SUMMARY
The emergence of multidrug-resistant cancers and the lack of targeted therapies for many cancers underscore an unmet need for new therapeutics with novel modes of action towards cancer cells. Host-defense peptides often exhibit selective cytotoxicity towards cancer cells and show potential as anti-cancer therapeutics. Here, we screen 26 naturally occurring variants of the peptide pleurocidin for cytotoxic and anti-cancer activities, and investigate the underlying mechanism of action. Cytotoxicities were assessed in vitro using cell-based assays and in vivo using zebrafish embryos. Morphological changes were assessed by both transmission and scanning electron microscopy, and functional assays were performed on zebrafish embryos to investigate the mechanism of cell death. A total of 14 peptides were virtually inactive against HL60 human leukemia cells, whereas 12 caused >50% death at ≤32 μg/ml. Morphological changes characteristic of oncosis were evident by electron microscopy after only 1 minute of treatment with 32 μg/ml of variant NRC-03. Only two peptides were hemolytic. Four peptides showed no toxicity towards zebrafish embryos at the highest concentration tested (25 μM; ∼64 μg/ml) and one peptide was highly toxic, killing 4-hour-post-fertilization (hpf) embryos immediately after exposure to 1 μM peptide. Four other peptides killed embryos after 24 hours of exposure at 1 μM. Most peptides caused mortality at one or more developmental stages only after continuous exposure (24 hours) with higher lethal doses (≥5 μM). Pleurocidin NRC-03 bound to embryos and induced the release of superoxide, caused an increase in the number of TUNEL-positive nuclei, and caused membrane damage and the loss of embryonic epithelial integrity, marked by the exclusion of cells from the outer epithelium and the appearance of F-actin within the circumferential cells of the repair site. Our results indicate that specific pleurocidin variants are attractive cancer-selective agents that selectively induce cell death in target cells but leave non-target cells such as erythrocytes and non-transformed cells unaffected.
INTRODUCTION
Most currently used anti-cancer therapeutics rely on the rapid cell division of neoplastic cells rather than on other cancer-cell-specific traits to exert their actions. Unfortunately, normal healthy cells that divide rapidly are also killed, and conversely slow-growing or dormant cancer cells are left unaffected. Another drawback of current chemotherapeutic agents is their lack of efficacy against multidrug-resistant tumors. Thus, novel approaches to cancer drug treatment that selectively target cancer cells are urgently needed. Cancer-selective membrane-lytic peptides such as cationic antimicrobial (host defense) peptides (CAPs) offer great promise because their main mode of action is physical disruption of cancer cell membranes or cancer cell mitochondrial membranes, resulting in cell death (Mader and Hoskin, 2006). Magainin has been shown to selectively kill bladder cancer cells, although the concentration required (198 μM) was quite high (Lehmann et al., 2006). Necrotic peptides isolated from Australian frogs and toads, insect cecropins and various defensins, as well as apoptotic peptides from various sources also possess moderate anti-cancer activity (Bhutia and Maiti, 2008).
CAPs are crucial components of the innate immune system of most organisms (Nijnik and Hancock, 2009) and form the first line of defense against invading pathogens through their direct killing capabilities (Zasloff, 2002). Their ability to kill not only microbes but also cancer cells makes them attractive candidates as therapeutic agents in human medicine (Zhang and Falla, 2010). CAPs aggregate and associate with anionic membranes, such as those found on bacteria and cancer cells, causing pore formation and leakage of cell contents (Brogden, 2005; Dennison et al., 2006). Because the interaction with cells is based on charge properties rather than cell proliferation, both rapidly dividing and quiescent cancer cells are likely to be targeted by CAPs. By contrast, normal non-transformed cells with less-negatively charged membranes should not be susceptible. Furthermore, because CAPs are larger than currently used anti-cancer small-molecule inhibitors such as tyrosine kinase inhibitors, multidrug efflux pumps are ineffective against CAPs. In addition, because CAPs do not target specific receptors, mutations or overexpression of such receptors in certain cancers are not likely to present problems, unlike the case with various immunotherapeutics. They are also much smaller than chemotherapeutic antibodies and demonstrate superior penetration of cancer cells (Bhutia and Maiti, 2008).
Pleurocidins are a family of positively charged α-helical CAPs secreted by the mucosal surfaces lining the gut and skin of pleuronectid flatfish (Douglas et al., 2001), and have widely differing properties with respect to charge, molecular weight and antimicrobial efficacy (Patrzykat et al., 2003). Most studies on the mechanism of action of CAPs have been performed with model membranes (Nguyen et al., 2009) or in vitro assays; reports using in vivo systems are limited. A number of models have been advanced to explain how CAPs cause pore formation or membrane instability (Hale and Hancock, 2007). For example, lysis of the anionic mitochondrial and/or plasma membranes by α-helical CAPs has been proposed to occur by the carpet model and be dependent on physical properties of the CAP such as α-helicity (Dennison et al., 2006). The well-studied CAPs magainin-2 and lactoferrin have been shown to cross bacterial membranes and become localized in the cytoplasm (Haukland et al., 2001). In artificial (Matsuzaki et al., 1996) and bacterial (Imura et al., 2008) membranes, magainin-2 is believed to aggregate and form short-lived toroidal pores 2–3 nm in diameter and then translocate inside the cell. By contrast, in Chinese hamster ovary K1 (CHO K1) cells, magainin-2 forms pores that are 23 nm in diameter, and this is accompanied by membrane budding, lipid flip-flop and localization of peptide in the nucleus and mitochondria (Imura et al., 2008). Bladder cancer cells treated with magainin exhibit disrupted cell membranes containing pores, possibly caused by peptide carpeting the membrane and causing vesiculation prior to pore formation (Lehmann et al., 2006). Structural studies have shown that pleurocidin forms an amphipathic α-helix in membrane-mimicking environments (Syvitski et al., 2005) and that it forms pores in lipid bilayers (Yoshida et al., 2001; Saint et al., 2002). The closely related piscidins also form pores in fungal membranes (Sung et al., 2008). Various analogs and enantiomers of pleurocidin have been synthesized in order to improve activity and stability (Jung et al., 2007; Lee and Lee, 2008), thereby making them more efficacious. The processes resulting in CAP-mediated death of cancer cells remain elusive. In general, there are three phases of cellular response to cell injury: prelethal, cell death and necrosis. Both apoptosis and oncosis are prelethal responses leading to cell death and can be distinguished morphologically and biochemically. Apoptosis is energy dependent and characterized by cell shrinkage and nuclear fragmentation resulting in apoptotic bodies (Trump et al., 1997). Caspase-dependent apoptosis can be mediated by cell membrane death receptors or via mitochondria, and caspase-independent apoptosis has also been described (Lee et al., 2006). Oncosis, by contrast, is induced by energy depletion and failure of membrane ionic pumps, resulting in cell swelling (Van Cruchten and Van Den Broeck, 2002). Necrosis is the process of cellular degradation following cell death by apoptosis or oncosis (Majno and Joris, 1995). Using HL-60 cells as an in vitro model, we have investigated which of these processes is involved in pleurocidin-mediated cytotoxicity of cancer cells.
For in vivo studies, zebrafish embryos provide an affordable and relatively rapid approach to both screening pleurocidins for cytotoxicity and probing mechanism-of-action. There are many experimental advantages of the zebrafish, including the ability to generate large numbers of optically translucent embryos by external fertilization, rapid embryonic maturation, a high degree of cellular and developmental homology to mammals, permeability to small molecules, a nearly complete genome sequence, and a variety of mutants and functional genomic and genetic tools. Its widespread use for chemical screening makes it particularly attractive and relevant for in vivo studies of newly identified biologically active compounds to complement in vitro cell-based assays.
The zebrafish has shown great promise not only as a developmental model (Amsterdam and Hopkins, 2006) but also as a tool for the study of various human diseases, including cancer (Meeker and Trede, 2008). Zebrafish embryos have been used to screen a library of over 100 triazine compounds as anti-cancer drugs targeting tubulin (Moon et al., 2002). Fin reduction in zebrafish embryos was used as a readout for the anti-proliferative activity of ruthenium derivatives, which are promising alternatives to platinum-based chemotherapeutic agents such as cisplatin (Wang et al., 2009). The anti-proliferative organometallic compound vanadocene has also been assessed in early-stage zebrafish embryos (Navara et al., 2001). Numerous techniques and methods have also been developed and utilized to study the mechanisms of apoptosis during normal embryonic development as well as in the context of cellular stressors in the zebrafish embryo (Eimon and Ashkenazi, 2010).
Here, we describe the screening of 26 naturally occurring members of the pleurocidin family, as well as an enantiomer of NRC-03 (containing D-lysine and D-arginine amino acids to enhance stability), for their toxicity against human leukemia cells and erythrocytes in vitro. Examination of cells treated with NRC-03 by both scanning and electron microscopy elucidated the mode of cell death. Pleurocidins were also screened in an in vivo model, the zebrafish embryo, and the molecular mechanism of cell death initiated by the pleurocidin variant NRC-03 was probed using various functional assays, comparing pleurocidin with the relatively well-studied α-helical CAP magainin-2 (Baker et al., 1993; Imura et al., 2008).
RESULTS AND DISCUSSION
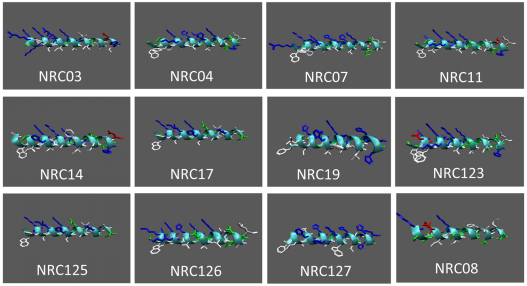
Exposure of human leukemia (HL60) cells to various concentrations of each pleurocidin variant (Table 1) revealed that 14 of the 26 pleurocidin peptides (NRC-01, -02, -05, -06, -08, -09, -10, -12, -13, -15, -18, -20, -124 and -128) were virtually inactive, whereas 12 peptides (NRC-03, -04, -07, -11, -14, -16, -17, -19, -123, -125, -126 and -127) were highly toxic (lytic activity values below 32 μg/ml), as assessed by MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay. By comparison, magainin-2 was inactive at 128 μg/ml (52 μM), in agreement with previous studies involving bladder cancer cells (Lehmann et al., 2006). Structural modeling showed that the active peptides adopted a typical amphipathic α-helical structure with the positively charged amino acids on one face and the hydrophobic amino acids on the opposite face (Fig. 1). By contrast, none of the inactive peptides were amphipathic when modeled as helical wheels (data not shown). The active peptides also tended to be much more highly charged, at least +6.5, whereas most of the inactive peptides, including magainin-2, had charges of only +2 to +4.5 (Table 1). NRC-03B showed similar activity to its unbiotinylated counterpart (8 μg/ml) and the NRC-03 enantiomer that contained D-lysine and D-arginine was inactive.
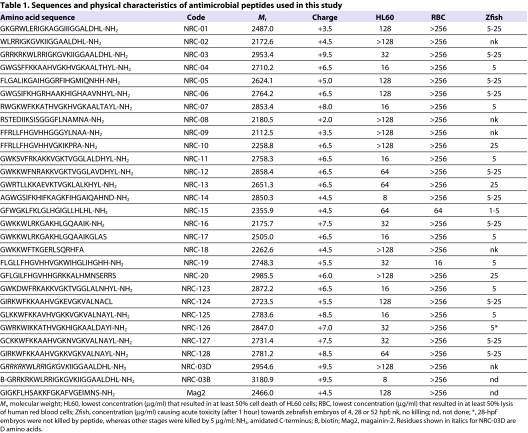
Table 1.
Sequences and physical characteristics of antimicrobial peptides used in this study
Fig. 1.
Structural modeling of pleurocidin variants with high cytotoxicity to HL60 cells. NRC-16 is not shown because it is a truncated version of NRC-17 (missing the last four amino acids). NRC-08, which is inactive, is included to show the lack of amphipathicity compared with the active peptides. The amino acid sequence is modeled as an α-helix with positively charged amino acids shown in blue, negatively charged amino acids shown in red, hydrophobic amino acids shown in white and polar residues shown in green.
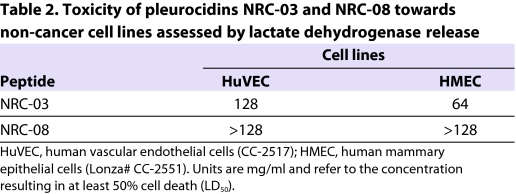
We next studied the effect of one of the highly toxic peptides, NRC-03, and one of the inactive peptides, NRC-08, against epithelial and endothelial non-cancer cell lines. As with HL60 cells, NRC-08 exhibited no toxicity towards any of the cell lines tested (Table 2). NRC-03 showed low toxicity towards the human epithelial and endothelial cell lines tested. NRC-03 has also been tested against a variety of breast cancer cell lines and shown high toxicity (Ashley L. Hilchie, Carolyn Doucette, Aleks Patrzykat, S.E.D. and David Hoskin, unpublished).
Table 2.
Toxicity of pleurocidins NRC-03 and NRC-08 towards non-cancer cell lines assessed by lactate dehydrogenase release
Hemolysis assays showed that only two of the pleurocidin peptides (NRC-15 and NRC-19) caused some erythrocyte lysis, whereas the rest were non-hemolytic at >256 μg/ml. Interestingly, NRC-19 contains seven histidine residues, and histidine-rich CAPs such as histatins have been shown to be hemolytic (Stallmann et al., 2005). This specificity of pleurocidins for cancer cells rather than non-cancer cells combined with the low concentration required for cell killing indicates that they show good potential as anti-cancer agents.
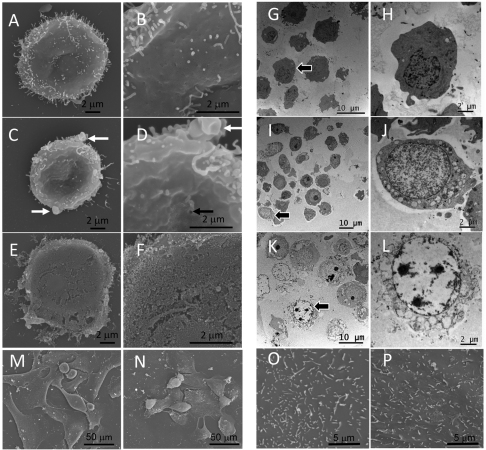
Normal HL60 cells have been shown to exhibit a background level of apoptosis, probably due to the spontaneous differentiation and maturation of this cell line (Martin et al., 1990). In agreement with this, we found that approximately 15% of the control untreated HL60 cells in scanning electron microscopy (SEM) images were shrunken, which is characteristic of apoptotic cells. No significant DNA laddering, a hallmark of apoptosis, was detected relative to positive controls even after 24 hours of exposure of HL60 cells to increasing concentrations of NRC-03 (Fig. 2). However, treatment with 32 μg/ml NRC-03 for as little as 1 minute resulted in loss of microvilli and the appearance of membrane pores and cell swelling typical of oncosis in SEM images (Fig. 3C,D) compared with controls (Fig. 3A,B). Treated cells had an average size of 16 μm, compared with an average size of 12.3 μm for untreated cells, whereas apoptotic cells had an average size of 9 μm. After 5 minutes there was extensive damage to the cell membrane in treated cells (Fig. 3E,F) and, at later time points, only cellular debris was present, with very few intact cells. Transmission electron microscopy (TEM) images also showed that the cells suffered damage after 1 minute of exposure to NRC-03 (Fig. 3I,J), with many cells showing severe vacuolation of the cytoplasm and cell swelling that is characteristic of oncosis rather than apoptosis. After 5 minutes (Fig. 3K,L), karyolysis and cell lysis were evident. Inspection of over 100 cells in semi-thin sections showed that the percentage of dying cells increased from 15% in untreated controls to 46% after 1 minute and 95% after 5 minutes of treatment, and the corresponding numbers of lysed cells increased from 0% to 12% and 30%, respectively. By contrast, the same treatment of human mammary epithelial cells (HMECs) with 32 μg/ml NRC-03 for as long as 4 hours did not result in loss of microvilli or cell lysis (Fig. 3M,N), although the microvilli appeared somewhat shorter (Fig. 3O,P). Instead, those cells that were affected by peptide showed a rounded up appearance indicative of apoptotic cells. Untreated controls also contained a small proportion of apoptotic cells. High-power SEM images showed no evidence of pores or membrane destruction in HMECs treated with NRC-03 compared with untreated controls (Fig. 3O,P).
Fig. 2.
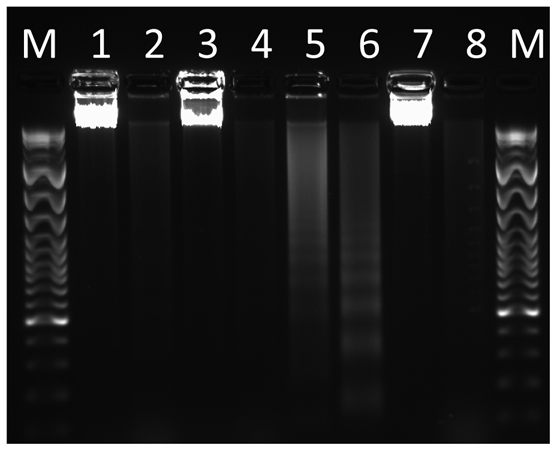
DNA fragmentation assay with HL60 cells exposed to NRC-03 for 24 hours with increasing concentrations of NRC-03. M, molecular weight markers; lane 1, pellet, 8 μg/ml treatment; lane 2, supernatant, 8 μg/ml treatment; lane 3, pellet, 16 μg/ml treatment; lane 4, supernatant, 16 μg/ml treatment; lane 5, pellet, positive control; lane 6, supernatant, positive control; lane 7, pellet, negative control; lane 8, supernatant, negative control.
Fig. 3.
SEM and TEM images of HL60 cells treated with NRC-03. (A–F) Treatment was carried out for 0 (A,B), 1 (C,D) and 5 (E,F) minutes with 32 μg/ml NRC-03. Note the loss of microvilli and appearance of membrane blebs (white arrows) and membrane damage (black arrow) after 1 minute, and disruption of membrane after 5 minutes. (G–L) TEM images of HL60 cells treated with NRC-03 for 0 (G,H), 1 (I,J) and 5 (K,L) minutes. Black arrows in G, I and K indicate cells shown at high magnification in plates H, J and L. (M–P) SEM images of HMECs treated for 0 (M,O) or 4 (N,P) hours with 32 μg/ml NRC-03 do not show membrane blebs or damage after treatment, although some cell shrinkage and rounding is evident.
Oncosis is known to occur within seconds to minutes following injury to the cell or damage to the plasma membrane (Trump et al., 1997). During this mode of cell death, cytoplasmic blebs appear, the chromatin condenses, and intracellular calcium increases resulting in the swelling of organelles such as mitochondria, endoplasmic reticulum and Golgi. Neither TEM nor SEM analysis of pleurocidin-treated HL60 cells provided evidence of cell shrinkage, which is characteristic of apoptosis, and apoptotic bodies were also not seen.
Examples of both apoptotic and oncotic cell death in response to other CAPs have been reported. Hepatocarcinoma cells treated with cecropin for 24 hours underwent caspase-mediated apoptosis (Jin et al., 2010), as did Jurkat leukemia cells treated with HNP1-3 (Aarbiou et al., 2006). Magainin-2 has been shown to cross the cell membrane by an energy- and receptor-independent mechanism (Takeshima et al., 2003), and to induce the mitochondrial-mediated apoptosis pathway by dissipating the membrane potential, promoting cytochrome c release and activating proteasomes (Westerhoff et al., 1989). By contrast, bladder tumor cells treated with magainin-2 showed disrupted cell membranes (Lehmann et al., 2006) similar to those seen after a 5-minute treatment with NRC-03, whereas fibroblasts were unaffected. Cell death was non-apoptotic and proposed to be due to the formation of ion-dissipating channels in the cell membrane, leading to depolarization and cell lysis. Oncosis has been described in several cases in which the membranes of mammalian cells were damaged by anti-cancer agents (see Sun et al., 2010). Mitochondrial uncoupling protein 2 (UCP2) has been implicated in the regulation of oncosis in HeLa cells (Mills et al., 2002), as have MAP kinase pathways (Romashko et al., 2003) and NFκB (Franek et al., 2004). Future studies will examine the possible involvement of these pathways in pleurocidin-mediated anti-cancer activity.
The cytotoxic effects of CAPs have been tested mainly in in vitro systems such as cell lines (Lehmann et al., 2006); few reports of in vivo studies exist and those that do typically use the relatively expensive sentient adult mouse models (Baker et al., 1993; Papo et al., 2003; Papo et al., 2004; Makovitzki et al., 2009). Because of the large family of pleurocidin peptide variants in our collection, we required a higher-throughput in vivo screen for cytotoxicity to complement our in vitro studies. Thus, we chose the zebrafish embryo as a model to assess the cytotoxicity of the pleurocidins in vivo, and as a tool to examine the mode of action of these compounds. Zebrafish are increasingly being used for screening pharmaceuticals and have been validated as a model for studying the mechanisms of action of anti-cancer drugs (Langheinrich, 2003).
The effects of administering the different pleurocidin variants at three different stages of development [4, 28 and 52 hours post-fertilization (hpf)] are shown in supplementary material Table S1. No cytotoxicity was evident in embryos of any stage after treatment with all three concentrations of NRC-02, -08, -09 or -18, as was seen with HL60 cells. The remaining peptides that were inactive in HL60 cells (NRC-01, -05, -06, -10, -12, -13, -20, -124 and -128) were slightly toxic to embryos, usually requiring at least 25 μM to kill all embryos. Similarly, the 12 peptides that were cytotoxic towards HL60 cells (NRC-03, -04, -07, -11, -14, -16, -17, -19, -123, -125, -126 and -127) were similarly toxic to the zebrafish embryo. Most of these peptides killed all embryos at 5 μM or at least 10% of the embryos at 1 μM at the three developmental stages, with the exception of NRC-16, which required 25 μM. Of the two hemolytic pleurocidins, NRC-15 was highly toxic, killing 4-hpf embryos after exposure to peptide at 1 μM, whereas NRC-19 killed at 5 μM. For mechanistic studies, we chose the moderately toxic NRC-03 for further evaluation.
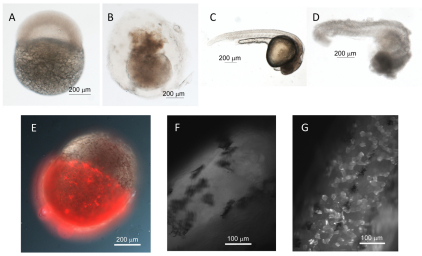
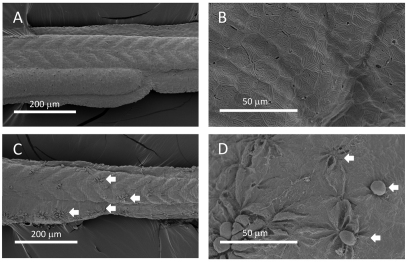
The effect of adding NRC-03 to 4-hpf and 28-hpf embryos is shown in Fig. 4A–D (representative embryos). There was a significant effect after treatment of 4-hpf embryos with 20 μM NRC-03 for 1 hour. The yolk sac ruptured and the animal pole cells began to dissociate. After treatment for 1 hour with 10 μM NRC-03, the 28-hpf embryos became more opaque, the integrity of the outer epithelial layer was disrupted and individual cells began to slough off, and finally the yolk-sac ruptured. All embryos treated with NRC-03 progressed as described above. Using fluorescently labeled streptavidin–Alexa-Fluor-546 to visualize NRC-03, peptide was seen to bind preferentially to the animal pole of 5-hpf embryos (Fig. 4E), and not to the extraembryonic yolk cytoplasmic layer, demonstrating the specificity of the pleurocidin for cell membranes. Similarly, NRC-03 binds to individual cells throughout the epithelial layer of 24-hpf embryos treated with 25 μM pleurocidin. Interestingly, not all cells were uniformly bound by NRC-03 during each treatment (Fig. 4G). Control experiments with non-biotinylated NRC-03 did not show any fluorescently labeled cells. To further examine the site of pleurocidin-induced cellular damage, we examined NRC-03-treated and non-treated 48-hpf embryos by SEM. Untreated embryos displayed the characteristic regularly patterned cell layer of the outer epithelium along with the outline of the chevron-shaped somites in the trunk and tail beneath the epithelium (Fig. 5A,B). After treatment with NRC-03, multiple wound repair events were evident and many cells had rounded up and dissociated from the outer epithelial layer (Fig. 5C,D). Characteristic of embryonic wound healing, healthy cells flanking the affected area migrated towards the site of the lesion to maintain the integrity of the epithelium and protect exposed underlying tissues. It seems that the presence of the dying cell, caused by pleurocidin treatment, triggers the wound healing event rather than loss of epithelial integrity itself or interactions with the extracellular matrix. The physical force required for this collective cell movement is mediated at least in part by the supercellular F-actin myosin cable or ‘purse string’ (visualized by phalloidin staining; see below) drawing the surrounding cells inward towards the site of the damage (Slattum et al., 2009). This occurs within minutes of wounding, before the activation of caspases (Rosenblatt et al., 2001), and requires activation of Rho GTPase (Brock et al., 1996). The signal originating in the dying cell that results in the movement of the surrounding cells has not been identified; it is possible that pleurocidin could trigger chemical or mechanosensing responses that induce the surrounding cells to form the actin purse string and extrude the damaged cell. Given the rapid formation of purse string structures, and the lack of evidence of caspase involvement or DNA fragmentation after pleurocidin treatment, it is unlikely that anoikis, which involves extrusion of apoptotic cells in response to the absence of cell-matrix interactions, is occurring. Hallmarks of anoikis include nucleosomal DNA ladder formation, cell shrinkage, caspase activation and/or cleavage of caspase substrates, and cytochrome c release from mitochondria (Frisch and Screaton, 2001).
Fig. 4.
Pleurocidin binds to and damages zebrafish embryos. (A–D) Effect of pleurocidin NRC-03 on 4-hpf and 28-hpf zebrafish embryos. (A) 4-hpf untreated control embryo. (B) 4-hpf embryo 1 hour after treatment with 20 μM NRC-03. (C) 28-hpf untreated control embryo. (D) 28-hpf embryo 1 hour after treatment with 10 μM NRC-03. (E-G) Localization of NRC-03 by fluorescence microscopy. (E) Composite bright-field and fluorescence image of a 5-hpf embryo treated with 1 μM NRC-03B for 3 hours and stained with streptavidin–Alexa-Fluor-546, showing signal concentrated on the animal pole. (F,G) Fluorescence images of the trunk of a 24-hpf untreated embryo (F) and embryo treated with 25 μM NRC-03B for 2 hours (G). Dark staining patches in F and G are melanophores.
Fig. 5.
SEM images of the tails of 48-hpf embryos treated with NRC-03. (A,B) Untreated. (C,D) 2 μM NRC-03 treatment for 25 minutes followed by 2 μM NRC-03 for an additional 25 minutes. Arrows indicate wound repair events. Anterior is to the left and dorsal is up.
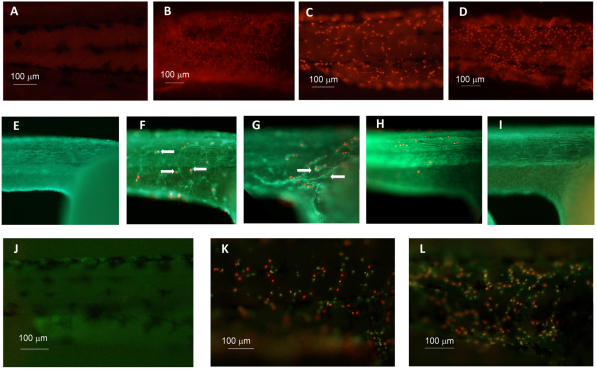
The mechanism by which pleurocidin mediates cell death was probed using a number of functional assays, including dihydroethidium staining of the reactive oxygen species (ROS) superoxide, TUNEL staining of nicked DNA, and Vybrant staining of dying cells (Fig. 6).
Fig. 6.
Functional assays of the cytotoxicity of NRC-03 and magainin-2 on zebrafish embryos. (A–D) DHE staining for superoxide in 48-hpf embryos. (A) Untreated. (B) 2 mM KCN treatment for 4 hours. (C) 3.75 μM NRC-03 treatment for 2 minutes. (D) 7.5 μM magainin-2 treatment for 10 minutes. (E–I) TUNEL staining for nicked DNA with actin counterstaining in the trunk of 24-hpf embryos. (E) Untreated. (F) 2.5 μM NRC-03 treatment for 20 minutes. (G) 3.75 μM magainin-2 treatment for 20 minutes. (H) Positive control (DNase I treated). (I) Negative control (no TUNEL label added). (J–L) Vybrant staining for apoptotic and oncotic or necrotic cells of 48-hpf embryos. (J) Untreated. (K) 3.75 μM NRC-03 treatment for 2 minutes. (L) 10 μM magainin-2 treatment for 6 minutes. Anterior is to the right and dorsal is up.
ROS are known to induce apoptosis (Pelicano et al., 2004), but both apoptosis and oncosis can occur together in response to differing concentrations of ROS such as hydrogen peroxide, with oncosis predominating at high concentrations (Lecoeur et al., 2001). In a mouse model of renal ischemia, hydrogen peroxide was shown to cause both apoptosis and oncosis as well as apoptotic nuclei in oncotic cytoplasm in S3 proximal straight tubule cells (Takeda et al., 1999). Esophageal squamous carcinoma tumors exhibit an increased number of oncotic rather than apoptotic cells in regions distant from microvessels, where oxygen, blood supply and cellular energy stores are limited (Zhao et al., 2007). This reduced cellular energy content might explain the enhanced susceptibility of cancer cells to CAPs such as pleurocidin and the ensuing oncotic cell death. Using the superoxide-sensitive dye dihydroethidium (DHE), we demonstrated that superoxide is released from mitochondria into the cytoplasm of affected zebrafish cells shortly after treatment with NRC-03 and magainin-2, resulting in red fluorescing nuclei (Fig. 6C,D). We were unable to find reference in the literature to the use of DHE in zebrafish, and therefore validated our assay using KCN, a known inhibitor of superoxide dismutase (Shearer et al., 2003). Superoxide dismutase inhibits superoxide-mediated cytochrome c reduction (Forman and Kennedy, 1975) and has been studied in zebrafish (Mendelsohn et al., 2008). Treatment with KCN yielded a similar result and the negative control containing no peptide showed no red nuclei (Fig. 6A,B). These results are in agreement with previous studies with magainin-2 (Westerhoff et al., 1989) and lactoferrin B, which also induces ROS production and cytochrome c release in Jurkat cells (Mader et al., 2007).
Superoxide anions have also been shown to induce a caspase-independent apoptotic pathway in T lymphocytes (Hildeman et al., 1999). Similarly, oncosis usually does not require caspases. Using the caspase-9 inhibitor Z-LEHD-FMK, we were unable to inhibit NRC-03-mediated cell death (data not shown), indicating that either oncosis or caspase-9-independent apoptosis is induced by NRC-03 in zebrafish embryos. Unfortunately, this is impossible to definitively confirm because we were unable to find a positive control for Z-LEHD-FMK inhibition of caspase-9 in zebrafish.
Although these results demonstrate that NRC-03 targets mitochondria, it is unlikely that the release of superoxide and possibly other ROS are the sole mediators of cytotoxicity. Pre-incubation of embryos with reduced glutathione, which protects against peroxides, did not affect survival of NRC-03-treated embryos (data not shown). Furthermore, we did not see wound repair events in KCN-treated embryos, which also demonstrates that NRC-03 is more than simply interrupting the electron transport chain.
TUNEL-staining of nicked DNA ends, once thought to be the hallmark of apoptosis, is sometimes found during the process of oncosis as well (Saikumar et al., 1999). Exposure of embryos to either NRC-03 or magainin-2 generated DNA strand breaks in affected cells as judged by TUNEL staining (Fig. 6F,G). Subsequent staining of filamentous actin with Alexa-Fluor-488-conjugated phalloidin revealed that these damaged cells were almost always (99.7%) associated with actin condensation and cellular rearrangements that occur during embryonic epithelial wound repair. It is remarkable that not all cells are undergoing apoptosis, or actin rearrangement, indicating that only a fraction of cells are affected at a given time. This is in agreement with our fluorescence data that showed that NRC-03 bound to only some cells (Fig. 4). We do not know whether this is due to a predisposition by these cells for NRC-03 binding, or whether it is simply a random, concentration-dependant event. These rearrangements include the extension and migration of neighboring cells towards the wound site, and the formation of the characteristic actin-myosin purse string that accompanies embryonic wound repair (Slattum et al., 2009). Whereas 99.7% of TUNEL-positive cells were associated with wound repair events, only 34.8% of wound repair events were associated with TUNEL-positive cells (31 fields counted, 538 wound repair events, 198 TUNEL-positive cells), presumably because the damaged cell inducing the wound repair event had already been extruded from the epithelium and could no longer be detected by TUNEL staining. Production of TUNEL-positive cells has been documented for several other CAPs, including cecropin (Jin et al., 2010), lactoferrin B (Mader et al., 2007), cathelicidin LL-37 and HNP1-3 (Aarbiou et al., 2006). Interestingly, in the latter work, although both HNP1-3 and LL-37 were capable of generating TUNEL-positive cells, caspase involvement was both cell-line- and peptide-specific.
As discussed above, both apoptotic and oncotic cells are often found together in tissues that are subjected to stress, ROS and ischemia. In agreement with this, rodent ischemic astrocytes showed a high incidence of oncotic cell death with concomitant nuclear labeling with propidium iodide (PI), but little labeling with annexin V, a marker of apoptosis (Simard et al., 2006). There are no published reports of successful annexin V staining of zebrafish embryos and unfortunately we were also unable to obtain reliable annexin V staining. To this end, the distinction between apoptotic and oncotic or necrotic cells was further investigated using the Vybrant apoptosis assay (Fig. 6K,L). Whereas healthy cells are impermeable to both dyes, the apoptotic cells resulting from treatment of embryos with NRC-03 or magainin-2 were permeable to the YO-PRO-1 dye, but impermeable to PI, resulting in green nuclei. Conversely, oncotic or necrotic cells, which lose membrane integrity, are permeable to both dyes, resulting in red or yellow nuclei in overlay images. Both NRC-03- and magainin-2-treated embryos contained a mixture of apoptotic and oncotic or necrotic cells. It is difficult to interpret the significance of the nuclei that took up both stains (yellow). Although they might simply be cells with membrane damage that are permeant to both dyes, the presence of PI-only nuclei (red) might suggest that the yellow nuclei represent cells that were damaged just after the onset of apoptosis.
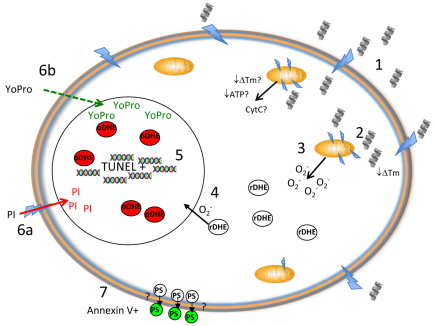
In summary, the most amphipathic and positively charged members of the pleurocidin family exhibit potent and selective oncotic activity against human leukemia cells in vitro. In vivo studies in the zebrafish embryo indicate that NRC-03 binds to the membrane and forms holes, as summarized in Fig. 7. DHE staining demonstrates the production of superoxide from damaged mitochondria, which might be accompanied by decreased intracellular energy reserves. Vybrant staining of NRC-03-treated embryos indicates that cell death is caused by membrane permeabilization, and TUNEL staining suggests that the DNA in damaged cells becomes fragmented. In response to this cell injury, the embryo forms purse string structures that draw in the cells surrounding the injury in order to contain the damage. These features of NRC-03-mediated cytotoxicity of cancer cells make it an attractive candidate for development in anti-cancer therapy.
Fig. 7.
Proposed mechanism of pleurocidin-mediated cytotoxicity. Peptide causes the formation of small pores in the cell membrane (1), disrupting transmembrane potential. Intracellular peptide targets mitochondria (2), causing the generation of superoxide, and possibly the loss of mitochondrial membrane potential (Tm), ATP generation (ATP) and the release of cytochrome c (CytC) (3). Oxidation of reduced DHE (4) results in red fluorescing nuclei and DNA fragmentation occurs, resulting in TUNEL-positive nuclei (5). PI enters through small pores of oncotic or necrotic cells, resulting in red fluorescing nuclei (6a), whereas YO-PRO-1 enters apoptotic cells that have intact membranes, resulting in green fluorescing nuclei (6b). Phosphatidylserine translocates to the outer leaflet of the plasma membrane in both apoptotic and oncotic cells, resulting in green annexin-V-positive staining (7). ATP, adenosine triphosphate; CytC, cytochrome c; O2–, superoxide; PI, propidium iodide; PS, phosphatidylserine; rDHE, reduced dihydroethidium; oDHE, oxidized dihydroethidium; ?, hypothetical actions not confirmed in this study. Jagged arrows represent pores in membrane; gray helices represent pleurocidin α-helical peptide; colored double helices represent fragmented DNA; yellow ovals represent mitochondria.
METHODS
Pleurocidin peptides
The amino acid sequences of pleurocidin peptides were predicted from nucleic acid sequences as previously described (Patrzykat et al., 2003) and those that were predicted to contain a C-terminal glycine were amidated (Table 1). Peptides NRC-01 to NRC-20 were synthesized by N-9-fluorenylmethoxycarbonyl (Fmoc) chemistry at Dalton Chemical Laboratories (Toronto, ON, Canada) and NRC-123 to NRC-128 were synthesized at the Nucleic Acid Protein Service unit at the University of British Columbia (Vancouver, BC, Canada). The remaining peptides were synthesized by BioBasic (Markham, ON, Canada). NRC-03D contained D-lysine and D-arginine residues to prevent protease digestion, and NRC-03B contained biotin at the N-terminus for subsequent fluorescent detection with streptavidin–Alexa-Fluor-546. Peptide purity (75–95%) was confirmed by high-performance liquid chromatography (HPLC) and mass spectrometry analysis. All peptide stocks were prepared in ddH2O and stored at −20°C until use. For peptide exposure of embryos, stocks were diluted to the working concentration in E3 medium (see below).
Cell lines
Human leukemia (HL60) cells were obtained from ATCC (CCL-240) and cultured at 37°C and 5% CO2 in RPMI media containing 10% fetal bovine serum (FBS). Prior to pleurocidin exposure, cells were washed and resuspended in media containing 2.5% FBS to minimize adsorption of peptide by serum components. HMECs and human umbilical vein endothelial cells (HuVECs) were obtained from Cambrex Bio Science Walkersville (Walkersville, MD) and maintained in supplemented MEBM according to the manufacturer’s protocol.
Cytotoxicity assay
Cytotoxicity of peptides against HL60 cells was measured using the standard MTT assay in microtitre plates. Cells (2×105) were incubated with twofold dilutions of peptide (1 μg/ml to 128 μg/ml) and MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; Sigma, Oakville, ON, Canada] for 2 hours at 37°C and 5% CO2. The negative control contained no peptide and the positive control contained 1% Triton X-100. Cytotoxicity was recorded as the lowest concentration that resulted in at least 50% cell death. Cytotoxicity was also assessed by measuring the release of lactate dehydrogenase (LDH) into the media from damaged cells. The level of LDH was assayed by using the Cytotoxicity Detection kit (Roche Molecular Biochemicals, Laval, PQ, Canada). The reduction of NAD to NADH was evaluated by detecting changes in absorbance of formazan salt at 490 nm.
Hemolysis assay
The ability of peptides to lyse human erythrocytes was assessed using standard procedures (Bulmus et al., 2003). Erythrocytes were incubated with twofold dilutions of peptide (2 μg/ml to 256 μg/ml) for 1 hour at 37°C. The negative control contained no peptide, the blank contained no cells, and the positive control contained 1% Triton X-100. Hemolytic activity was recorded as the lowest concentration that resulted in at least 50% hemolysis.
DNA fragmentation assay for apoptosis
The ability of NRC-03 to cause nucleosomal DNA fragmentation in HL60 cells was assessed in 12-well plates using a modification of the published method (Herrmann et al., 1994). HL60 cells (5×106) were incubated for 24 hours in the presence of NRC-03 (8 and 16 μg/ml; higher concentrations were not possible owing to extensive cell lysis and DNA degradation that would occur after 24 hours of incubation). Cells were pelleted by centrifugation, lysed, treated with proteinase K and RNase, and the DNA ethanol-precipitated. DNA in cell supernatants was also ethanol-precipitated and all samples were analyzed by agarose gel electrophoresis for fragmentation into 200-bp fragments. The negative control contained no peptide and the positive control contained a mixture of actinomycin D (1 μM) and A23187 (1 μM), known inducers of apoptosis.
Zebrafish husbandry and embryo collection
All experiments used wild-type AB zebrafish exclusively. Zebrafish were maintained at 28°C on a 14 hour light:10 hour dark cycle according to standard culture conditions (Westerfield, 2000) in a ZebTEC housing system (Tecniplast, Exton, PA). All animals were treated in accordance with Canadian Council for Animal Care guidelines. Fertilized embryos were collected and maintained at 28°C at a density of <100 embryos per 100×15 mm dish in E3 medium until the appropriate developmental stage was reached.
Zebrafish embryo toxicity assay
Zebrafish embryos at 4, 28 and 52 hpf were used to test potential toxic effects of pleurocidins in 96-well plates. Each peptide was tested on ten embryos at 0, 1, 5 and 25 μM in 100 μl of E3 medium, in triplicate. Plates were monitored for survival after 1-and 24-hour treatments. The level of treatment was considered ‘toxic’ at first indications of lethality (i.e. >10% lethality). Untreated wild-type controls routinely showed no teratogenic effects or toxicity associated with 24-hour incubation. In the event that the wild-type untreated controls displayed any adverse effects, the experimental replicates were discarded. 4-hpf embryos treated with 20 μM NRC-03 for 1 hour were fixed overnight in 4% paraformaldehyde (PFM) or without PFM, dechorionated and imaged using a Nikon AZ100 stereomicroscope, with an AZ Plan Apo 1× lens and 7× zoom. All images were processed using NIS Elements BR 2.30 software (Nikon Instruments, Melville, NY). Embryos beyond 24 hpf were manually dechorionated and treated with or without 10 μM NRC-03 for 1 hour, prior to being fixed, and treated as described above.
DHE assay
The presence of superoxide in the cytoplasm of live embryos was detected by adding DHE (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Embryos (48 hpf) were exposed to 3.75 μM NRC-03 or 7.5 μM magainin-2 and immediately received 3 μM DHE. Embryos were imaged within 5 minutes to demonstrate the presence of oxidized DHE (red). As a positive control for superoxide release, embryos were treated with KCN (2 mM) for 4 hours in 10 mM Tris-buffered E3 (pH 7.5).
TUNEL assay and phalloidin staining
DNA fragmentation was detected using the In Situ Cell Death Detection Kit (Roche Molecular Biochemicals, Laval, PQ, Canada) according to the manufacturer’s protocol. Dechorionated 24-hpf embryos were treated for 20–60 minutes with 2.5 μM NRC-03 or 3.75 μM magainin-2 in E3, and then fixed overnight at 4°C in 4% PFM. Fixative was removed by washing in PBS and embryos were incubated in permeabilization solution for 1 minute at room temperature followed by 2 minutes on ice. Embryos were treated with TUNEL labeling solution for 60 minutes at 37°C in the dark, washed and then labeled with Alexa-Fluor-488-conjugated phalloidin (Invitrogen) for 20 minutes at room temperature, followed by three 10-minute washes in PBS prior to imaging. For the positive control, one group of untreated embryos received 3000 U of DNAase I (Invitrogen) for 10 minutes. Similarly, a no TUNEL enzyme negative control was performed on untreated embryos. Wound repair events and TUNEL-positive cells for each treatment were counted in fields derived from multiple fish, and from multiple locations on the embryos.
Vybrant apoptosis assay
The detection of apoptotic and oncotic or necrotic cells in peptide-treated embryos was analyzed using the Vybrant Apoptosis Kit #4 (Invitrogen). NRC-03 (0.25–5 μM) or magainin-2 (5–10 μM) were used in order to observe both healthy and dying cells within the same embryos. Treated embryos were washed in PBS and stained simultaneously with 0.2 μM of the green-fluorescent nucleic acid stain YO-PRO-1 (Invitrogen) and 0.5 μg/ml PI, and were immediately visualized.
Fluorescent in vivo detection of biotinylated NRC-03
Dechorionated embryos treated with NRC-03B were fixed in 4% PFM, washed in PBS, dehydrated in methanol, rehydrated in PBS and blocked in PBT-N (10% normal goat serum in PBT) at 4°C for 2–12 hours, followed by treatment with Endogenous Biotin-Blocking Kit (Molecular Probes, Eugene, OR) according to the manufacturer’s recommendations. Embryos were incubated overnight at 4°C with conjugated streptavidin–Alexa-Fluor-546 (Molecular Probes) diluted 1/2000 in PBT-N. Embryos were washed in PBT and mounted in PBS for observation.
Fluorescent microscopy
DHE, TUNEL, phalloidin and Vybrant-treated embryos were visualized on a Nikon AZ100 fluorescent microscope, with an AZ Plan Apo 4× lens, and 3–6× zoom, using the appropriate filter modules and settings (TE-DAPI/Hoechst, TE-FITC/EGFP Bodipy, TE-TRITC–HQ/Rhodamine). All images were processed using NIS Elements BR 2.30 software. 5-hpf embryos treated with biotinylated NRC-03B were imaged using a Nikon AZ100 fluorescent microscope using NIS elements BR 2.30 software, whereas the images of 24-hpf embryos were obtained using a Leica DMRE microscope (Wetzlar GmbH, Germany), with Simple PCI software (Hamamatsu, Sewickley, PA).
Scanning electron microscopy
HL60 cells were treated with 32 μg/ml NRC-03 for 1–30 minutes, fixed in 4% PFM and prepared for SEM. Samples were dehydrated in a graded ethanol series, critical point drying was performed using a BOMAR SPC-900 (The Bomar Co., Tacoma, WA), gold/palladium coating was performed in a SC7620 sputter coater (Quorum Technologies, East Sussex, England) and images were acquired using an S300N scanning electron microscope (Hitachi, Tokyo, Japan). Average cell size was calculated by measuring the diameters of at least 50 cells in several fields of control and 1-minute treatments with NRC-03. Apoptotic cells were identified as small cells with numerous small membrane protrusions in samples of untreated cells.
HMECs were seeded (8×104 cells/well) and allowed to settle overnight on 15-mm Thermonox plastic coverslips (Nunc, Rochester, NY) in 12-well plates in 1 ml of supplemented MEBM media. Cells were treated with 32 μg/ml NRC-03 for 5 minutes, 1 hour and 4 hours, washed twice with 2 ml DPBS and fixed with 1 ml of 1G4F for at least 24 hours at 4°C and prepared for SEM as described above.
To test the effect of pleurocidin activity, 48-hpf embryos were manually dechorionated in E3 and treated with 2 μM NRC-03 for 30 minutes, prior to fixation in 4% PFM. All samples were then prepared for SEM as described above.
TRANSLATIONAL IMPACT.
Clinical issue
There is an unmet need for new anti-cancer therapeutics with novel modes of action – particularly with respect to drug-resistant cancers. Some host-defense peptides, many of which make up families of peptides that differ slightly in amino acid sequence, exhibit selective cytotoxicity towards cancer cells and are therefore promising anti-cancer therapeutics. However, methods and models required to screen them and ascertain their mechanism of action are currently lacking. Although assays based on cell lines are useful, whole animal model systems provide much more relevant information on the activity and possible side effects of novel compounds. Zebrafish embryos provide an affordable and relatively rapid system both for screening compounds for cytotoxicity and probing their mechanism of action.
Results
Here, the authors present the results of screening cationic antimicrobial peptides of the pleurocidin family (consisting of 26 different variants) for anticancer activity in a cell line as well as in the zebrafish embryo model system. They find that the most amphipathic and positively charged pleurocidin variants exhibit potent and selective oncotic activity against human leukemia cells in vitro, but not hemolytic activity. Electron microscopy reveals that leukemia cells treated with one of these pleurocidin variants, NRC-03, exhibit severe vacuolation of the cytoplasm, cell swelling, loss of microvilli, and the appearance of membrane pores and blebs typical of oncosis. There is no evidence of apoptotic cell death. A variety of functional assays performed in zebrafish suggest that the active peptides cause membrane damage and the loss of embryonic epithelial integrity, and selectively induce oncosis and apoptosis in cancer cells in vivo.
Implications and future directions
These results indicate that some pleurocidin peptides show selective and potent anti-cancer activity that make them attractive candidates for the development of novel therapeutic agents. Owing to the fast killing ability, potent activity and membranolytic mechanism of action of these compounds, resistant cancer cells are unlikely to arise. Future screens of synthetic libraries of short peptides with anti-cancer potential in zebrafish embryos hold great promise for finding the highly selective and active variants, and for discerning their mode-of-action.
Transmission electron microscopy
HL60 cells were treated with 32 μg/ml NRC-03 for 1–30 minutes and fixed in 1% glutaraldehyde/4% formaldehyde in 0.1 M cacodylate buffer (pH 7.4) overnight at 4°C. Cells were then rinsed, embedded in a 3% agarose pellet and post-fixed in 2% OsO4/0.1M cacodylate buffer for 2 hours. After rinsing in ddH2O, they were dehydrated through acetone and embedded in Epon Araldite. Semi-thin sections (0.3 μm) were stained with 1% Toluidine Blue. Ultra-thin sections (80 nm) were stained with 2% aqueous uranyl acetate and lead citrate. Cells were counted in several fields of semi-thin sections and scored as oncotic if >50% of the cytoplasm was vacuolated, apoptotic if shrunken, and necrotic if plasma membranes were ruptured and organelles severely damaged.
HMECs were grown, treated and fixed in 1G4F as described for SEM studies. They were then prepared for TEM as described above.
Supplementary Material
Acknowledgments
We thank Cindy Leggiadro and David O’Neil, Institute for Marine Biosciences, for performing transmission and scanning electron microscopy, respectively. Structural modeling of pleurocidin variants by Ray Syvitski, Institute for Marine Biosciences, is gratefully acknowledged. Caspase-9 inhibitor and biotinylated NRC-03 were kindly provided by David Hoskin, Dalhousie University. This work was funded by the National Research Council of Canada and is NRCC publication number 51776.
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
S.E.D. and K.H.S. conceived and designed the study. M.G.M. performed all functional assays in zebrafish, and J.W.G. performed all hemolysis and HL60 experiments. A.R. and M.G.M. performed cytotoxicity assays on zebrafish embryos and C.M.R. performed fluorescence peptide localization studies. S.E.D., K.H.S. and M.G.M. wrote the paper..
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.007310/-/DC1
REFERENCES
- Aarbiou J., Tjabringa G. S., Verhoosel R. M., Ninaber D. K., White S. R., Peltenburg L. T., Rabe K. F., Hiemstra P. S. (2006). Mechanisms of cell death induced by the neutrophil antimicrobial peptides alpha-defensins and LL-37. Inflamm. Res. 55, 119–127 [DOI] [PubMed] [Google Scholar]
- Amsterdam A., Hopkins N. (2006). Mutagenesis strategies in zebrafish for identifying genes involved in development and disease. Trends Genet. 22, 473–478 [DOI] [PubMed] [Google Scholar]
- Baker M. A., Malog W. L., Zasloff M., Jacob L. (1993). Anticancer efficacy of magainin 2 and analogue peptides. Cancer Res. 53, 3052–3057 [PubMed] [Google Scholar]
- Bhutia S. K., Maiti T. K. (2008). Targeting tumors with peptides from natural sources. Trends Biotechnol. 26, 210–217 [DOI] [PubMed] [Google Scholar]
- Brock J., Midwinter K., Lewis J., Martin P. (1996). Healing of incisional wounds in the embryonic chick wing bud: characterization of the actin purse-string and demonstration of a requirement for Rho activation. J. Cell Biol. 135, 1097–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden K. A. (2005). Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3, 238–250 [DOI] [PubMed] [Google Scholar]
- Bulmus V., Woodward M., Lin L., Murthy N., Stayton P., Hoffman A. (2003). A new pH-responsive and glutathione-reactive, endosomal membrane-disruptive polymeric carrier for intracellular delivery of biomolecular drugs. J. Control. Release 93, 105–120 [DOI] [PubMed] [Google Scholar]
- Dennison S. R., Whittaker M., Harris F., Phoenix D. A. (2006). Anticancer alpha-helical peptides and structure/function relationships underpinning their interactions with tumour cell membranes. Curr. Protein Pept. Sci. 7, 487–499 [DOI] [PubMed] [Google Scholar]
- Douglas S. E., Gallant J. W., Gong Z., Hew C. (2001). Cloning and developmental expression of a family of pleurocidin-like antimicrobial peptides from winter flounder, Pleuronectes americanus (Walbaum). Dev. Comp. Immunol. 25, 137–147 [DOI] [PubMed] [Google Scholar]
- Eimon P. M., Ashkenazi A. (2010). The zebrafish as a model organism for the study of apoptosis. Apoptosis 15, 331–349 [DOI] [PubMed] [Google Scholar]
- Forman H. J., Kennedy J. (1975). Superoxide production and electron transport in mitochondrial oxidation of dihydroorotic acid. J. Biol. Chem. 250, 4322–4326 [PubMed] [Google Scholar]
- Franek W. R., Morrow D. M., Zhu H., Vancurova I., Miskolci V., Darley-Usmar K., Simms H. H., Mantell L. L. (2004). NF-kappaB protects lung epithelium against hyperoxia-induced nonapoptotic cell death-oncosis. Free Radic. Biol. Med. 37, 1670–1679 [DOI] [PubMed] [Google Scholar]
- Frisch S. M., Screaton R. A. (2001). Anoikis mechanisms. Curr. Opin. Cell Biol. 13, 555–562 [DOI] [PubMed] [Google Scholar]
- Hale J. D., Hancock R. E. (2007). Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev. Anti Infect. Ther. 5, 951–959 [DOI] [PubMed] [Google Scholar]
- Haukland H. H., Ulvatne H., Sandvik K., Vorland L. H. (2001). The antimicrobial peptides lactoferricin B and magainin 2 cross over the bacterial cytoplasmic membrane and reside in the cytoplasm. FEBS Lett. 508, 389–393 [DOI] [PubMed] [Google Scholar]
- Herrmann M., Lorenz H. M., Voll R., Grunke M., Woith W., Kalden J. R. (1994). A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 22, 5506–5507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildeman D. A., Mictchell T., Teague T. K., Henson P., Day B. J., Kappler J., Marrack P. C. (1999). Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity 10, 35–44 [DOI] [PubMed] [Google Scholar]
- Imura Y., Choda N., Matsuzaki K. (2008). Magainin 2 in action: distinct modes of membrane permeabilization in living bacterial and mammalian cells. Biophys. J. 95, 5757–5765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Mei H., Li X., Ma Y., Zeng A. H., Wang Y., Lu X., Chu F., Wu Q., Zhu J. (2010). Apoptosis-inducing activity of the antimicrobial peptide cecropin of Musca domestica in human hepatocellular carcinoma cell line BEL-7402 and the possible mechanism. Acta Biochim. Biophys. Sin. (Shanghai) 42, 259–265 [DOI] [PubMed] [Google Scholar]
- Jung H. J., Park Y., Sung W. S., Suh B. K., Lee J., Hahm K. S., Lee D. G. (2007). Fungicidal effect of pleurocidin by membrane-active mechanism and design of enantiomeric analogue for proteolytic resistance. Biochim. Biophys. Acta 1768, 1400–1405 [DOI] [PubMed] [Google Scholar]
- Langheinrich U. (2003). Zebrafish: a new model on the pharmaceutical catwalk. BioEssays 25, 904–912 [DOI] [PubMed] [Google Scholar]
- Lecoeur H., Prevost M. C., Gougeon M. L. (2001). Oncosis is associated with exposure of phosphatidylserine residues on the outside layer of the plasma membrane: a reconsideration of the specificity of the annexin V/propidium iodide assay. Cytometry 44, 65–72 [DOI] [PubMed] [Google Scholar]
- Lee J., Lee D. G. (2008). Structure-antimicrobial activity relationship between pleurocidin and its enantiomer. Exp. Mol. Med. 40, 370–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. J., Kim E. J., Kim S., Jung E. M., Park J. W., Jeong S. H., Park S. E., Yoo Y. H., Kwon T. K. (2006). Caspase-dependent and caspase-independent apoptosis induced by evodiamine in human leukemic U937 cells. Mol. Cancer Ther. 5, 2398–2407 [DOI] [PubMed] [Google Scholar]
- Lehmann J., Retz M., Sidhu S. S., Suttmann H., Sell M., Paulsen F., Harder J., Unteregger G., Stockle M. (2006). Antitumor activity of the antimicrobial peptide magainin II against bladder cancer cell lines. Eur. Urol. 50, 141–147 [DOI] [PubMed] [Google Scholar]
- Mader J. S., Hoskin D. W. (2006). Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin. Invest. Drugs 15, 933–946 [DOI] [PubMed] [Google Scholar]
- Mader J. S., Richardson A., Salsman J., Top D., de Antueno R., Duncan R., Hoskin D. W. (2007). Bovine lactoferricin causes apoptosis in Jurkat T-leukemia cells by sequential permeabilization of the cell membrane and targeting of mitochondria. Exp. Cell Res. 313, 2634–2650 [DOI] [PubMed] [Google Scholar]
- Majno G., Joris I. (1995). Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 146, 3–15 [PMC free article] [PubMed] [Google Scholar]
- Makovitzki A., Fink A., Shai Y. (2009). Suppression of human solid tumor growth in mice by intratumor and systemic inoculation of histidine-rich and pH-dependent host defense-like lytic peptides. Cancer Res. 69, 3458–3463 [DOI] [PubMed] [Google Scholar]
- Martin S. J., Bradley J. G., Cotter T. G. (1990). HL-60 cells induced to differentiate towards neutrophils subsequently die via apoptosis. Clin. Exp. Immunol. 79, 448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki K., Murase O., Fujii N., Miyajima K. (1996). An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry 35, 11361–11368 [DOI] [PubMed] [Google Scholar]
- Meeker N. D., Trede N. S. (2008). Immunology and zebrafish: spawning new models of human disease. Dev. Comp. Immunol. 32, 745–757 [DOI] [PubMed] [Google Scholar]
- Mendelsohn B. A., Kassebaum B. L., Gitlin J. D. (2008). The zebrafish embryo as a dynamic model of anoxia tolerance. Dev. Dyn. 237, 1780–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E. M., Xu D., Fergusson M. M., Combs C. A., Xu Y., Finkel T. (2002). Regulation of cellular oncosis by uncoupling protein 2. J. Biol. Chem. 277, 27385–27392 [DOI] [PubMed] [Google Scholar]
- Moon H. S., Jacobson E. M., Khersonsky S. M., Luzung M. R., Walsh D. P., Xiong W., Lee J. W., Parikh P. B., Lam J. C., Kang T. W., et al. (2002). A novel microtubule destabilizing entity from orthogonal synthesis of triazine library and zebrafish embryo screening. J. Am. Chem. Soc. 124, 11608–11609 [DOI] [PubMed] [Google Scholar]
- Navara C. S., Benyumov A., Vassilev A., Narla R. K., Ghosh P., Uckun F. M. (2001). Vanadocenes as potent anti-proliferative agents disrupting mitotic spindle formation in cancer cells. Anticancer Drugs 12, 369–376 [DOI] [PubMed] [Google Scholar]
- Nguyen K. T., Le Clair S. V., Ye S., Chen Z. (2009). Molecular interactions between magainin 2 and model membranes in situ. J. Phys. Chem. B 113, 12358–12363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijnik A., Hancock R. E. (2009). Host defence peptides: antimicrobial and immunomodulatory activity and potential applications for tackling antibiotic-resistant infections. Emerg. Health Threats J. 2, e1–e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papo N., Shahar M., Eisenbach L., Shai Y. (2003). A novel lytic peptide composed of DL-amino acids selectively kills cancer cells in culture and in mice. J. Biol. Chem. 278, 21018–21023 [DOI] [PubMed] [Google Scholar]
- Papo N., Braunstein A., Eshhar Z., Shai Y. (2004). Suppression of human prostate tumor growth in mice by a cytolytic D-, L-amino acid peptide: membrane lysis, increased necrosis, and inhibition of prostate-specific antigen secretion. Cancer Res. 64, 5779–5786 [DOI] [PubMed] [Google Scholar]
- Patrzykat A., Gallant J. W., Seo J. K., Pytyck J., Douglas S. E. (2003). Novel antimicrobial peptides derived from flatfish genes. Antimicrob. Agents Chemother. 47, 2464–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicano H., Carney D., Huang P. (2004). ROS stress in cancer cells and therapeutic implications. Drug Resist. Updat. 7, 97–110 [DOI] [PubMed] [Google Scholar]
- Romashko J., 3rd, Horowitz S., Franek W. R., Palaia T., Miller E. J., Lin A., Birrer M. J., Scott W., Mantell L. L. (2003). MAPK pathways mediate hyperoxia-induced oncotic cell death in lung epithelial cells. Free Radic. Biol. Med. 35, 978–993 [DOI] [PubMed] [Google Scholar]
- Rosenblatt J., Raff M. C., Cramer L. P. (2001). An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr. Biol. 11, 1847–1857 [DOI] [PubMed] [Google Scholar]
- Saikumar P., Dong Z., Mikhailov V., Denton M., Weinberg J. M., Venkatachalam M. A. (1999). Apoptosis: definition, mechanisms, and relevance to disease. Am. J. Med. 107, 489–506 [DOI] [PubMed] [Google Scholar]
- Saint N., Cadiou H., Bessin Y., Molle G. (2002). Antibacterial peptide pleurocidin forms ion channels in planar lipid bilayers. Biochim. Biophys. Acta 1564, 359–364 [DOI] [PubMed] [Google Scholar]
- Shearer J., Fitch S. B., Kaminsky W., Benedict J., Scarrow R. C., Kovacs J. A. (2003). How does cyanide inhibit superoxide reductase? Insight from synthetic FeIIIN4S model complexes. Proc. Natl. Acad. Sci. USA 100, 3671–3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard J. M., Chen M., Tarasov K. V., Bhatta S., Ivanova S., Melnitchenko L., Tsymbalyuk N., West G. A., Gerzanich V. (2006). Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat. Med. 12, 433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattum G., McGee K. M., Rosenblatt J. (2009). P115 RhoGEF and microtubules decide the direction apoptotic cells extrude from an epithelium. J. Cell Biol. 186, 693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallmann H. P., Faber C., Bronckers A. L., de Blieck-Hogervorst J. M., Brouwer C. P., Amerongen A. V., Wuisman P. I. (2005). Histatin and lactoferrin derived peptides: antimicrobial properties and effects on mammalian cells. Peptides 26, 2355–2359 [DOI] [PubMed] [Google Scholar]
- Sun L., Zhao Y., Yuan H., Li X., Cheng A., Lou H. (2010). Solamargine, a steroidal alkaloid glycoside, induces oncosis in human K562 leukemia and squamous cell carcinoma KB cells. Cancer Chemother. Pharmacol. 67, 813–821 [DOI] [PubMed] [Google Scholar]
- Sung W. S., Lee J., Lee D. G. (2008). Fungicidal effect of piscidin on Candida albicans: pore formation in lipid vesicles and activity in fungal membranes. Biol. Pharm. Bull. 31, 1906–1910 [DOI] [PubMed] [Google Scholar]
- Syvitski R. T., Burton I., Mattatall N. R., Douglas S. E., Jakeman D. L. (2005). Structural characterization of the antimicrobial peptide pleurocidin from winter flounder. Biochemistry 44, 7282–7293 [DOI] [PubMed] [Google Scholar]
- Takeda M., Shirato I., Kobayashi M., Endou H. (1999). Hydrogen peroxide induces necrosis, apoptosis, oncosis and apoptotic oncosis of mouse terminal proximal straight tubule cells. Nephron 81, 234–238 [DOI] [PubMed] [Google Scholar]
- Takeshima K., Chikushi A., Lee K.-K., Yonehara Y., Matsuzaki K. (2003). Translocation of analogues of the antimicrobial peptides magainin and buforin across human cell membranes. J. Biol. Chem. 278, 1310–1315 [DOI] [PubMed] [Google Scholar]
- Trump B. F., Berezesky I. K., Chang S. H., Phelps P. C. (1997). The pathways of cell death: oncosis, apoptosis, and necrosis. Toxicol. Pathol. 25, 82–88 [DOI] [PubMed] [Google Scholar]
- Van Cruchten S., Van Den Broeck W. (2002). Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat. Histol. Embryol. 31, 214–223 [DOI] [PubMed] [Google Scholar]
- Wang Y. H., Cheng C. C., Lee W. J., Chiou M. L., Pai C. W., Wen C. C., Chen W. L., Chen Y. H. (2009). A novel phenotype-based approach for systematically screening antiproliferation metallodrugs. Chem. Biol. Interact. 182, 84–91 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (2000). The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). Eugene, OR: University of Oregon Press [Google Scholar]
- Westerhoff H. V., Hendler R. W., Zasloff M., Juretic D. (1989). Interactions between a new class of eukaryotic antimicrobial agents and isolated rat liver mitochondria. Biochim. Biophys. Acta 975, 361–369 [DOI] [PubMed] [Google Scholar]
- Yoshida K., Mukai Y., Niidome T., Takashi C., Tokunaga Y., Hatakeyama Y., Aoyagi H. (2001). Interaction of pleurocidin and its analogs with phospholipid membrane and their antibacterial activity. J. Pept. Res. 57, 119–126 [DOI] [PubMed] [Google Scholar]
- Zasloff M. (2002). Antimicrobial peptides of multicellular organisms. Nature 415, 389–395 [DOI] [PubMed] [Google Scholar]
- Zhang L., Falla T. J. (2010). Potential therapeutic application of host defense peptides. Methods Mol. Biol. 618, 303–327 [DOI] [PubMed] [Google Scholar]
- Zhao G. F., Seng J. J., Zhao S., Hu W., Li A., Li X. N., Qi Y. (2007). Oncosis in human esophageal squamous cell carcinoma and its relationship with apoptosis and microvessel density. Chin. Med. J. (Engl.) 120, 1999–2001 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.