Abstract
We have screened human adenoviruses (Ads) for oncolytic activity against a variety of mouse and hamster cell lines and have found a number that are susceptible to a variety of Ad serotypes. A20 lymphoma is derived from BALB/c mice and is susceptible to infection and killing by a variety of human Ads. A20 is also a suitable cancer vaccine model, because these cells express a unique immunoglobulin variable region that can be targeted by vaccination. To compare Ads as cancer vaccines versus Ads as oncolytics, A20 tumors were initiated in immunocompetent BALB/c mice. Mice immunized with first-generation Ad5 expressing the A20 immunoglobulin ScFv immunogen (Ad-A20) were protected against A20 lymphomas only when the vaccine was delivered before tumor. In contrast, vaccination after tumor initiation failed to increase survival or delay tumor growth. When Ad serotypes from species B, C, D, and E were tested as oncolytics in vitro, A20 cells were most efficiently killed by species D Ads, with intermediate activity by species B Ads. When tested in vivo in immunocompetent BALB/c mice bearing A20 tumors, single intratumoral injection of species D Ad26 and Ad48 were effective at controlling tumor growth. These data demonstrate that in this immunocompetent mouse cancer model, the oncolytic activity of adenoviruses is more potent than their use as a cancer vaccine. These data in immunocompetent mice lend further support to species D Ads as promising oncolytic viruses against B cell cancers.
Weaver and colleagues compare adenoviruses as cancer vaccines versus adenoviruses as oncolytics in an immunocompetent mouse cancer model. They find that mice immunized with first-generation adenovirus serotype 5 (Ad5) expressing the A20 immunoglobulin ScFv immunogen (Ad-A20) are protected against A20 lymphomas only when the vaccine is delivered before tumor initiation. In contrast, vaccination after tumor initiation failed to increase survival or to delay tumor growth. When adenoviral serotypes from species B, C, D, and E are tested in vivo in immunocompetent BALB/c mice bearing A20 tumors, single intratumoral injection of species D Ad26 and Ad48 are effective at controlling tumor growth.
Introduction
Bcell lymphomas are potentially unique cancer vaccine targets, because they can express unique rearranged immunoglobulin idiotypes. Previous studies have shown that protective immunity can be generated against this unique feature (Stevenson et al., 1995; Hakim et al., 1996; Syrengelas et al., 1996; King et al., 1998). Immunization with DNA vaccines expressing the single-chain FV (scFv) and the scFv fused to cholera toxin have been shown to induce protective immunity against lymphoma and myeloma (King et al., 1998; Syrengelas and Levy, 1999). Hawkins and colleagues fused the scFv of the lymphoma cell line A20 to the human IgG1 Fc fragment (A20scFv-hFc) (Armstrong et al., 2002). Recombinant adenovirus expressing A20scFv-hFc was used to immunize BALB/c mice. On challenge >40% of mice were protected against a challenge with a lethal dose of A20 cells (Armstrong et al., 2002). DNA immunizations of scFv fused to the fragment C from tetanus toxin protected mice against A31 lymphoma and 5T33 myeloma cell lethal challenges (King et al., 1998). Technological advances in the sequencing of immunoglobulin scFv of tumors are promising and it has been suggested that individualized tumor vaccinations could be generated against lymphomas and myelomas (Doenecke et al., 1997).
Although cancer vaccines are attractive, the ability to stimulate protective immunity after tumor formation is the ultimate goal. A comparable system would be that of the rabies vaccine. In this system the infection has already occurred and postexposure prophylaxis (PEP) consists of anti-rabies immunoglobulin (RIG) and three to five immunizations with killed inactivated rabies virus (Rupprecht and Gibbons, 2004). In this system, the rabies virus is slow-growing and requires several weeks to months to travel through the CNS and reach the brain, ultimately resulting in death (Faber et al., 2009). However, before the rabies virus travels through the CNS and reaches the brain the RIG slows it down and the immune responses stimulated by the vaccine catch up, control, and eradicate the infection (Rupprecht and Gibbons, 2004). Cancer vaccines that use the unique immunoglobulin of lymphomas and myelomas need to be able to achieve this type of PEP.
Although chemotherapeutics and surgery are the standard for cancer treatment, they are invasive and toxic to healthy tissue. Researchers have been searching for alternatives to these standards and have extensively explored the use of viruses to control, reduce, or eliminate tumors. Modifications in the adenovirus serotype 5 (Ad5) genome have increased the efficiency with which tumor cells are targeted and destroyed (Fueyo et al., 2000; Nettelbeck et al., 2002; Reid et al., 2002). Measles virus was also modified to target tumor specific cells by displaying anti-CD38 and epidermal-like growth factor receptor (EGFR) scFv (Nakamura et al., 2005). Others have exploited the natural ability of vesicular stomatitis virus (VSV) to replicate in cancer cells in the presence of interferon that would protect normal primary cells (Stojdl et al., 2000). Vaccinia virus, herpesvirus, reovirus, and poliovirus have also been modified and studied for their ability to effectively replicate in and destroy tumor cells (Gromeier et al., 2000; Todo et al., 2001; Hirasawa et al., 2002; Parato et al., 2005; Kirn et al., 2007). In this study we explored the use of an adenovirus-vectored scFv vaccine against an A20 lymphoma tumor cell line in an immunocompetent BALB/c mouse model. Here we show that the prevention of lymphoma tumors can be achieved only if the mice are immunized before tumor formation, and that PEP is not achievable once tumors are established. However, virotherapy using wild-type adenoviruses (wtAds) was achievable in established tumors and offers a new platform for vaccine vector development and cancer therapy.
Materials and Methods
Viruses and cell lines
A20 is a B cell lymphoma cell line derived from an old BALB/cAnN mouse (Kim et al., 1979). A20 cells were purchased from the American Type Culture Collection (TIB-208; ATCC, Manassas, VA) and were cultured in complete RPMI containing 10% fetal bovine serum. The wild-type adenoviruses Ad4, Ad5, Ad6, Ad17, Ad24, Ad26, Ad28, Ad30, Ad35, Ad45, and Ad48 were purchased from the ATCC and grown in HEK-293 cells (CRL-1573; ATCC). The A20 scFv-expressing virus (Ad-A20) was made with an AdEasy expression system (Agilent Technologies, Santa Clara, CA). The A20 scFv was purchased from GenScript (Piscataway, NJ) and cloned into the AdEasy expression system as previously described (Armstrong et al., 2002). Ad-A20 virus was rescued and amplified in HEK-293 cells. The viruses were purified using two consecutive CsCl centrifugations and quantitated by determining the optical density at 260 nm (OD260).
Mice
Female BALB/c mice (6–8 weeks old) were purchased from Charles River Laboratories (Wilmington, MA) and housed in the Mayo Clinic (Rochester, MN) Animal Facility under the Association for Assessment and Accreditation of Laboratory Animal Care (AALAC) guidelines with animal use protocols approved by the corresponding Mayo Clinic Institutional Animal Care and Use Committee. All animal experiments were carried out according to the provisions of the Animal Welfare Act, PHS Animal Welfare Policy, the principles of the NIH Guide for the Care and Use of Laboratory Animals, and the policies and procedures of the Mayo Clinic.
Anti-A20 immunization
Mice were anesthetized intraperitoneally with ketamine (140 mg/kg)–xylazine (5.55 mg/kg) and were immunized intramuscularly with 1010 viral particles (VP) of Ad-A20 in a volume of 50 μl (n=10). Twenty-five microliters was injected into each mouse quadriceps. Three weeks postimmunization the mice were boosted with 1010 VP of Ad-A20. Control mice were injected with Dulbecco's phosphate-buffered saline (DPBS) or a nonspecific green fluorescent protein (GFP)/luciferase-expressing Ad (Ad-GL). Two weeks postimmunization 106 A20 cells were injected subcutaneously to induce tumors. Tumors were measured weekly and mice were humanely killed when tumors reached a volume of 2000 μl.
Analysis of in vitro infection and killing of A20 cells by wild-type Ads
A20 cells were infected with 10,000 VP per cell for 1 hr at 4°C. Cells were then washed three times with Hanks' buffered saline solution (Invitrogen, Carlsbad, CA) and placed in a 37°C incubator for the indicated length of time. Loss of membrane integrity was assessed by trypan blue uptake as described by Barry and colleagues (1990).
Tumor control by wild-type Ads
A20 cells (106) were injected subcutaneously into the hind flank of 6- to 8-week-old BALB/c mice. When the tumors reached an average size of 200 μl they were injected intratumorally with 3×1010 VP of wild-type virus in a 100-μl total volume of DPBS. Control groups were injected with buffer alone. Tumors were observed every other day and measured weekly. Volume was calculated as ½ length×width×width. Animals were killed when tumor volume exceeded 2000 μl, were found with ulcerated tumors, or had weight loss greater than 20%.
Results
Prophylactic immunization by Ad against A20 tumors
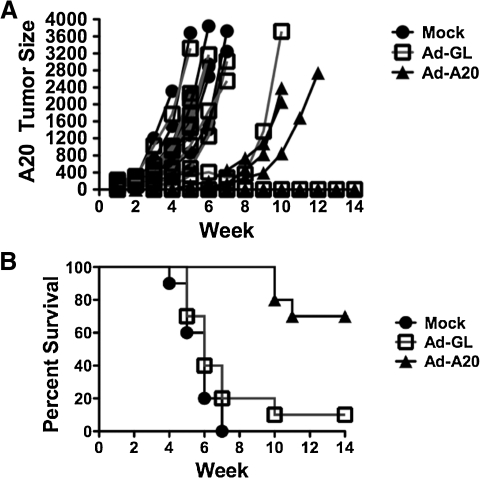
Groups of female BALB/c mice were immunized intramuscularly with 1010 VP of replication-defective Ad-A20 expressing the A20 ScFv immunogen (n=10). Three weeks later, the mice were boosted with 1010 VP of Ad-A20. Control mice were injected with DPBS or a nonspecific GFP/luciferase-expressing Ad (Ad-GL). Two weeks after immunization A20 tumors were initiated by subcutaneous injection of 106 A20 cells into the flanks.
Mice that received a prime–boost immunization of Ad-A20 virus were capable of inhibiting A20 tumor growth (Fig. 1A). In fact, all Ad-A20-immunized mice suppressed A20 tumor growth for 4 weeks after injection as compared with control mice. However, three of the immunized mice lost control of tumor growth and A20 tumors reestablished at a growth rate similar to the controls (Fig. 1A). The survival curves for the Ad-A20-immunized mice were significantly different as compared with the Ad-GL and DPBS mice (p=0.0005 and p≤0.0001, respectively). Seven of the immunized mice survived the A20 tumor challenge whereas none of the control DPBS-immunized mice and only one of the control Ad-GL immunized mice survived (Fig. 1B).
FIG. 1.
Prophylactic immunization against A20 tumor growth. Mice that received a prime–boost immunization with 1010 VP of Ad-A20 virus were capable of inhibiting A20 tumor growth (A). The survival curves for the Ad-A20-immunized mice were significantly different as compared with the Ad-GL and DPBS mice (p=0.0005 and p≤0.0001, respectively). Seven of the immunized mice survived the A20 tumor challenge whereas none of the control DPBS immunized mice, and only one of the control Ad-GL-immunized mice, survived (B).
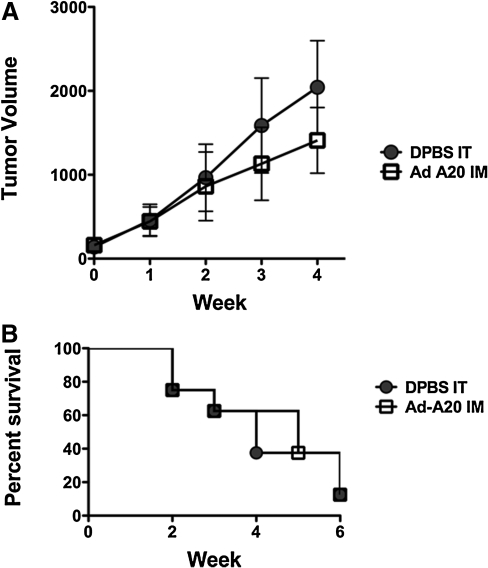
Established A20 tumors are not controlled by Ad-A20 immunization
Groups of eight mice were injected with A20 cells. When the tumors reached an average size of 200 μl, the mice were immunized intramuscularly with 3×1010 VP of Ad-A20 or control DPBS and tumor growth and survival were monitored. Control of established tumors by immunization with Ad-A20 was not achieved (Fig. 2). Although the tumor growth in Ad-A20-immunized mice appeared to be delayed (Fig. 2A), the ultimate survival rates among the immunized and control mice were equivalent (Fig. 2B). There were no significant differences in tumor size or survival between immunized and control animals.
FIG. 2.
Therapeutic control of established tumors. Groups of eight mice were injected with 106 A20 cells. When the tumors reached an average size of 200 μl, the mice were immunized intramuscularly with 3×1010 VP of Ad-A20 or control DPBS. Control of established tumors by immunization with Ad-A20 was not achieved. Although the tumor growth in Ad-A20-immunized mice appeared to be delayed (A) the ultimate survival rates of the immunized and control mice were equivalent (B). There were no significant differences in tumor sizes or survival between immunized and control.
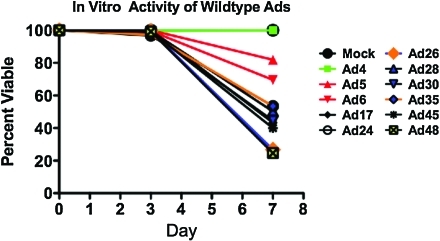
Analysis of in vitro infection and killing of A20 cells by human adenoviruses
We tested a panel of adenoviruses from species B, C, D, E, and F against human solid and B cell cancers (Shashkova et al., 2009; Senac et al., 2010; Chen et al., 2011a,b). To test this for mouse B cell-derived A20 lymphoma cells, A20 cells were infected with 10,000 VP per cell for 1 hr at 4°C. The cells were then washed three times with Hanks' buffered saline solution (Invitrogen) and placed in a 37°C incubator for the indicated length of time. Cell killing, as demonstrated by loss of membrane integrity, was assessed by trypan blue uptake as in Barry and colleagues (1990) (Fig. 3). Consistent with data in human cells (Senac et al., 2010; Chen et al., 2011a,b), species D Ads had highest activity against these B cell cancers, with lower killing by species B Ad35, species C Ad5 and Ad6, and species E Ad4. Of these viruses, species D Ad26 and Ad48 appeared most robust.
FIG. 3.
Analysis of in vitro infection and killing of A20 cells by wild-type Ads. A20 cells were infected with 10,000 VP per cell for 1 hr at 4°C. Cells were then washed three times with Hanks' buffered saline solution (Invitrogen, Carlsbad, CA) and placed in a 37°C incubator for the indicated length of time. Loss of membrane integrity was assessed by trypan blue uptake. Color images available online at www.liebertonline.com/hum
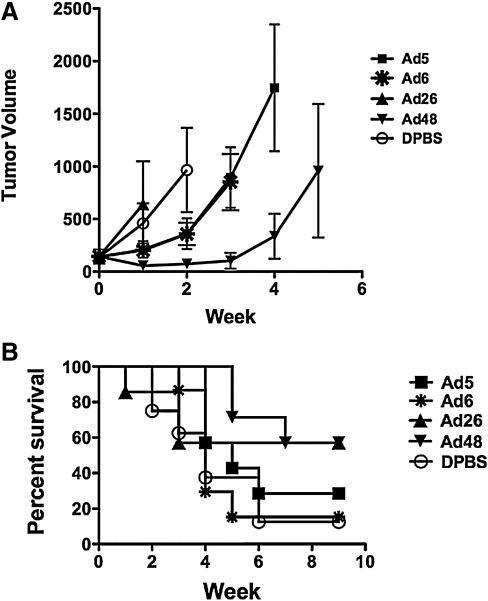
Control of tumor growth by wild-type Ads
Given that the wild-type Ads appeared to have oncolytic activity in vitro against A20 cells, the oncolytic activities of a select set of viruses were tested in vivo in immunocompetent BALB/c mice (Fig. 4). Groups of seven or eight mice were injected subcutaneously with 106 A20 cells. When the tumors averaged 200 μl, they were injected intratumorally with 3×1010 VP of wild-type Ad5, Ad6, Ad26, and Ad48 viruses and tumor size and survival were monitored. Under these conditions, Ad48 induced the greatest delay in tumor growth (Fig. 4A). When survival was monitored, 60% of animals still survived through 9 weeks after single treatment with either Ad26 or Ad48 (Fig. 4B); however, only Ad48 mouse survival was statistically different from that of buffer-treated animals (p=0.0299). In contrast, Ad6-treated mouse survival was no better than that of buffer-treated animals and Ad5-treated mouse survival was only slightly better. The individual tumor volumes for the wild-type Ad-treated groups are shown in Supplementary Fig. S1 (supplementary data are available online at www.liebertonline.com/hum).
FIG. 4.
Control of tumor growth by wild-type Ads. Groups of seven or eight mice were injected with A20 cells. When the tumors averaged 200 μl, they were injected intratumorally with 3×1010 VP of the wild-type viruses that induced greatest levels of killing in vivo. Ad48 induced the greatest delay in tumor growth (A). Ad26 and Ad48 had the greatest levels of survival against A20 tumor growth (B). Both Ad26 and Ad48 were capable of clearing established tumors in mice and protected four of seven mice from death. However, only Ad48 was statistically significant as compared with DPBS (p=0.0299).
Discussion
The vast majority of studies of Ad as a vaccine or of Ad as an oncolytic have used species C Ad5 as a vector. Although Ad5 is robust, it is arguably one of the least practical viruses for use in humans, because 27–100% of humans are immune to the virus. Given these issues, we and others have explored other low-seroprevalence Ads for use as oncolytic and vaccine vectors. In the course of our studies (Senac et al., 2010; Chen et al., 2011a,b), we have found that the relatively unstudied species D adenoviruses have surprisingly high activity as oncolytics against B cell cancers.
At the same time, studies of Ad5 and other Ad serotypes have been hampered by the necessity to use the viruses in immunodeficient mouse xenograft models, because these viruses do not propagate well in most nonhuman cells. Given this, we have tested human Ads from species B, C, D, E, and F for their ability to infect nonhuman cancer cell lines to allow testing in immunocompetent animal models. In this work, we have found that the popular HaK kidney cancer cell line from Syrian hamsters is indeed permissive as reported for Ad5 infection, but that the only other virus that would kill these cells was another species C virus, Ad6 (Chen et al., 2011a). In contrast, all other tested Ad serotypes failed to kill HaK cells, rendering them unsuitable for testing non-Ad6 or Ad6 viruses. Subsequent testing in a range of mouse cell lines including A20 identified several that support infection of a variety of Ad serotypes. Given the permissivity of A20 to various Ad serotypes and prior experience in using it as a cancer vaccine model (Armstrong et al., 2002), in this study we compared Ad vaccine and Ad oncolytic activities in this unique immunocompetent model.
In this work, we found that adenovirus expressing the A20 tumor cell line scFv was capable of inducing protective immune responses against a lethal A20 tumor challenge. The protective immune response was most likely enhanced by the anamnestic responses induced by the subsequent injection of mice with A20 tumor cells. We found that we were able to protect 70% of mice from tumor formation and death as compared with previous reports of <20% protection (Armstrong et al., 2002). Although delayed, three of the immunized mice did establish tumors. As there are no known correlates of protection against these tumors, the mechanism of protection and failure to control tumor progression is unknown. However, we speculate that the delayed tumor formation may indicate the establishment of tolerance in mice that were unable to completely clear the tumor cells. Although this was promising, this cancer vaccine approach was effective only if the animals were immunized before exposure to the tumor, a situation that generally does not exist in human cancer patients. In contrast to prophylactic protection, vaccination after tumors had already formed failed to inhibit tumor progression. This does not mean that such a cancer vaccine could never have utility. Rather, in this setting it was less effective than other interventions. A20 tumors are rapidly established and progress with a doubling approximately every week. In this model, tumors form and become lethal (>2 cm3) within 4 to 7 weeks. The A20 lymphoma model represents an aggressive form of disease. A less aggressive, slower model may result in a different outcome and may be more representative of slower-progressing lymphomas. Alternatively, combining vaccination with treatments such as chemotherapy, immunotherapy, or alternative approaches such as statin inhibition of tumor progression may allow for the induction of protective immune responses and result in greater levels of treatment efficacy (Czuczman et al., 1999; Demierre et al., 2005). It should also be noted that this tumor vaccine model is in the context of an immunocompetent mouse; it cannot be directly translated to humans. It is possible that protective immune responses against established tumors could be generated in a different genetic background.
Because vaccination against established A20 tumors was unsuccessful we tested whether replication-competent adenoviruses might instead have utility as oncolytics rather than vaccines. When 11 wild-type human Ads were tested in vitro, A20 cells were killed most efficiently by species D Ads. This is consistent with a number of studies against human B cell cancers using cell lines and primary patient samples. Previous studies on primary multiple myeloma patient samples showed that species D Ad26 and Ad48 killed CD138+ myeloma cells better than species C Ad5 and Ad6, which were better than species B Ad11 and Ad35 (Senac et al., 2010). Subsequent studies in lymphoma and myeloma cell lines and patient samples have demonstrated that many species D viruses have higher oncolytic activity than Ads from other species in vitro and in immunodeficient mouse xenografts of lymphoma (Chen et al., 2011b). When Ad26 and Ad48 were compared with Ad5 and Ad6 in this immunocompetent mouse model of lymphoma, the species D viruses were again most effective in controlling these B cell cancers. Importantly, Ad26 and Ad48 were effective after single treatment and in the context of an intact immune system.
In summary, these data suggest that adenovirus may be more effective when applied as an oncolytic rather than a cancer vaccine, at least in this model. Previous data against primary patient samples suggest these viruses can kill the cancer cells that are present in patients. The present data suggest they can act as effective oncolytics in the face of a fully functional immune system. These data suggest that species D adenoviruses may have utility as oncolytic agents against B cell cancers.
Supplementary Material
Acknowledgments
This project was supported by a Developmental Project to M.A.B. from the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE) in Lymphoma (P50 CA097274), a grant to M.A.B. from the Fraternal Order of Eagles Cancer Research Fund, and by the Predolin Foundation.
Author Disclosure Statement
E.A.W., C.Y.C., S.M.M., M.E.B., and M.A.B. have no competing financial interests.
References
- Armstrong A.C. Dermime S. Allinson C.G., et al. Immunization with a recombinant adenovirus encoding a lymphoma idiotype: Induction of tumor-protective immunity and identification of an idiotype-specific T cell epitope. J. Immunol. 2002;168:3983–3991. doi: 10.4049/jimmunol.168.8.3983. [DOI] [PubMed] [Google Scholar]
- Barry M.A. Behnke C.A. Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem. Pharmacol. 1990;40:2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- Chen C.Y. Weaver E.A. Khare R., et al. Mining the adenovirus virome for effective oncolytics against breast and ovarian cancer. Cancer Gene Ther. 2011a:1–7. doi: 10.1038/cgt.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.Y. Weaver E.A. Khare R., et al. Species D adenoviruses as oncolytics against B cell cancers. Clin. Cancer Res. 2011b doi: 10.1158/1078-0432.CCR-11-0968. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuczman M.S. Grillo-Lopez A.J. White C.A., et al. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J. Clin, Oncol. 1999;17:268–276. doi: 10.1200/JCO.1999.17.1.268. [DOI] [PubMed] [Google Scholar]
- Demierre M.F. Higgins P.D. Gruber S.B., et al. Statins and cancer prevention. Nat. Rev. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- Doenecke A. Winnacker E.L. Hallek M. Rapid amplification of cDNA ends (RACE) improves the PCR-based isolation of immunoglobulin variable region genes from murine and human lymphoma cells and cell lines. Leukemia. 1997;11:1787–1792. doi: 10.1038/sj.leu.2400781. [DOI] [PubMed] [Google Scholar]
- Faber M. Li J. Kean R.B., et al. Effective preexposure and postexposure prophylaxis of rabies with a highly attenuated recombinant rabies virus. Proc. Natl. Acad. Sci. U.S.A. 2009;106:11300–11305. doi: 10.1073/pnas.0905640106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fueyo J. Gomez-Manzano C. Alemany R., et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- Gromeier M. Lachmann S. Rosenfeld M.R., et al. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6803–6808. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim I. Levy S. Levy R. A nine-amino acid peptide from IL-1β augments antitumor immune responses induced by protein and DNA vaccines. J. Immunol. 1996;157:5503–5511. [PubMed] [Google Scholar]
- Hirasawa K. Nishikawa S.G. Norman K.L., et al. Oncolytic reovirus against ovarian and colon cancer. Cancer Res. 2002;62:1696–1701. [PubMed] [Google Scholar]
- Kim K.J. Kanellopoulos-Langevin C. Merwin R.M., et al. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J. Immunol. 1979;122:549–554. [PubMed] [Google Scholar]
- King C.A. Spellerberg M.B. Zhu D., et al. DNA vaccines with single-chain Fv fused to fragment C of tetanus toxin induce protective immunity against lymphoma and myeloma. Nat. Med. 1998;4:1281–1286. doi: 10.1038/3266. [DOI] [PubMed] [Google Scholar]
- Kirn D.H. Wang Y. Le Boeuf F., et al. Targeting of interferon-β to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T. Peng K.W. Harvey M., et al. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat. Biotechnol. 2005;23:209–214. doi: 10.1038/nbt1060. [DOI] [PubMed] [Google Scholar]
- Nettelbeck D.M. Rivera A.A. Balague C., et al. Novel oncolytic adenoviruses targeted to melanoma: Specific viral replication and cytolysis by expression of E1A mutants from the tyrosinase enhancer/promoter. Cancer Res. 2002;62:4663–4670. [PubMed] [Google Scholar]
- Parato K.A. Senger D. Forsyth P.A. Bell J.C. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- Reid T. Galanis E. Abbruzzese J., et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): Phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62:6070–6079. [PubMed] [Google Scholar]
- Rupprecht C.E. Gibbons R.V. Clinical practice: Prophylaxis against rabies. N. Engl. J. Med. 2004;351:2626–2635. doi: 10.1056/NEJMcp042140. [DOI] [PubMed] [Google Scholar]
- Senac J.S. Doronin K. Russell S.J., et al. Infection and killing of multiple myeloma by adenoviruses. Hum. Gene Ther. 2010;21:179–190. doi: 10.1089/hum.2009.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashkova E.V. May S.M. Barry M.A. Characterization of human adenovirus serotypes 5, 6, 11, and 35 as anticancer agents. Virology. 2009;394:311–320. doi: 10.1016/j.virol.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson F.K. Zhu D. King C.A., et al. Idiotypic DNA vaccines against B-cell lymphoma. Immunol. Rev. 1995;145:211–228. doi: 10.1111/j.1600-065x.1995.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Stojdl D.F. Lichty B. Knowles S., et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- Syrengelas A.D. Levy R. DNA vaccination against the idiotype of a murine B cell lymphoma: Mechanism of tumor protection. J. Immunol. 1999;162:4790–4795. [PubMed] [Google Scholar]
- Syrengelas A.D. Chen T.T. Levy R. DNA immunization induces protective immunity against B-cell lymphoma. Nat. Med. 1996;2:1038–1041. doi: 10.1038/nm0996-1038. [DOI] [PubMed] [Google Scholar]
- Todo T. Martuza R.L. Rabkin S.D. Johnson P.A. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc. Natl. Acad. Sci. U.S.A. 2001;98:6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.