Abstract
Background
The evolution of the Alphaproteobacteria and origin of the mitochondria are topics of considerable debate. Most studies have placed the mitochondria ancestor within the Rickettsiales order. Ten years ago, the bacterium Odyssella thessalonicensis was isolated from Acanthamoeba spp., and the 16S rDNA phylogeny placed it within the Rickettsiales. Recently, the whole genome of O. thessalonicensis has been sequenced, and 16S rDNA phylogeny and more robust and accurate phylogenomic analyses have been performed with 65 highly conserved proteins.
Methodology/Principal Findings
The results suggested that the O. thessalonicensis emerged between the Rickettsiales and other Alphaproteobacteria. The mitochondrial proteins of the Reclinomonas americana have been used to locate the phylogenetic position of the mitochondrion ancestor within the Alphaproteobacteria tree. Using the K tree score method, nine mitochondrion-encoded proteins, whose phylogenies were congruent with the Alphaproteobacteria phylogenomic tree, have been selected and concatenated for Bayesian and Maximum Likelihood phylogenies. The Reclinomonas americana mitochondrion is a sister taxon to the free-living bacteria Candidatus Pelagibacter ubique, and together, they form a clade that is deeply rooted in the Rickettsiales clade.
Conclusions/Significance
The Reclinomonas americana mitochondrion phylogenomic study confirmed that mitochondria emerged deeply in the Rickettsiales clade and that they are closely related to Candidatus Pelagibacter ubique.
Introduction
Proteobacteria are one of the best-studied phyla within bacteria. According to the 16S rDNA phylogeny, Proteobacteria are subdivided into five classes: α, β, γ, δ and ε [1]. Alphaproteobacteria biodiversity and evolution has been well studied through phylogenetic analyses [2]. Current phylogenomic analysis allows the subdivision of the Alphaproteobacteria into six major orders: Rhodospirillales, Caulobacterales, Sphingomonadales, Rickettsiales, Rhodobacterales and Rhizobiales. Among them, Sphingomonadales, Rhodobacterales and Rhizobiales have a strong record of free-living organisms and are widespread in aquatic and terrestrial habitats; these organisms also have intracellular lifestyles as plant mutualists or pathogens and animal pathogens [3]. Unlike the three previous orders, Rickettsiales members are mostly obligate intracellular bacteria, and either parasitic, for Rickettsia and Orientia, or symbiotic for Wolbachia. Gene losses often occurred during the evolution of the intracellular species, which explains the small genome sizes of intracellular versus free-living Alphaproteobacteria [4]. Wolbachia is a special case study that lives in symbiosis with arthropods and annelids. This species shows evidence of genome reduction, but it also experienced several gene integration events from the symbiont genome to the host nuclear genome [5]. It is thought that mitochondria originated through an endosymbiotic event that occurred between the proto-Rickettsiales and a pro-eukaryotic cell [6], [7]. Based on biological arguments [8], the endosymbiotic event occurred during the early stages of eukaryotic evolution approximately one billion years ago. Phylogenetic analyses have attempted to reveal the nature of the engulfed bacterium, but this remains a subject of debate [9]. Molecular phylogenomic analyses of whole mitochondrial proteins rooted the mitochondrion among the Alphaproteobacteria [10]–[12] but revealed that the heterogeneous origin of mitochondrial genes did not clearly locate the position of the mitochondrion ancestor within the Alphaproteobacteria tree. Studies of mitochondrial proteins that are congruent with the Alphaproteobacteria evolution place the mitochondrion at the root of the Rickettsiales order [13]. Candidatus Pelagibacter ubique is a marine free-living bacterium, member of the SAR11 clade, with a small genome and an AT rich genome [14] that was included in the Rickettsiales clade since 2007 [2] although there is still discussion on whether a free-living bacterium could be part of a clade including obligate intracellular species [15]. However, phylogenomic studies including Candidatus Pelagibacter ubique located the mitochondrion ancestor within the Rickettsiales order [2]. Furthermore, its very small and AT rich genome constitute two features that are typical of mitochondria and related obligate intracellular parasites such as the Rickettsiales [15]. More recent studies, on the mitochondria of Chlamydomonas reinhardtii [10] and Saccharomyces cerevisiae [16] find Rhizobiales and Rhodobacterales as sister taxa of the mitochondria more often that Rickettsiales. Therefore, because of limitations in phylogenomic methods and data availability, the origin of the mitochondrial ancestor remains unclear.
Ten years ago, the intra-amoebal gram-negative bacteria, Odyssella thessalonicensis, was isolated from Acanthamoeba spp. [17]; the 16S rDNA was sequenced and phylogenetic analysis was performed. The resulting tree placed O. thessalonicensis in the same clade as Paraholospora and in a sister clade to Rickettsiales. It was suggested that Holosporaceae comprised O. thessalonicensis, Holospora obtusa, NHP Bacterium and Caedibacter caryophilus, and that it was within the Rickettsiales order.
Whole genome shotgun sequencing of O. thessalonicensis recently yielded genomic data on a new intracellular Alphaproteobacteria. In this study, we have used the sequenced O. thessalonicensis genome and the available alphaproteobacterial genomes to reanalyze the phylogenetic position of O. thessalonicensis and the evolutionary relationship between the Alphaproteobacteria and the Reclimonas americana mitochondrion which resembles the most the ancestral proto-mitochondrial genome than any other mitochondrial DNA investigated to date [18].
Results
Alphaproteobacteria 16S rDNA phylogeny
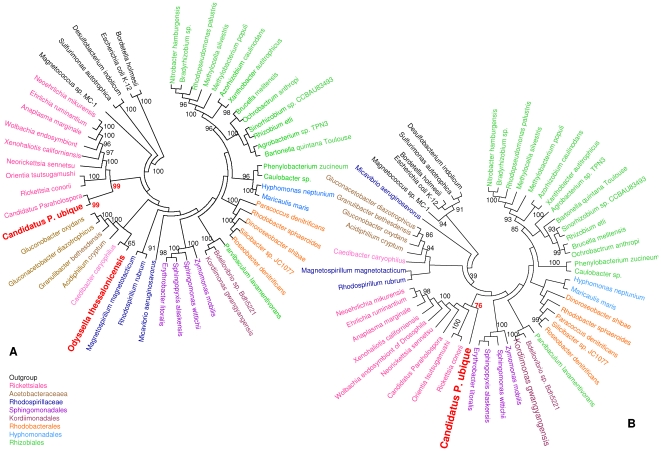
Phylogenies to recover the position of Candidatus Pelagibacter ubique and O. thessalonicensis, were built with 53 sequences of 16S rDNA, including 49 Alphaproteobacteria 16S rDNA sequences, comprising O. thessalonicensis, one Gammaproteobacteria (Escherichia coli K-12), one Betaproteobacteria (Bordetella holmesii), one Epsilonproteobacteria (Sulfurimonas autotrophica) and one Deltaproteobacteria (Desulfobacterium indolicum). Three phylogenetic methods were used: Maximum Likelihood (ML), Maximum Parsimony (MP) and Neighbor Joining (NJ). All three methods yielded the same topology, although branches were better supported by ML and MP methods. The monophyly of Rhodospirillales was not supported ( Figure 1A ). Instead, this order was split into two clades corresponding to the Acetobacteraceae and Rhodospirillaceae families. It appears that O. thessalonicensis is a sister taxon to the clade formed by Caedibacter caryophilus and the four Acetobacteraceae species (Bootstrap (BP) = 100). Phylogenies placed Paraholospora deep in the Rickettsiales clade (BP = 99), while Candidatus Pelagibacter ubique is a sister taxon to Paraholospora. Candidatus Pelagibacter ubique was grouped with Rickettsiales. These results were also consistent with those obtained on the phylogenetic tree realized without the O. thessalonicensis 16S rDNA sequence ( Figure 1B ), however, the topology of Candidatus Pelagibacter ubique branching right outside the Rickettsiales was not well supported (BP = 76). Both ribosomal DNA phylogenies (with or without O. thessalonicensis) also showed that the Magnetococcus sp. was the first diverging Alphaproteobacteria.
Figure 1. Alphaproteobacteria 16S rDNA phylogeny.
A. A ML phylogenetic tree of 49 Alphaproteobacteria ribosomal DNA sequences is rooted with a non-Alphaproteobacteria as outgroup. B. Alphaproteobacteria 16S rDNA phylogeny without Odyssella thessalonicensis. A ML phylogenetic tree of Alphaproteobacteria ribosomal DNA sequences is rooted with a non-Alphaproteobacteria as outgroup. Bootstrap values are indicated near branches as a percentage. Different colors correspond to different orders. Candidatus Pelagibacter ubique and Odyssella thessalonicensis are shown in red.
Alphaproteobacteria phylogenomic tree
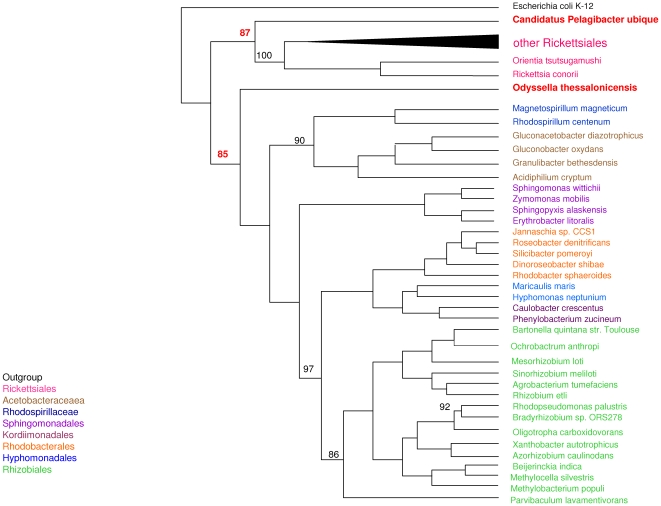
Because the 16S rDNA does not guarantee an accurate delineation of bacterial species [4], 19,20, we performed a phylogenomic analysis involving highly conserved proteins among 42 Alphaproteobacteria. We selected non-duplicated proteins in the Alphaproteobacteria proteomes and performed an all-against-all BLAST analysis. Proteins present in all Alphaproteobacteria with high-scoring segment pair lengths of more than 150 amino acids and 20% identity were selected; only 65 proteins matched these criteria. The 65 corresponding alignments were performed, conserved blocks were selected, and the resulting cured alignments were concatenated in a single 12,437 amino acid alignment and used for phylogeny construction. The ML and MP methods showed similar topologies with high branch supports, while the NJ method gave very low bootstrap values. The O. thessalonicensis clustered together with Alphaproteobacteria other than the Rickettsiales clades, with high support values (BP = 85), even though the absence of Holosporaceae from the dataset does not allow a strong confirmation of this topology ( Figure 2 ). Candidatus Pelagibacter ubique topology as sister taxon to Rickettsiales however, was confirmed, as it formed a deep branch alongside Rickettsiales also with high support values (BP = 87), and there was an early divergence between the intracellular Rickettsiales and the free-living Pelagibacter ( Figure 2 ). The phylogenomic tree suggested that all Alphaproteobacteria have evolved from an ancestor located between the Rickettsiales clade and the other Alphaproteobacteria.
Figure 2. Phylogenomic tree of Alphaproteobacteria.
Phylogenomic tree of 65 concatenated highly conserved proteins representing the evolution of 42 Alphaproteobacteria species. Important bootstrap values are indicated near branches as a percentage. The tree is rooted on Escherichia coli. Different orders of Alphaproteobacteria are labeled by different colors. Candidatus Pelagibacter ubique and Odyssella thessalonicensis are shown in red. Some of the Rickettsiales species are collapsed.
Mitochondrion and Alphaproteobacteria relationship
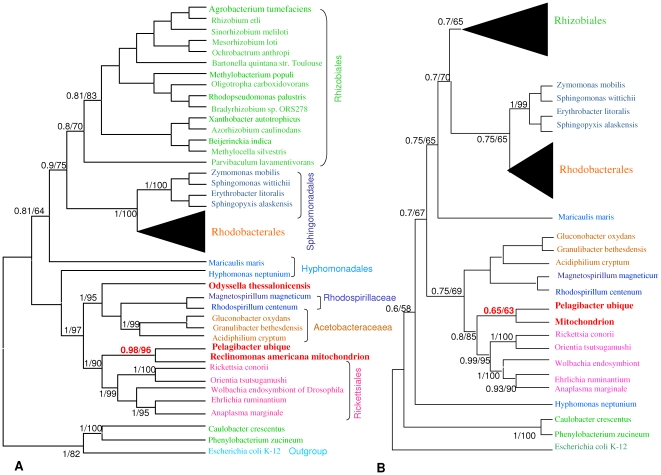
Mitochondrion-encoded proteins whose phylogeny is closest to the previous Alphaproteobacteria phylogenomic tree were selected according to the K tree score method ( Table 1 ) and used to place the mitochondrion within the Alphaproteobacteria tree. The nine best protein alignments were concatenated, and ML and Bayesian phylogenies were inferred ( Figure 3A ). The Bayesian tree had the same topology as the ML tree, although the Bayesian tree branches were better supported. O. thessalonicensis was located alongside the group formed by the Rhodospirillaceae and the Acetobacteraceaea (Posterior Probability (PP) = 1, BP = 95), and its branch appeared early in the Alphaproteobacteria evolution. ML and Bayesian methods showed that R. americana mitochondrion grouped with the free-living Candidatus Pelagibacter ubique (PP = 0.98, BP = 96), and that together they branched deeply alongside Rickettsiales. The mitochondrion phylogenomic tree also suggested an early divergence between Candidatus Pelagibacter ubique and the mitochondrion as shown by the length of the branches and the position of the node. Mitochondrion trees without O. thessalonicensis presented the same topology for the Candidatus Pelagibacter ubique and the mitochondrion, but the branches were not well supported (BP = 63) ( Figure 3B ).
Table 1. Selection of the nine mitochondrion proteins whose phylogeny is closest to the Alphaproteobacteria phylogenomic tree.
| K-score | Function |
| 0.48375 | LSU ribosomal protein L2p (L8e) |
| 0.51416 | NADH-ubiquinone oxidoreductase chain G |
| 0.64953 | SSU ribosomal protein S4p (S9e) |
| 0.6564 | Cyytochrome c-type biosynthesis protein CcmC |
| 0.68246 | LSU tribosomal protein L6p (L9e) |
| 0.68879 | NADH-ubiquinone oxidoreductase chain I |
| 0.71266 | LSU ribosomal protein L5p (L11e) |
| 071299 | Succinate deshydrogenase iron-sulfur protein |
| 0.72572 | SSU ribosomal protein S3p (S3e) |
Figure 3. Phylogenomic tree of Alphaproteobacteria and the Reclinomonas americana mitochondrion.
A. ML and Bayesian tree of nine concatenated proteins whose phylogeny is closest to the Alphaproteobacteria phylogenomic tree. The Rhodobacterales are collapsed. B. Phylogenomic tree of 42 Alphaproteobacteria and the Reclinomonas americana mitochondrion without Odyssella thessalonicensis. The Rhizobiales and Rhodobacterales are collapsed. Both trees are rooted on Escherichia coli. Values near nodes are Bayesian posterior probabilities and ML bootstraps, respectively. Different orders of Alphaproteobacteria are labeled by different colors. Candidatus Pelagibacter ubique and Odyssella thessalonicensis are shown in red.
Discussion
Ten years ago, 16S rDNA phylogeny studies described O. thessalonicensis as belonging to the Rickettsiales order [17]. Here, except from the 16S rDNA tree, we constructed a phylogenomic analysis, more accurate for species delineation [4], and we used the three classic inference methods (ML, MP, NJ), as well as the Bayesian approach, not used in the original paper [17]. The phylogenetic positioning of O. thessalonicensis within the Rickettsiales clade was only in part verified by the 16S rDNA phylogenetic tree that grouped O. thessalonicensis with the Acetobacteraceae and as sister taxon of the Holosporaceae member, Caedibacter caryophilus. In the original paper, the 16S rDNA datasets used were not the same, as the study [17] did not include Acetobacteraceaea, Candidatus Paraholospora, or Candidatus Pelagibacter ubique. Phylogenomic analyses of Alphaproteobacteria allowed more robust trees to be built, which help to establish a more reliable position of O. thessalonicensis in the Alphaproteobacteria family. However, we recognize that the unavailability of the Holosporaceae genomes may raise questions on the accuracy of the topology of O. thessalonicensis. Nevertheless, the phylogeny of the 65 proteins undoubtedly supports that Candidatus Pelagibacter ubique emerged deeply alongside Rickettsiales, while the O. thessalonicensis branch was well supported between Rickettsiales and other Alphaproteobacteria. As previously described [2], [13], the selection of the mitochondrion proteins whose phylogeny was closest to the Alphaproteobacteria phylogenomic tree was a powerful approach for locating the ancestor of mitochondria. Most studies argued that mitochondria are closely related to the Rickettsiales order. However, recent studies on the mitochondrion of the green algae, C. reinhardtii, have proposed that most of its mitochondrial protein sister taxon were members of the Rhizobiales and the Rhodobacterales [11] more often than the Rickettsiales order, while a study on S. cerevisiae mitochondrion proposes that its sister taxa are more often members of the Rhizobiales [16]. The latter study argues the possibility that mitochondrial genomes have a mosaic structure [16], so maybe their origin and evolution is dictated by different elements according to the organism they belong to. Mitochondria are heterogenous and their genomes structure suggests possible genome fusions, addition of different elements and recombination. Different analyses using mitochondria of different organisms would give different results and comparison would not be possible (data not shown). Therefore, for this study, we decided to focus only on the origins of R. americana mitochondrion that resembles the most to the proto-mitochondrion ancestor, avoiding to add noise to the phylogenies with the addition of many mitochondria.
It has been proven that adding characters while constructing phylogenetic trees increases the probability that the topology of the obtained tree is correct. The more signals are tested, the more the branches are well supported [21]. O. thessalonicensis is, most probably, a non-Rickettsiales species the closest related to Rickettsiales, as raised by our phylogenomic analyses, it is therefore legitimate to account the O. thessalonicensis genome when studying the origin of mitochondria. The addition of new data from O. thessalonicensis whole-genome sequencing and the mitochondrial protein selection method using the K tree score partially confirmed the results found by Williams three years ago [2], as well as the ones found by other studies supporting the grouping of the mitochondria with Alphaproteobacteria [12], and more specifically with Rickettsiales [13] and reinforced the topology presenting Pelagibacter ubique in the Rickettsiales order [2]. Further and more surprisingly, in our study, the mitochondrion branch emerged as a sister taxon of Candidatus Pelagibacter ubique, a result strongly supported by the chosen approach. In the studies mentioned above the mitochondrion branch does not emerge as a sister taxon of Candidatus Pelagibacter ubique. Differences are probably due to the different datasets used by each study that do not allow a fair comparison. Moreover, mitochondria seem to have chimeric and heterogenous structures [15] that vary from one organism to another, introducing different results according on which mitochondrion is used in every study. There still is an important debate on whether the free-living organism Candidatus Pelagibacter ubique is a member of the Rickettsiales order or not, because it is not an intracellular species [15]. Our results link, for the first time undoubtedly, Candidatus Pelagibacter ubique to the Rickettsiales order and furthermore, with the Reclimonas americana mitochondrial ancestor. Trees with or without O. thessalonicensis present the same topology, but the positioning of Candidatus Pelagibacter ubique as a sister taxon of Reclinomonas americana mitochondrion is better supported when O. thessalonicensis is used for the tree reconstruction. The node presenting Candidatus Pelagibacter ubique and the mitochondrion as sister taxa are better supported when O. thessalonicensis is used ( Figure 3A,B ). O. thessalonicensis data reinforce previous results.
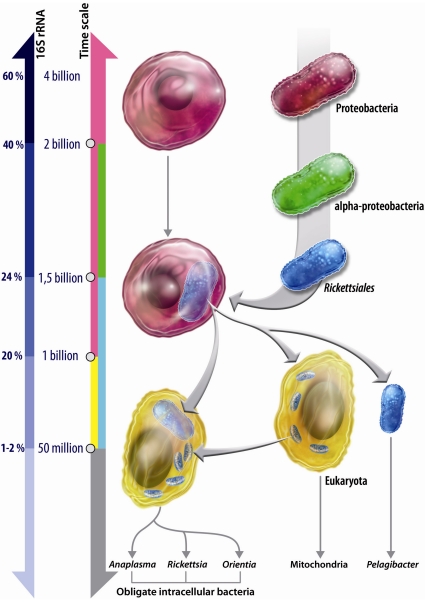
From the R. americana mitochondrion phylogenomic tree, we can suggest two hypotheses. In the first, the Rickettsiales (also including Candidatus Pelagibacter ubique and the proto-mitochondrion ancestor) had a free-living common ancestor with a rather small genome. There were two endosymbiotic events, one for the Rickettsiaceae and one for mitochondria. In the second and more parsimonious hypothesis, there was a single facultative intracellular Rickettsiales common ancestor with two clades evolving into a strict intracellular species contemporary to the emergence of eukaryotes and to the creation of proto-mitochondria. In contrast, Candidatus Pelagibacter ubique later evolved into a free-living form due to environmental changes that facilitated its adjustment to a relatively stable extracellular environment. Candidatus Pelagibacter ubique is the smallest free-living bacterium. Such a massive genome reduction can only be explained by extreme specialisation [22]. However, Pelagibacter ubique is a bacterium found in water everywhere in the world. Its small size may therefore witness its previous lifestyle. If its ancestor was a facultative intracellular species the genome reduction took place during its association with a proto-eukaryote ( Figure 4 ). One single endosymbiotic event is by itself complicated enough and absolutely more parsimonious than two simultaneous endosymbiotic events, so we believe that the most plausible hypothesis is the latter. Moreover, a scenario suggesting that the Rickettsiales ancestor became intracellular after diverging from Candidatus Pelagibacter ubique would not explain this species extremely small genome. Many hypotheses were described on Rickettsiales and mitochondria, in our study we argument on our hypothesis that was never explored before and which was well-supported by our results and by the use of data used for the first time in a study on mitochondrial origins.
Figure 4. The hypothesis for mitochondrion and free-living Candidatus Pelagibacter ubique emergence.
From a single facultative intracellular Rickettsiales common ancestor, two clades evolved into strict, intracellular species contemporary to the emergence of eukaryotes. Candidatus Pelagibacter ubique later evolved into a free-living form. Arrows on the left represent the 16S rRNA percentage divergence scale and the time scale in million of years. A 16S rRNA percentage divergence of 1–2% corresponds to about 50 million years [36]. The arrows on the right represent the emergence events, divergence events and endosymbiotic events.
The use of new data from O. thessalonicensis whole-genome sequencing in the reconstruction of Alphaproteobacteria phylogenies, strongly confirmed the emergence of the R. americana mitochondrion branch between Candidatus Pelagibacter ubique and the other Rickettsiales genera. Indeed, the topology of the trees built without O. thessalonicensis that presented the Candidatus Pelagibacter ubique in the Rickettsiales clade was not well supported; therefore, proper determination of its taxonomy was not possible. By adding O. thessalonicensis, the classification of Candidatus Pelagibacter ubique as member of Rickettsiales is strongly sustained by all topologies obtained by classic phylogenetic methods, such as ML, and by the Bayesian method. Finally, positioning the R. americana mitochondrion ancestor within Alphaproteobacteria has proven that the monophyly of Rickettsiales and the R. americana mitochondrion, and the evolution of Candidatus Pelagibacter ubique, emerged from an intracellular to a free-living organism. Currently, the most numerous and successful extracellular bacterial species on Earth, Candidatus Pelagibacter ubique, which is highly dominant in both salt and fresh water worldwide [23], is actually a member of the intracellular Rickettsiales order.
Materials and Methods
Sequence data
O. thessalonicensis [Genome Project: ID63085] was isolated from Acanthamoeba spp. as described by Britles et al. [17]. Genomic DNA was extracted and sequenced using the same method as for Legionella drancourtii [24]. The first genome assembly was performed using Newbler software (454 Life Sciences, Roche) and produced 106 contigs (20 scaffolds). Contigs were sent to the RAST platform [25] for rapid gene prediction and annotation. The 16S rDNA sequences from the 49 Alphaproteobacteria were extracted from the Ribosomal Database Project [26]. The Alphaproteobacteria, Escherichia coli K-12 substr. MG1655 [27] and the Reclinomonas americana mitochondrion proteomes [28] were downloaded from the NCBI database.
Alphaproteobacteria 16 rDNA phylogeny
The 53 16S rDNA sequences were aligned using MUSCLE [29], and conserved blocks were selected using Gblocks [30]. The curated alignments were realized and used for phylogeny construction. Phylogeny inference was constructed using three different methods, ML, MP, NJ, and a four-category gamma distribution was fit for among-site rate variation. One hundred bootstrap replicates were completed, and the resulting trees were summarized using the majority-rule consensus method. Bootstrap values were considered high when they were higher that 85. Trees were displayed using MEGA4 [31].
Alphaproteobacteria phylogenomics
We used a stringent method in order to establish a protein list that would be representative of all the Alphaproteobacteria used in the study. Duplicated genes were discarded from Alphaproteobacteria proteomes using the BLASTClust program [32] with a minimum overlap of 70% and a minimum identity of 30%. Proteins considered as non-paralogous were then gathered and used for the cluster of orthologous group (COG) searches. An all-against-all NCBI-BLASTp search was performed on the 42 Alphaproteobacteria dataset. All of the proteins present in all species with an identity of 20% and a high-scoring segment pair (HSP) length over 150 amino acids were considered orthologous. Through this method, 65 clusters were identified. Corresponding proteins were aligned with MUSCLE, and conserved blocks were selected with Gblocks. The 65-curated alignments were concatenated and used for phylogeny construction. Phylogenies were constructed using three different methods, ML, MP, NJ, and 100 bootstrap replicates were sampled. Holosporaceae were not included because of unavailability of their proteomes. Bootstrap values were considered high when they were higher that 85. The 65 protein sequences from the Odyssella were submitted to the GenBank database (File S1).
Mitochondrion phylogeny relationships
The 67 proteins coded by the mitochondrial DNA of Reclinomonas americana were compared to the Alphaproteobacteria proteomes using NCBI4 BLASTp. Mitochondrial proteins with the best blast hits (BBHs) and an e-value under e-20 were selected; 59 proteins matched these criteria. For each of the 59 successful proteins, corresponding BBHs were aligned, and an ML tree was built using PhyML [33]. Trees with 42 leaves were compared to the Alphaproteobacteria multiprotein tree using the K tree score. Only 43 trees had 42 leaves. The nine best trees were determined according to the K tree score [34]. Mitochondrion-encoded proteins were added to each of the nine successful alignments. The conserved blocks were concatenated in a single 728-amino acid alignment, and the mitochondrion phylogeny was inferred by ML and Bayesian inference methods. For the Bayesian approach, phylogeny was performed using MrBayes [35]; the GTR matrix was used, and model parameters (gamma shape and proportion of invariant) were allowed to vary through the Markov Chain Monte Carlo Chain (MCMC). Four MCMC chains were run for one million generations and sampled every 100th generation. The first 100,000 trees were discarded, and the “sumt” command of MrBayes was used to compute the clade posterior probabilities. Holosporaceae were not included because of unavailability of their proteomes. Bootstrap values were considered high when they were higher that 85 and PP higher that 0.85.The trees were rendered with MEGA4.
Supporting Information
65 Odyssella thessalonicensis proteins. http://www.biomedcentral.com/imedia/1660338084382525/supp1.txt
(TXT)
Acknowledgments
We thank Ghislain Fournous for technical support and Christelle Forzale for her help with the Figure 4. We would also like to thank the reviewers for their strong comments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Woese CR. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams KP, Sobral BW, Dickerman AW. A robust species tree for the Alphaproteobacteria. J Bacteriol. 2007;189:4578–4586. doi: 10.1128/JB.00269-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batut J, Andersson SG, O Callaghan D. The evolution of chronic infection strategies in the alpha-proteobacteria. Nat Rev Microbiol. 2004;2:933–945. doi: 10.1038/nrmicro1044. [DOI] [PubMed] [Google Scholar]
- 4.Merhej V, El Karkouri K, Raoult D. Whole genome-based phylogenetic analysis of Rickettsiae. Clin Microbiol Infect. 2009;15:336–337. doi: 10.1111/j.1469-0691.2008.02265.x. [DOI] [PubMed] [Google Scholar]
- 5.Nikoh N, Tanaka K, Shibata F, Kondo N, Hizume M, et al. Wolbachia genome integrated in an insect chromosome: evolution and fate of laterally transferred endosymbiont genes. Genome Res. 2008;18:272–280. doi: 10.1101/gr.7144908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavalier-Smith T. The phagotrophic origin of eukaryotes and phylogenetic classification of protozoa. Int J Syst Evol Microbiol. 2002;52:297–354. doi: 10.1099/00207713-52-2-297. [DOI] [PubMed] [Google Scholar]
- 7.Emelyanov VV. Rickettsiaceae, Rickettsia-like endosymbionts and the origin of mitochondria. Biosc Rep. 2001;21:1–17. doi: 10.1023/a:1010409415723. [DOI] [PubMed] [Google Scholar]
- 8.Cavalier-Smith T. Only six kingdoms of life. Proc Biol Sci. 2004;271:1251–1262. doi: 10.1098/rspb.2004.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R, et al. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. Plos Biol. 2004;2:e69. doi: 10.1371/journal.pbio.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esser C, Ahmadinejad N, Wiegand C, Rotte C, Sebastiani F, et al. A genome phylogeny for mitochondria among alpha-proteobacteria and a predominantly eubacterial ancestry of yeast nuclear genes. Mol Biol Evol. 2004;21:1643–1660. doi: 10.1093/molbev/msh160. [DOI] [PubMed] [Google Scholar]
- 11.Atteia A, Adrait A, Brugiere S, Tardif M, van Lis R, et al. A proteomic survey of Chlamydomonas reinhardtii mitochondria sheds new light on the metabolic plasticity of the organelle and on the nature of the alpha-proteobacterial mitochondrial ancestor. Mol Biol Evol. 2009;26:1533–1548. doi: 10.1093/molbev/msp068. [DOI] [PubMed] [Google Scholar]
- 12.Esser C, Martin W. Supertrees and symbiosis in eukaryote genome evolution. Trends Microbiol. 2007;15:435–437. doi: 10.1016/j.tim.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick DA, Creevey CJ, McInerney JO. Genome phylogenies indicate a meaningful alpha-proteobacterial phylogeny and support a grouping of the mitochondria with the Rickettsiales. Mol Biol Evol. 2006;23:74–85. doi: 10.1093/molbev/msj009. [DOI] [PubMed] [Google Scholar]
- 14.Giovannoni SJ, Tripp HJ, Givan S, Podar M, Vergin KL, et al. Genome streamlining in a cosmopolitan oceanic bacterium. Science. 2005;309:1242–1245. doi: 10.1126/science.1114057. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez Ezpeleta I, Embley TM. Data from: The closest Alphaproteobacteria relative to mitochondria: insights from Candidatus Pelagibacter ubique. Mol Biol Evol. 2011:doi:10.5061/dryad.6477. [Google Scholar]
- 16.Abhishek A, Bavishi A, Bavishi A, Choudhary M. Bacterial genome chimaerism and the origin of mitochondria. Can J Microbiol. 2011;57:49–61. doi: 10.1139/w10-099. [DOI] [PubMed] [Google Scholar]
- 17.Birtles RJ, Rowbotham TJ, Michel R, Pitcher DG, La Scola B, et al. 'Candidatus Odyssella thessalonicensis' gen. nov., sp. nov., an obligate intracellular parasite of Acanthamoeba species. Int J Syst Evol Microbiol. 2000;50:63–72. doi: 10.1099/00207713-50-1-63. [DOI] [PubMed] [Google Scholar]
- 18.Lang BF, Burger G, O Kelly CJ, Cedergren R, Golding GB, et al. An ancestral mitochondrial DNA resembling a eubacterial genome in miniature. Nature. 1997;387:493–497. doi: 10.1038/387493a0. [DOI] [PubMed] [Google Scholar]
- 19.Fox GE, Wisotzkey JD, Jurtshuk P. How close is close- 16S ribosomal RNA sequence identity may not be sufficient to guarantee species identity. Int J Syst Bacteriol. 1992;42:166–170. doi: 10.1099/00207713-42-1-166. [DOI] [PubMed] [Google Scholar]
- 20.Rossello Mora R, Amann R. The species concept for prokaryotes. FEMS Microbiol Rev. 2001;25:39–67. doi: 10.1111/j.1574-6976.2001.tb00571.x. [DOI] [PubMed] [Google Scholar]
- 21.Soltis SP, Soltis ED. Applying the bootstrap in phylogeny reconstruction. Statist Sci. 2003;18:256–267. [Google Scholar]
- 22.Merhej V, Royer Carenzi M, Pontarotti P, Raoult D. Massive comparative genomic analysis reveals convergent evolution of specialized bacteria. Biol Direct. 2009;4:13. doi: 10.1186/1745-6150-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris MB, Rappé SM, Connon SA, Vergin LK, Siebold WA, et al. SAR11 dominates ocean surface bacterioplankton communities. Nature. 2002;420:806–810. doi: 10.1038/nature01240. [DOI] [PubMed] [Google Scholar]
- 24.Moliner C, Raoult D, Fournier PE. Evidence that the intra-amoebal Legionella drancourtii acquired a sterol reductase gene from eukaryotes. BMC Res Notes. 2009;2:51. doi: 10.1186/1756-0500-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75–90. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsen N, Olsen GJ, Maidak BL, McCaughey MJ, Overbeek R, et al. The ribosomal database project. Nucleic Acids Res. 1993;21:3021–3023. doi: 10.1093/nar/21.13.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blattner FR, Plunkett G, Bloch CA, Perna NT, Burland V, et al. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 28.Lang BF, Burger G, O Kelly CJ, Cedergren R, Golding GB, et al. An ancestral mitochondrial DNA resembling a eubacterial genome in miniature. Nature. 1997;387:493–497. doi: 10.1038/387493a0. [DOI] [PubMed] [Google Scholar]
- 29.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113–132. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007;56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 31.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 32.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 34.Soria Carrasco V, Talavera G, Igea J, Castresana J. The K tree score: quantification of differences in the relative branch length and topology of phylogenetic trees. Bioinformatics. 2007;23:2954–2956. doi: 10.1093/bioinformatics/btm466. [DOI] [PubMed] [Google Scholar]
- 35.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 36.Ogata H, Audic S, Renesto-Audiffren P, Fournier PE, Barbe V, et al. Mechanisms of evolution in Rickettsia conorii and Rickettsia prowazekii. Science. 2001;293:2093–2098. doi: 10.1126/science.1061471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
65 Odyssella thessalonicensis proteins. http://www.biomedcentral.com/imedia/1660338084382525/supp1.txt
(TXT)