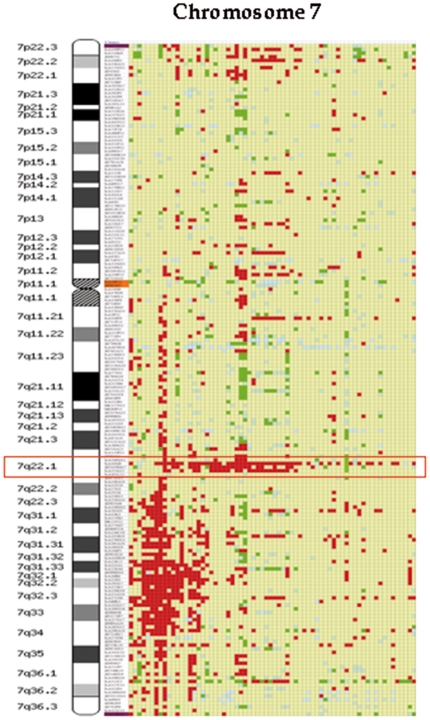
Interestingly, BAC CGH arrays detected two regions of losses on chromosome 7q (Fig. 2). One of the regions was located in the cytoband 7q22.1 and was deleted in 49% of patients with SMZL. This region was 1.51 Mb long, located between the BAC clones bA44M6 and bA333G13. The second region was larger, and localized between the 7q31 and 7q35 cytobands. This region was 43.15 Mb in size and situated between the BAC clones bA5N18 (7q31.1) and dJ558L10 (7q35). This second region was less frequent, affecting 30% of SMZL patients, and was located on 7q31–q35. In addition, one patient showed a deletion of only the 7q32 region.
Figure 2. BAC CGH array analysis of chromosome 7 from 68 patients with SMZL.
Each patient is shown in columns while the location of the BAC clones is shown in rows. Red colour indicates the presence of a loss, while green indicates a gain of genomic material. Yellow represents a normal amount of DNA, and blue indicates a deficient hybridization. The common deleted region on 7q22.1 is shown in a red box.
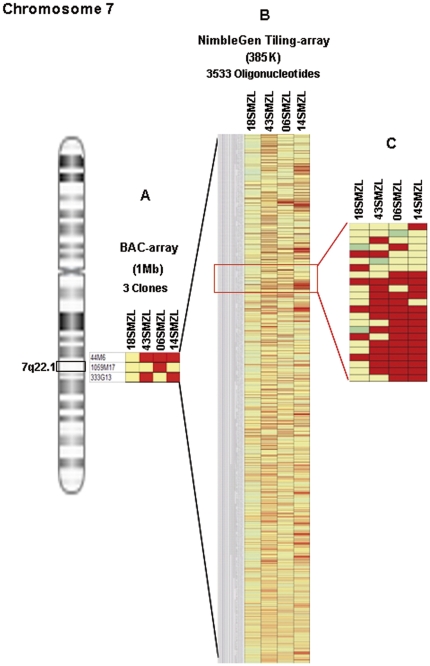
Chromosome 7 tiling-array analysis enabled the boundaries of rearrangements found by CGH array to be refined. Chromosome 7 tiling array NimbleGen 385K was used in four SMZL patients. We found three patients with losses on band 7q22.1, where genes such as MUC3, MUC12 and CUX1 were localized. This focused array was used to obtain a better analysis of the deleted region on 7q. Figure 3 A shows a probe-level view of (A) the BAC CGH array, (B) the CGH array NimbleGen 385K and (C) illustrate the detail of the area of the deletion (7q22.1). The focused array has more probes covering the same genomic area, so it enables a better and more accurate estimate of the exact break-point boundaries compared with the whole-genome array.
Figure 3. Detailed analysis of the commonly deleted region at 7q22.1 by use of two different CGH arrays.
Three cases showed loss in the region (43SMZL, 06SMZL and 14SMZL) between 99925039–101348479 bp (size 1.5 Mb), while the CGH array did not reveal any abnormality of the 7q22.1 region in the control SMZL (18SMZL). (Red, losses; green, gains; yellow, normal; blue, non-informative oligonucleotide hybridization). Figure 3C shows a close-up of a selected region, illustrating the loss on 7q22.1 in three SMZL patients.
Determination of the commonly lost region on chromosome 7
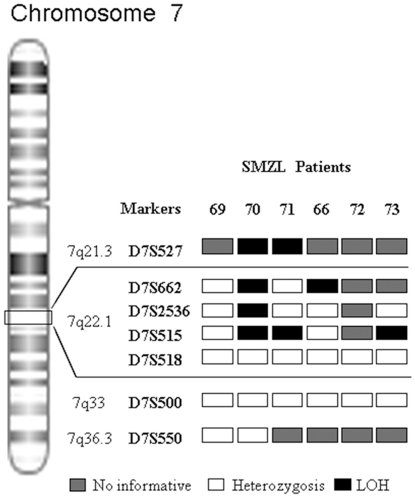
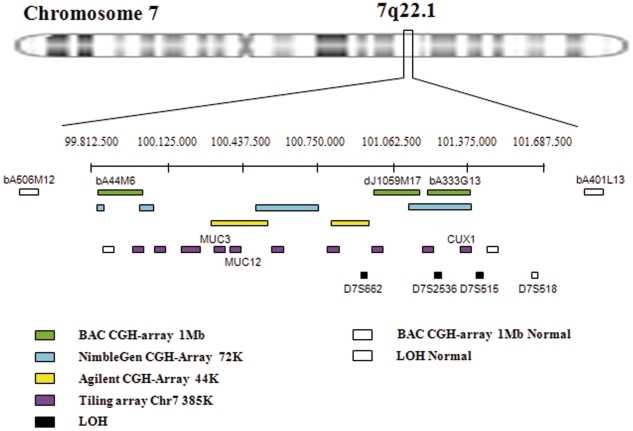
We analyzed the extent of the region identified on 7q22.1 by the CGH array and tried to define the minimum region of molecular allelic loss. Nine markers were analyzed in six patients with SMZL. The chosen markers have been described in previously published studies of LOH on chromosome 7q [17], [22], [23]. Four of the six patients analyzed (67%) showed LOH on various markers analyzed on chromosome 7. Likewise, the LOH revealed that the markers D7S662, D7S2536 and D7S515, located at 100950585, 101235892 and 101490495, had losses of 50%, 20% and 60%, respectively (Fig. 4). Therefore, based on the analysis of the CGH array and LOH, the region commonly lost in SMZL is located between 99925039 and 101348479 bp (Fig. 5).
Figure 4. Mapping of LOH in region 7q.
(Left) Representation of human chromosome 7, including band assignments. (Right) Names of polymorphic markers used and chromosome band assignments. Four of the six patients analyzed (70, 71, 66 and 73) show LOH for one or more of the markers included in the region 7q22.1 (D7S662, D7S2536, D7S515).
Figure 5. Genetic analysis combined in cytoband 7q22.1 in splenic marginal zone lymphoma.
Each method used to analyze the 7q22.1 region commonly deleted in the SMZL is represented by a separate colour. The green boxes correspond to losses detected by BAC mapping to this region; the blue, yellow and purple boxes represent the fragments of the chromosome 7q22.1 region in which losses were detected by commercial NimbleGen arrays. Black boxes show the losses observed with LOH. White boxes define the position of the BAC clones, oligonucleotides and markers of LOH that were not deleted in SMZL.
Discussion
The present study assessed DNA copy number alterations in SMZL by using a CGH array and refined the region lost at 7q. In addition to the previous region located at 7q32, the results showed the presence of another small lost region located on 7q22.1. Previous studies using chromosome banding, FISH, LOH, chromosomal-based CGH and oligonucleotide CGH arrays have shown that the partial deletion of the long arm of chromosome 7 is a recurrent abnormality in SMZL [5], [6], [12], [13], [17]–[21], [24], [25].
CGH array results from three different platforms confirmed that partial loss of 7q is among the most frequent imbalances in SMZL. It should be noted that this deletion was precisely delimited in two regions: 7q22.1 with 1.51 Mb size and another well-known region from 7q31 to 7q35 spanning 43.15 Mb. Interestingly, we found the deletion on 7q22.1 by BAC array in 49% of the SMZL samples. This region has usually been out of the lost region on 7q in most of the previous studies [5], [6], [11], [13], [19], [20], [26]–[29]. In our study the BAC CGH array approach, with a resolution of 1 Mb, showed that the deletion on 7q22.1 was delimited by two BAC clones, bA44M6 and bA333G13, and involved MUC3, MUC12 and CUX1 genes. This recurrent abnormality suggests that this region may contain genes involved in SMZL that are yet to be identified. The genes of the MUC family, which includes MUC3 and MUC12, code for transmembrane mucins, and are involved in epithelial cell protection, adhesion modulation, and signalling; their aberrant expression could be associated with human cancers [30], [31]. The protein encoded by the CUX1 gene is a member of the homeodomain family of DNA-binding proteins. It may regulate gene expression, morphogenesis and differentiation, and may also play a role in cell-cycle progression. Moreover, it has a function as a transcriptional repressor of the c-MYC proto-oncogene. The chromosomal position and function of CUX1 suggest that it may act as a tumour-suppressor gene [22]. This gene regulates normal B lymphopoiesis, and its alteration is associated with lymphoid abnormalities in mice [32], [33]. Aberrant expression of CUX1 could be related to tumour progression in a number of cancers [22], [23], [34]–[40], but to date studies of SMZL have not demonstrated any relationship between CUX1 and this NHL.
In a previous study, 13 markers covering the region 7q21–7q36 were analyzed. The marker D7S487 located on 7q31.3 was frequently lost (45%), while D7S518 (7q22.1) did not show LOH in the cases analyzed [17]. Our study confirmed that the marker D7S518 was heterozygous in all the patients analyzed. However, a more detailed analysis of the 7q22.1 region showed LOH in markers D7S527 (7q21.3; 2 patients), D7S662 (7q22.1; 2 patients), D7S2536 (7q22.1; 1 patient) and D7S515 (7q22.1; 3 patients). It is notable that two of the markers used in the LOH analysis are in the CUX1 gene located in this region (7q22.1): CUX1 D7S515 (intron 3) and CUX1 D7S518 (intron 20) [22]. Therefore, the LOH complements the findings obtained from the CGH array, which revealed a frequent loss of genomic material in the 7q22.1 region. When we analyzed this region in greater depth, through tiling arrays and LOH studies, it was possible to refine the mapping of the region to Chr7: 99925039–101348479 bp.
CGH arrays showed a second region, located on 7q31–q35, that has been found to be deleted in SMZL in previous studies [5], [6], [12], [13], [17]–[21], [25], [24]. This region was 43.15 Mb in size, ranging between the BAC clones bA5N18 and dJ558L10. In the present study the region was lost in 30% of SMZL patients. This anomaly is considered a relatively specific genetic marker of SMZL and has been associated with an aggressive clinical course [17]. The region includes genes such as THAP5, IMMP2L, FOXP2, TES, MET, ST7, PTPRZ1, GRM8, UBE2H, MLKN1, BPGM, CALD1, PTN, SVOPL, AGK, KEL, TPK1 and CNTNAP2. The MET gene is a proto-oncogene and an important regulator of cell proliferation and differentiation, organ regeneration, embryogenesis and oncogenesis. It may be one of the longest-sought oncogenes controlling progression of primary cancers to metastasis. By contrast, the ST7 gene is a tumour suppressor gene that is underexpressed in mantle cell lymphoma [41].
In summary, the resolving power of the CGH array enabled us to confirm that the most frequent alterations in SMZL were losses on chromosome 7, and to identify a new region in the band 7q22.1 (between 99925039–101348479 bp).
Materials and Methods
Patients
Seventy-three patients with the diagnosis of SMZL were studied. All cases were reviewed by expert haemopathologists (TF, MM, MAP) and the diagnosis was established according to the WHO classification [3], [42], [43] Forty of the patients (52%) were women. The ages ranged from 44 to 86 years (median 69 years). The study was approved by the local ethical committees “Comité Ético de Investigación Clínica, Hospital Universitario de Salamanca”. Informed consent was obtained from each patient before they entered the study.
Methods
Of the 73 patients with SMZL included in the study, 68 were analyzed by BAC CGH arrays. To confirm the results from these, commercial oligonucleotide microarrays were used in 19 of these patients (a NimbleGen CGH array 72 k was used for 11 patients, while the remaining eight were analyzed with an Agilent CGH array 44 k). Four of the 68 cases were studied by tiling array. Six cases were studied by LOH, five of which were not studied by BAC CGH arrays. The array data has been deposited in the gene expression omnibus database, the accession number is GSE31203.
DNA isolation
All genomic DNA was extracted from fresh-frozen samples using the standard phenol-chloroform method. Tumour DNA was isolated from the spleen (49 cases), peripheral blood (27 cases) or bone marrow (3 cases). Of the 68 cases analyzed using the BAC arrays, most (44 patients) were studied in spleen while the remaining 24 were analyzed in PBL. In all samples the tumour cell percentage was greater than 50%. Normal DNA was extracted from the human placenta of healthy donors. All DNA was quantified using a NanoDrop spectrophotometer (ND-1000, NanoDrop Technologies, Wilmington, DE). DNA quality was assessed by the 260∶280 ratio and its integrity by agarose gel ethidium bromide visualization [44].
CGH array studies
1. BAC CGH array
A genome-wide analysis of DNA copy number changes of patients was performed using a BAC CGH array. Slides containing 3528 BACs spanning the genome were produced at the Cancer Research Centre (Salamanca, Spain) as previously described [45]. Briefly, to test for labelling reactions, 2 µg of unamplified genomic DNA, test (tumour DNA) and reference material (placental DNA) were digested separately with DpnII restriction enzyme (New England Biolabs, Beverly, MA) and separately labelled using random primers (BioPrime Labeling System, Invitrogen, Carlsbad, Calif) and Cy5-dCTP and Cy3-dCTP (CyDye™ 3-dCTP and CyDye™ 5-dCTP, Amersham Biosciences, Piscataway, NJ) fluorescent dye for paired hybridization samples, respectively. The incorporation of labelled nucleotide was quantified using a NanoDrop spectrophotometer (ND-1000, NanoDrop Technologies). Labelled test and reference DNA samples were mixed equitably, co-precipitated in the presence of Cot-1 human DNA (Roche, Indianapolis, IN) with ethanol, washed, and resuspended in hybridization solution (50% formamide, 10% Dextran sulphate, 2× standard saline citrate, 10 mM Tris pH 7.6, 2.7% sodium dodecyl sulphate and 10 µg/µl of yeast tRNA). DNA mixtures were cohybridized to the arrays in a GENETAC (Genomic Solutions, Ann Arbor, Michigan, USA) for 48 hours at 42°C in accordance with the manufacturer's recommended protocol.
Images and signal intensities were acquired using a GenePix 4000B (Axon Instruments, Burlingame, CA) dual laser scanner in combination with GenePix Pro4.0 (Axon Instruments, Burlingame, CA) imaging software. Fluorescence ratios were normalized using the median of the fluorescence ratios of every spot, computed as log2 values. The log2 ratio of each clone was normalized to the median log2 ratio of the 20 control hybridizations, after which the median of the three spots was calculated. Data from two-colour hybridizations for both sets of DNA were normalized using the DNMAD module of the GEPAS software [46]–[48]. Regions of copy number gain and loss for the BAC CGH array data were identified by creating sample-specific thresholds [49]. A cut-off value of 0.4 was used, based on the ratios of clones in ten hybridizations of normal male versus normal female DNA [45]. The clones with log2 ratios above or below a control sample's threshold value were considered as gains or losses, respectively. At least two contiguous BAC clones with a log2 ratio of −0.4 or less were defined as a region of loss and a log2 ratio of +0.4 or more was defined as a region of gain. Furthermore, spots with weak Cy3 or Cy5 intensity (R2<0.2) and clones with a standard deviation of more than 0.3 from measurement of the three spots were excluded from the analysis. Approximately 10% of clones were excluded in this way. All data sets were carefully reviewed for frequently affected chromosomal sites of physiological copy number polymorphisms (CNPs). Every clone on the array was compared with the ‘Database of Genomic Variants’ (available at: www.project.tcag.ca/cariation; accessed November 2009) and that of chromosomal imbalances and phenotype in humans using Ensembl Resources (DECIPHER: available at: http://www.sanger.ac.uk/PostGenomics/decipher/; accessed November 2009) [49]–[51].
2. Oligonucleotide CGH array platforms
To confirm the results from the BAC CGH array analysis, two types of oligonucleotide CGH array platforms were used. Eight SMZLs were analyzed with Agilent's Human Genome CGH Microarray 44 k (Agilent Technologies). The microarray contains 44255 in situ synthesized 60-mer probes spaced at 43000 bp intervals throughout the human genome and includes 3877 controls. The probes are, according to the manufacturer's description, enriched in cancer-relevant genes, representing both coding and non-coding sequences on the chromosomes. Experiments using Agilent arrays were performed using human placenta genomic DNA as reference and following Agilent's recommended standard protocol. The arrays were scanned using the Agilent scanner, and data were extracted, filtered and normalized using the Feature Extraction program (Agilent Technologies). The Agilent CGH Analytics Software 3.4 trial (Agilent Technologies) was used to export the CGH array data for use with Nexus Copy Number Professional trial software (version 4, BioDiscovery Inc) [52].
Eleven patients with SMZL were analyzed with NimbleGen human CGH 4×72K Whole Genome v2.0 array (Roche NimbleGen, Inc). Placenta DNA was used as the reference. The CGH array protocol from NimbleGen Systems was followed. Briefly, 500 ng of tumour and reference DNA were labelled with Cy3 and Cy5, respectively. Labelled material was co-hybridized to microarrays consisting of 71341 oligonucleotide probes spaced at approximately 40000 bp intervals throughout the human genome, washed and scanned at 10 µm resolution using the GenePix 4000B dual scanner (Axon Instruments, Burlingame, CA). Raw data were extracted using NimbleScan software v2.5 (Roche NimbleGen, Inc), which enables automated grid alignment, extraction and generation of data files [53].
3. Detailed analysis of chromosome 7
Fluorescence in situ hybridization (FISH) analysis. To confirm the losses identified by CGH array, FISH analysis was performed using the BAC clones bA44M6 mapped to 7q22.1 (99873610–100038722 bp), bA333G13 mapped to 7q22.1 (101175494–101390682 bp), bA36B6 mapped to 7q31.31 (130078078–130270796 bp) and bA371N6 mapped to 7q33 (134684519–134842787 bp) as previously described (NCBI36/hg18) [54]. These clones were selected from the same BAC CGH array clones library used for the BAC CGH array studies (Wellcome Trust Sanger Institute, Cambridge, UK). DNA from the BAC clones was isolated and directly labelled with either Spectrum Green-dUTP or Spectrum Orange-dUTP (Vysis/Abbott Molecular, Inc.) by nick translation and hybridized as previously described [54]. All BAC clones were first hybridized to normal human metaphase chromosomes in order to verify their location. A minimum of 200 interphase nuclei were scored using an E1000 microscope (Nikon, Tokyo, Japan) equipped with the Quips system (Vysis, Downers Grove, IL).
Tiling CGH array. In order to localize the lost region at 7q better, four patients with SMZL were analyzed using tiling-path CGH arrays. These arrays were designed at higher resolution with fine-tiling analysis of chromosome 7 (chromosome7:117711–158805222 bp). This array was constructed by maskless array synthesis technology (Roche NimbleGen, Inc.), with up to 385110 oligonucleotides, with a median probe spacing of 365 bp, and synthesized by photolithography on an array by previously described methods [55], [56]. Arrays were scanned at 5 µm resolution using the NimbleGen MS 200 Microarray scanner (Roche NimbleGen, Inc) data were extracted from the scanned images using NimbleScan software v2.5 (Roche NimbleGen, Inc) [53].
LOH analysis. DNA from patients was extracted from spleen and peripheral blood or bone marrow tissues as previously described. Seven microsatellite markers on chromosome 7 were used (Table 2). Four of these markers, D7S662, D7S2536, D7S515 and D7S518, were used to define more precisely the level of the deleted region 7q22.1 detected in a high percentage of SMZLs. All microsatellite markers were selected on the basis of their location (Genethon map release, GDB and RHdb) and on the frequent LOH detected in losses on 7q. PCR reactions were performed in a final volume of 25 µl containing approximately 30–50 ng/µl of template DNA, 5× PCR buffer, 2.5 mM dNTPs, 25 mM Mg2Cl, 10 µM of each primer, and 1 U of Taq DNA polymerase (Promega, Madison, WI, USA). PCR was performed in a thermal cycler. Amplification consisted of an initial denaturing step of 5 min at 94°C, 35 cycles of 30 s at 94°C, 30 s at 55–65°C and 30 s at 72°C, followed by a final step of 5 min at 72°C. The PCR products were separated by 3% of agarose gel (MS4 Metagel, Conda).
Table 2. Microsatellites markers selected for the LOH analysis.
| Markers | Region Chromosome | Size (bp) | Localization (bp) | Sequence | |
| D7S527 | 7q21.3 | 273–297 | 95453048 | Forward | CATTGCAAACTCAGGAGATA |
| Reverse | TAACAGAGGCATGAAAACCA | ||||
| D7S662 | 7q22.1 | 204–234 | 100950585 | Forward | GTTGACAGACAAGCACAGAC |
| Reverse | AGCTGTTTCCCATTTCCA | ||||
| D7S2536 | 7q22.1 | 118–141 | 101235892 | Forward | ACACTCCGCCACCTTG |
| Reverse | CAACAACTGTTCCTAAAGCCT | ||||
| D7S515 | 7q22.1 | 128–190 | 101490495 | Forward | GGGAGTTACTACCCTCACTTAATG |
| Reverse | GGACTGGGCAGCAAAG | ||||
| D7S518 | 7q22.1 | 179–201 | 101648938 | Forward | CAGTAGGCAGGGGTGG |
| Reverse | GGGTGTGTCTGTGTGACAAC | ||||
| D7S500 | 7q33 | 188–210 | 134759259 | Forward | CCAGAATTGAAAACTCAGCA |
| Reverse | ATTGATTGAGGAACTGAACTTACCT | ||||
| D7S550 | 7q36.3 | 177–200 | 155210270 | Forward | TCTCATCTGTGAATGCACTATC |
| Reverse | GCAGTTGGGTTATTTCAAGTC |
Allelic loss was scored only for informative patients whose normal DNA samples were polymorphic at a given locus. LOH was identified by visual analysis as a loss in intensity or complete loss of one allele in the tumour DNA when compared with the normal DNA from the same patient. All cases of LOH were agreed by three reviewers.
Acknowledgments
We thank Eva Lumbreras, Teresa Prieto, Irene Rodríguez and Sara González from the Centro de Investigación del Cáncer, Salamanca, Spain, for their technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was partially supported by grants from the Spanish Fondo de Investigaciones Sanitarias 02/1041 and FIS 09/01543, Fondo Social Caja de Burgos de Investigación Clínica, Proyectos de Investigación del SACYL 106/A/06 and by the Acción Transversal del Cáncer project, through an agreement between the Instituto de Salud Carlos III (ISCIII), Spanish Ministry of Science and Innovation, and the Cancer Research Foundation of Salamanca University and Redes de Investigación RTIIC (FIS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Schmid C, Kirkham N, Diss T, Isaacson PG. Splenic marginal zone cell lymphoma. Am J Surg Pathol. 1992;16:455–466. doi: 10.1097/00000478-199205000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 3.Jaffe ES, Harris NL, Stein H, Isaacson PG. Classification of lymphoid neoplasms: the microscope as a tool for disease discovery. Blood. 2008;112:4384–4399. doi: 10.1182/blood-2008-07-077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matutes E, Oscier D, Montalban C, Berger F, Callet-Bauchu E, et al. Splenic marginal zone lymphoma proposals for a revision of diagnostic, staging and therapeutic criteria. Leukemia. 2008;22:487–495. doi: 10.1038/sj.leu.2405068. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez JM, Garcia JL, Gutierrez NC, Mollejo M, Martinez-Climent JA, et al. Novel genomic imbalances in B-cell splenic marginal zone lymphomas revealed by comparative genomic hybridization and cytogenetics. Am J Pathol. 2001;158:1843–1850. doi: 10.1016/S0002-9440(10)64140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sole F, Salido M, Espinet B, Garcia JL, Martinez Climent JA, et al. Splenic marginal zone B-cell lymphomas: two cytogenetic subtypes, one with gain of 3q and the other with loss of 7q. Haematologica. 2001;86:71–77. [PubMed] [Google Scholar]
- 7.Oscier DG, Matutes E, Gardiner A, Glide S, Mould S, et al. Cytogenetic studies in splenic lymphoma with villous lymphocytes. Br J Haematol. 1993;85:487–491. doi: 10.1111/j.1365-2141.1993.tb03337.x. [DOI] [PubMed] [Google Scholar]
- 8.Troussard X, Mauvieux L, Radford-Weiss I, Rack K, Valensi F, et al. Genetic analysis of splenic lymphoma with villous lymphocytes: a Groupe Francais d'Hematologie Cellulaire (GFHC) study. Br J Haematol. 1998;101:712–721. doi: 10.1046/j.1365-2141.1998.00764.x. [DOI] [PubMed] [Google Scholar]
- 9.Gruszka-Westwood AM, Hamoudi RA, Matutes E, Tuset E, Catovsky D. p53 abnormalities in splenic lymphoma with villous lymphocytes. Blood. 2001;97:3552–3558. doi: 10.1182/blood.v97.11.3552. [DOI] [PubMed] [Google Scholar]
- 10.Callet-Bauchu E, Baseggio L, Felman P, Traverse-Glehen A, Berger F, et al. Cytogenetic analysis delineates a spectrum of chromosomal changes that can distinguish non-MALT marginal zone B-cell lymphomas among mature B-cell entities: a description of 103 cases. Leukemia. 2005;19:1818–1823. doi: 10.1038/sj.leu.2403909. [DOI] [PubMed] [Google Scholar]
- 11.Gazzo S, Baseggio L, Coignet L, Poncet C, Morel D, et al. Cytogenetic and molecular delineation of a region of chromosome 3q commonly gained in marginal zone B-cell lymphoma. Haematologica. 2003;88:31–38. [PubMed] [Google Scholar]
- 12.Salido M, Baro C, Oscier D, Stamatopoulos K, Dierlamm J, et al. Cytogenetic aberrations and their prognostic value in a series of 330 splenic marginal zone B-cell lymphomas: a multicenter study of the Splenic B-Cell Lymphoma Group. Blood. 2010;116:1479–88. doi: 10.1182/blood-2010-02-267476. [DOI] [PubMed] [Google Scholar]
- 13.Andersen CL, Gruszka-Westwood A, Atkinson S, Matutes E, Catovsky D, et al. Recurrent genomic imbalances in B-cell splenic marginal-zone lymphoma revealed by comparative genomic hybridization. Cancer Genet Cytogenet. 2005;156:122–128. doi: 10.1016/j.cancergencyto.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 14.Dierlamm J, Rosenberg C, Stul M, Pittaluga S, Wlodarska I, et al. Characteristic pattern of chromosomal gains and losses in marginal zone B cell lymphoma detected by comparative genomic hybridization. Leukemia. 1997;11:747–758. doi: 10.1038/sj.leu.2400635. [DOI] [PubMed] [Google Scholar]
- 15.Andersen CL, Gruszka-Westwood A, Ostergaard M, Koch J, Jacobsen E, et al. A narrow deletion of 7q is common to HCL, and SMZL, but not CLL. Eur J Haematol. 2004;72:390–402. doi: 10.1111/j.1600-0609.2004.00243.x. [DOI] [PubMed] [Google Scholar]
- 16.Matteucci C, Galieni P, Leoncini L, Lazzi S, Lauria F, et al. Typical genomic imbalances in primary MALT lymphoma of the orbit. J Pathol. 2003;200:656–660. doi: 10.1002/path.1386. [DOI] [PubMed] [Google Scholar]
- 17.Mateo M, Mollejo M, Villuendas R, Algara P, Sanchez-Beato M, et al. 7q31–32 allelic loss is a frequent finding in splenic marginal zone lymphoma. Am J Pathol. 1999;154:1583–1589. doi: 10.1016/S0002-9440(10)65411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vega F, Cho-Vega JH, Lennon PA, Luthra MG, Bailey J, et al. Splenic marginal zone lymphomas are characterized by loss of interstitial regions of chromosome 7q, 7q31.32 and 7q36.2 that include the protection of telomere 1 (POT1) and sonic hedgehog (SHH) genes. Br J Haematol. 2008;142:216–226. doi: 10.1111/j.1365-2141.2008.07176.x. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira BI, Garcia JF, Suela J, Mollejo M, Camacho FI, et al. Comparative genome profiling across subtypes of low-grade B-cell lymphoma identifies type-specific and common aberrations that target genes with a role in B-cell neoplasia. Haematologica. 2008;93:670–679. doi: 10.3324/haematol.12221. [DOI] [PubMed] [Google Scholar]
- 20.Watkins AJ, Huang Y, Ye H, Chanudet E, Johnson N, et al. Splenic marginal zone lymphoma: characterization of 7q deletion and its value in diagnosis. J Pathol. 2010;220:461–474. doi: 10.1002/path.2665. [DOI] [PubMed] [Google Scholar]
- 21.Novara F, Arcaini L, Merli M, Passamonti F, Zibellini S, et al. High-resolution genome-wide array comparative genomic hybridization in splenic marginal zone B-cell lymphoma. Hum Pathol. 2009;40:1628–1637. doi: 10.1016/j.humpath.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Zeng WR, Scherer SW, Koutsilieris M, Huizenga JJ, Filteau F, et al. Loss of heterozygosity and reduced expression of the CUTL1 gene in uterine leiomyomas. Oncogene. 1997;14:2355–2365. doi: 10.1038/sj.onc.1201076. [DOI] [PubMed] [Google Scholar]
- 23.Zeng WR, Watson P, Lin J, Jothy S, Lidereau R, et al. Refined mapping of the region of loss of heterozygosity on the long arm of chromosome 7 in human breast cancer defines the location of a second tumor suppressor gene at 7q22 in the region of the CUTL1 gene. Oncogene. 1999;18:2015–2021. doi: 10.1038/sj.onc.1202519. [DOI] [PubMed] [Google Scholar]
- 24.Oscier DG, Gardiner A, Mould S. Structural abnormalities of chromosome 7q in chronic lymphoproliferative disorders. Cancer Genet Cytogenet. 1996;92:24–27. doi: 10.1016/s0165-4608(96)00025-8. [DOI] [PubMed] [Google Scholar]
- 25.Sole F, Woessner S, Florensa L, Espinet B, Mollejo M, et al. Frequent involvement of chromosomes 1, 3, 7 and 8 in splenic marginal zone B-cell lymphoma. Br J Haematol. 1997;98:446–449. doi: 10.1046/j.1365-2141.1997.2163033.x. [DOI] [PubMed] [Google Scholar]
- 26.Algara P, Mateo MS, Sanchez-Beato M, Mollejo M, Navas IC, et al. Analysis of the IgV(H) somatic mutations in splenic marginal zone lymphoma defines a group of unmutated cases with frequent 7q deletion and adverse clinical course. Blood. 2002;99:1299–1304. doi: 10.1182/blood.v99.4.1299. [DOI] [PubMed] [Google Scholar]
- 27.Boonstra R, Bosga-Bouwer A, van Imhoff GW, Krause V, Palmer M, et al. Splenic marginal zone lymphomas presenting with splenomegaly and typical immunophenotype are characterized by allelic loss in 7q31–32. Mod Pathol. 2003;16:1210–1217. doi: 10.1097/01.MP.0000095895.19756.77. [DOI] [PubMed] [Google Scholar]
- 28.Camacho FI, Mollejo M, Mateo MS, Algara P, Navas C, et al. Progression to large B-cell lymphoma in splenic marginal zone lymphoma: a description of a series of 12 cases. Am J Surg Pathol. 2001;25:1268–1276. doi: 10.1097/00000478-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Chacon JI, Mollejo M, Munoz E, Algara P, Mateo M, et al. Splenic marginal zone lymphoma: clinical characteristics and prognostic factors in a series of 60 patients. Blood. 2002;100:1648–1654. [PubMed] [Google Scholar]
- 30.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duraisamy S, Ramasamy S, Kharbanda S, Kufe D. Distinct evolution of the human carcinoma-associated transmembrane mucins, MUC1, MUC4 and MUC16. Gene. 2006;373:28–34. doi: 10.1016/j.gene.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Climent JA, Alizadeh AA, Segraves R, Blesa D, Rubio-Moscardo F, et al. Transformation of follicular lymphoma to diffuse large cell lymphoma is associated with a heterogeneous set of DNA copy number and gene expression alterations. Blood. 2003;101:3109–3117. doi: 10.1182/blood-2002-07-2119. [DOI] [PubMed] [Google Scholar]
- 33.Sinclair AM, Lee JA, Goldstein A, Xing D, Liu S, et al. Lymphoid apoptosis and myeloid hyperplasia in CCAAT displacement protein mutant mice. Blood. 2001;98:3658–3667. doi: 10.1182/blood.v98.13.3658. [DOI] [PubMed] [Google Scholar]
- 34.Ueda Y, Su Y, Richmond A. CCAAT displacement protein regulates nuclear factor-kappa beta-mediated chemokine transcription in melanoma cells. Melanoma Res. 2007;17:91–103. doi: 10.1097/CMR.0b013e3280a60888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neville PJ, Thomas N, Campbell IG. Loss of heterozygosity at 7q22 and mutation analysis of the CDP gene in human epithelial ovarian tumors. Int J Cancer. 2001;91:345–349. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1050>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 36.Narahari J, Fisk JC, Melendy T, Roman A. Interactions of the cellular CCAAT displacement protein and human papillomavirus E2 protein with the viral origin of replication can regulate DNA replication. Virology. 2006;350:302–311. doi: 10.1016/j.virol.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 37.Sato K, Takeuchi T, Kukimoto I, Mori S, Yasugi T, et al. Human papillomavirus type 16 P670 promoter is negatively regulated by CCAAT displacement protein. Virus Genes. 2007;35:473–481. doi: 10.1007/s11262-006-0074-8. [DOI] [PubMed] [Google Scholar]
- 38.Michl P, Ramjaun AR, Pardo OE, Warne PH, Wagner M, et al. CUTL1 is a target of TGF(beta) signaling that enhances cancer cell motility and invasiveness. Cancer Cell. 2005;7:521–532. doi: 10.1016/j.ccr.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 39.Michl P, Knobel B, Downward J. CUTL1 is phosphorylated by protein kinase A, modulating its effects on cell proliferation and motility. J Biol Chem. 2006;281:15138–15144. doi: 10.1074/jbc.M600908200. [DOI] [PubMed] [Google Scholar]
- 40.Ripka S, Konig A, Buchholz M, Wagner M, Sipos B, et al. WNT5A–target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer. Carcinogenesis. 2007;28:1178–1187. doi: 10.1093/carcin/bgl255. [DOI] [PubMed] [Google Scholar]
- 41.Pal S, Baiocchi RA, Byrd JC, Grever MR, Jacob ST, et al. Low levels of miR-92b/96 induce PRMT5 translation and H3R8/H4R3 methylation in mantle cell lymphoma. EMBO J. 2007;26:3558–3569. doi: 10.1038/sj.emboj.7601794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 43.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri S, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 2008. 4a Edition Volume 2.
- 44.Bredel M, Bredel C, Juric D, Kim Y, Vogel H, et al. Amplification of whole tumor genomes and gene-by-gene mapping of genomic aberrations from limited sources of fresh-frozen and paraffin-embedded DNA. J Mol Diagn. 2005;7:171–182. doi: 10.1016/S1525-1578(10)60543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robledo C, Garcia JL, Caballero D, Conde E, Arranz R, et al. Array comparative genomic hybridization identifies genetic regions associated with outcome in aggressive diffuse large B-cell lymphomas. Cancer. 2009;115:3728–3737. doi: 10.1002/cncr.24430. [DOI] [PubMed] [Google Scholar]
- 46.Herrero J, Al-Shahrour F, az-Uriarte R, Mateos A, Vaquerizas JM, et al. GEPAS: A web-based resource for microarray gene expression data analysis. Nucleic Acids Res. 2003;31:3461–3467. doi: 10.1093/nar/gkg591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montaner D, Tarraga J, Huerta-Cepas J, Burguet J, Vaquerizas JM, et al. Next station in microarray data analysis: GEPAS. Nucleic Acids Res. 2006;34:W486–W491. doi: 10.1093/nar/gkl197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conde L, Montaner D, Burguet-Castell J, Tarraga J, Medina I, et al. ISACGH: a web-based environment for the analysis of Array CGH and gene expression which includes functional profiling. Nucleic Acids Res. 2007;35:W81–W85. doi: 10.1093/nar/gkm257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollack JR, Perou CM, Alizadeh AA, Eisen MB, Pergamenschikov A, et al. Genome-wide analysis of DNA copy-number changes using cDNA microarrays. Nat Genet. 1999;23:41–46. doi: 10.1038/12640. [DOI] [PubMed] [Google Scholar]
- 50.Sebat J, Lakshmi B, Troge J, Alexander J, Young J, et al. Large-scale copy number polymorphism in the human genome. Science. 2004;305:525–528. doi: 10.1126/science.1098918. [DOI] [PubMed] [Google Scholar]
- 51.Iafrate AJ, Feuk L, Rivera MN, Listewnik ML, Donahoe PK, et al. Detection of large-scale variation in the human genome. Nat Genet. 2004;36:949–951. doi: 10.1038/ng1416. [DOI] [PubMed] [Google Scholar]
- 52.Baumbusch LO, Aaroe J, Johansen FE, Hicks J, Sun H, et al. Comparison of the Agilent, ROMA/NimbleGen and Illumina platforms for classification of copy number alterations in human breast tumors. BMC Genomics. 2008;9:379. doi: 10.1186/1471-2164-9-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Selzer RR, Richmond TA, Pofahl NJ, Green RD, Eis PS, et al. Analysis of chromosome breakpoints in neuroblastoma at sub-kilobase resolution using fine-tiling oligonucleotide array CGH. Genes Chromosomes Cancer. 2005;44:305–319. doi: 10.1002/gcc.20243. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez MB, Hernandez JM, Garcia JL, Lumbreras E, Castellanos M, et al. The value of fluorescence in situ hybridization for the detection of 11q in multiple myeloma. Haematologica. 2004;89:1213–1218. [PubMed] [Google Scholar]
- 55.Singh-Gasson S, Green RD, Yue Y, Nelson C, Blattner F, et al. Maskless fabrication of light-directed oligonucleotide microarrays using a digital micromirror array. Nat Biotechnol. 1999;17:974–978. doi: 10.1038/13664. [DOI] [PubMed] [Google Scholar]
- 56.Nuwaysir EF, Huang W, Albert TJ, Singh J, Nuwaysir K, et al. Gene expression analysis using oligonucleotide arrays produced by maskless photolithography. Genome Res. 2002;12:1749–1755. doi: 10.1101/gr.362402. [DOI] [PMC free article] [PubMed] [Google Scholar]