Abstract
Adenovirus (Ad)-based vectors are attractive candidates for a variety of gene-transfer applications. In this study, we found that decay-accelerating factor (DAF)–displaying Ads induce significantly decreased cellular immune responses to transgenes expressed from the vectors in both Ad5-naive and Ad5-immune mice. Specifically, we found a diminished ability of splenocytes to secrete interferon-γ after recall exposure to multiple peptides derived from antigens expressed by DAF-displaying Ads. We also confirmed that DAF-displaying Ads induce decreased numbers of antigen-specific, CD8+ effector memory and central memory CD8+ T cells, thereby uncovering a unique role of complement in modulating the induction of robust memory T-cell responses. We also confirmed that DAF-displaying Ads generate significantly reduced titers of Ad capsid–specific neutralizing antibodies after gene transfer in vivo. In conclusion, DAF-displaying Ad5-based vectors exhibit decreased induction of complement-dependent, innate immune responses, resulting in both an improved safety profile and a decreased propensity to induce humoral and cellular adaptive immune responses to Ad capsid proteins and Ad vector-expressed transgene products. This attractive combination of features will be beneficial in a variety of clinically relevant gene-transfer applications.
Novel adenoviral vectors displaying genetically engineered complement inhibitors such as human decay-accelerating factor (DAF) have been developed with the objective of averting vector-associated toxicity in vivo. In this report, Seregin and colleagues seek to assess the effect of DAF-modified adenoviral capsids on long-term transgene expression. They find that intravenously or intramuscularly delivered DAF-displaying adenovirus serotype 5 vectors induce significantly decreased cellular immune responses to transgenes both in Ad5-naive and Ad5-immune mice. DAF-displaying Ad5 also results in reduced adenoviral capsid-neutralizing antibody titers as well as antigen-specific CD8+ effector memory cells.
Introduction
Gene transfer-based therapeutics targeted for use in genetic diseases can only be successful if efficient gene transfer can be coupled with adequately sustained transgene expression. These objectives must be achieved without placing undo risk on the patient receiving the putative gene therapy, including risks such as those that can be associated with innate and adaptive immune responses induced by the specific vector itself, as well by the transgene expressed by the vector.
Currently, about 24% of all gene transfer–based clinical trials utilize adenovirus (Ad) vectors to deliver a variety of transgenes (www.wiley.com/legacy/wileychi/genmed/clinical/), confirming the significance of this platform for widespread human usage (Seregin and Amalfitano, 2009). However, at high doses, viral particles, including Ads, can trigger innate immune responses that result in global toxicities, such as thrombocytopenia (Wolins et al., 2003; Appledorn et al., 2008b), endothelial cell activation (Schiedner et al., 2000; Seregin et al., 2009b), release of systemic cytokines/chemokines (Kiang et al., 2006; Appledorn et al., 2008a), liver damage (Everett et al., 2003; Seregin et al., 2009b), as well as activation of pro-inflammatory gene expression in liver and/or splenic tissues (Hartman et al., 2007; Seregin et al., 2009b). More specifically, Ad vector interactions with the complement system have been proven to mediate many of these toxic responses, as loss of complement functionality in complement knockout animals results in ablation of these toxicities despite systemic administration of high levels of Ad vectors (Appledorn et al., 2008b; Seregin et al., 2009a; Tian et al., 2009). To address these safety limitations in a living animal with a normally functioning complement system, we have developed novel Ad vectors with capsid-displaying genetically engineered forms of known complement inhibitors (Seregin et al., 2010a,b). In one example, the Ad vector tolerated capsid display of the human decay-accelerating factor (DAF), a natural complement inhibitor. Introduction and in-frame genetic fusion of the complement inhibitory portions of the DAF protein onto the C-terminus of the Ad capsid cement protein pIX have been previously confirmed by our group to be viable (Seregin et al., 2010a). These DAF-displaying Ad vectors were not only confirmed to retain the native Ad vectors' ability to transduce genes both in vitro and in vivo, but the unique Ad vectors were also confirmed to be significantly safer; this was validated primarily by the vectors having a minimized induction of typical Ad capsid-triggered innate immune responses, coupled with a reduced activation of immune effector cell types early after administration (Seregin et al., 2010a).
In this study, we wished to determine the consequences of these improvements on the ability of DAF-displaying Ads to allow for long-term transgene expression. Specifically, we set out to determine if transgene- or Ad vector–specific adaptive immune responses were altered by the use of DAF-displaying Ad vectors, given the vectors' ability to mollify aspects of the antiviral innate immune response. Therefore, we have evaluated and compared both the cellular and humoral adaptive immune responses triggered by intravenously (IV) or intramuscularly (IM) administered conventional and DAF-displaying Ad5 vectors expressing immunogenic transgenes in vivo. We have now found that Ad capsid display of complement inhibitors, such as DAF, facilitates a reduced induction of transgene- as well as capsid- specific adaptive immune responses, after both systemic and localized (IM) administrations.
Materials and Methods
Ad vector construction, production, and characterization
Retro-DAF-displaying and control vectors were constructed as detailed (Seregin et al., 2010a). In brief, the following Ads were used: Ad5-Gag-IX-dDAF (Seregin et al., 2010a), Ad5-GFP-IX-dDAF (Seregin et al., 2010a), Ad5-GFP (Hodges et al., 2001; Ng and Graham, 2002), Ad5-Gag, and Ad5-Null. All Ads used in this study are human Ad type 5–derived replication-deficient vectors (deleted for the E1 and E3 genes). To construct Ad5-Gag, the HXB2 Gag gene (GenBank accession no. K03455, kindly supplied by Dr. Frank Jones, Etubics Corp., originally obtained from NIH Vaccine Research Center) was blunt-end subcloned from pVRC3900 into the EcoRV site of pShuttle-CMV. Restriction digests and sequencing were used to confirm the sequence integrity and correct orientation of the resulting shuttle (pShuttle-CMV-Gag). To construct Ad5-Gag-IX-dDAF_REO, the CMV-Gag-polyA expression cassette was subcloned into the multiple cloning site of the pShuttle-IX-DAF_REO as described for the green fluorescent protein (GFP) cassette (Hodges et al., 2001; Ng and Graham, 2002). Recombination and viral propagation were completed as described (Ng and Graham, 2002; Seregin et al., 2009a, 2010a). Ad5-Null vector was constructed by recombining pShuttle (with no transgene) with pAdEasyI and purified as described (Ng and Graham, 2002). Propagation and characterization of all Ads were performed as previously described (Seregin et al., 2010a). All viruses were found to be replication competent adenovirus (RCA)-free by both RCA PCR (E1 region amplification) and direct sequencing methods as previously described (Seregin et al., 2009b). All Ads have also been tested for the presence of bacterial endotoxin as previously described (Seregin et al., 2009b) and were found to contain <0.15 EU/ml.
Animal procedures
Adult C57BL/6 wild-type (WT) and BALB/c WT mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Ad5 vectors were injected IV (via the retro-orbital sinus, total volume 200 μl, diluted in PBS) or IM (into the tibialis anterior of the right hindlimb, total volume 25 μl) into 8-week-old male mice after being properly anesthetized with isofluorane. A total of 2 × 101 viral particles (vp) (high dose equals 8 × 1012 vp/kg) or 0.75 × 1011 vp (medium dose equals 3 × 1012 vp/kg) per mouse was used for IV injections, and 1 × 1010 vp/mouse (or 108 vl/mouse) was administered IM. The dose of HIV-Gag–expressing vectors for IM injections in Ad5-naive mice was selected based on our previous study (Appledorn et al., 2010). For Ad5-immune mice, IM injections were placed 2 weeks apart. For IV studies, n = 3–4 for Ad-injected groups and n = 3 for naive. For IM studies, n = 5 for terminal sacrifice time points (Ad-injected), n = 2–3 for naive (Ad5-immune), n = 4 for Ad-injected, and n = 3 for naive (experiments in Ad5-naive mice).
Plasma and tissue samples were collected and processed at the indicated time points in accordance with Michigan State University Institutional Animal Care and Use Committee. All procedures with recombinant Ads were performed under BSL-2, and all vector-treated animals were maintained under ABSL-2 conditions. All animal procedures were reviewed and approved by the Michigan State University ORCBS and IACUC. Care for mice was provided in accordance with PHS and AAALAC standards.
Neutralizing antibody (NAB) assay
HEK293-C7-31 cells were seeded in 48-well tissue culture plates at a concentration of 125,000 cells/well in 250 μl of complete medium per well [Dulbecco's modified Eagle's medium with 10% fetal bovine serum (FBS), 1 × penicillin/streptomycin/fungizone (PSF), 0.1 mg/ml hygromycin B]. Cells were cultured overnight in a 37°C, 5% CO2 incubator. To inactivate complement, plasma samples, collected from Ad-injected (and naive) mice, was heat-inactivated for 60 min at 56°C and brought to room temperature (RT). Plasma dilutions were made in complete medium in a total volume of 130 μl (1:15 to 1:400). A total of 7 × 106 vp of Ad5-GFP prepared in 130 μl of complete medium (∼55 vp/cell) was added next to each plasma dilution, mixed well, and incubated at RT for 60 min. Following plasma/Ad-GFP incubations, 250 μl of the medium/plasma/Ad-GFP mixtures was applied to cells (medium previously present in wells was aspirated prior to adding mixtures) and incubated for 14–16 hr. Control samples were prepared as follows: four wells had cells only (in 250 μl of complete medium, negative control), and four wells had Ad-GFP only (in 250 μl of complete medium, 100% transduction = 0% neutralization control). Following incubations, medium was aspirated, and cells were washed with 500 μl of PBS and harvested in 500 μl of cold FACS buffer [1 × PBS with 2% FBS and 0.9 g (per 1 L) of sodium azide]. Samples were analyzed on a BD LSR II instrument and analyzed using FlowJo software (Tree Star, San Carlos, CA). Propidium iodide (PI) (5 μl from 0.2 μg/μl stock) was added to each sample 2–3 min prior to analysis [fluorescein isothiocyanate (FITC)/PI]. Percent neutralization was determined as 100% – % transduction, where % transduction in Ad-GFP–only control samples was established as 100%.
Isolation of lymphocytes from spleen and peripheral blood mononuclear cells
Splenocytes from individual mice were harvested and processed as follows: Spleen tissues were physically disrupted (by passage through a 40-μm sieve), followed by red blood cell lysis by using 2 ml of ACK lysis buffer (Invitrogen, Carlsbad, CA) per homogenized spleen. Splenocytes were subsequently washed two times with RPMI 1640 medium (Invitrogen) supplemented with 10% FBS, 2 mM L-glutamine, and 1% PSF, resuspended, and counted. For isolation of PBMCs, blood was isolated by retro-orbital bleeds or at the time of terminal sacrifice, and peripheral blood mononuclear cells (PBMCs) were isolated using Lympholyte-Mammal (Cedarlane, Burlington, NC), as per the manufacturer's instructions.
Cell staining and flow cytometry
For Gag-tetramer staining, isolated splenocytes or PBMCs were used. Tetramer staining was completed using a phycoerythrin (PE)-conjugated major histocompatibility complex I tetramer folded with the AMQMLKETI H2Kd-restricted peptide generated at the NIH Tetramer Core Facility (working dilution 1:500). CD8-FITC and CD3-APC (6 μg/ml) were used in combination with Gag-tetramer. For memory T-cell staining, a mixture of the following antibodies was used: CD3-APC-Cy7, CD8-AlexaFluor700, CD127-PE-Cy7, CD62L-V450 (all 6 μg/ml), and tetramer-PE (1:500). All antibodies were purchased from BD Biosciences (San Diego, CA). For intracellular staining, 3 × 106 splenocytes were placed in U-bottom 96-well plates, washed two times with cold FACS buffer, and stimulated with AMQMLKETI peptide (200 ng/well). Brefeldin A was added to a final concentration of 1 μg/ml and incubated for 5 hr at 37°C. Following incubation, splenocytes were washed two times with FACS buffer, incubated for 15 min with purified rat anti-mouse CD16/CD32 Fcγ block (BD Biosciences), surface-stained with CD3-APCCy7 and CD8a-AlexaFluor700 (8 μg/ml) for 30 min at 4°C, washed with FACS buffer, fixed with 2% formaldehyde (Polysciences, Warrington, PA) for 20 min on ice, permeabilized with 0.5% saponin (Sigma-Aldrich, St. Louis, MO) for 20 min at RT, and incubated on ice with interferon-γ (IFNγ)-APC (8 μg/ml) for 2 hr. Samples were analyzed on a BD LSR II instrument and analyzed using FlowJo software (Tree Star).
ELISpot analysis
Ninety-six–well multiscreen high protein-binding Immobilon-P membrane plates (Millipore, Billerica, MA) were pretreated with ethanol, coated with mouse anti-IFNγ capture antibody, incubated overnight, and blocked with RPMI medium (with 10% FBS, 1% PSF) prior to the addition of 0.5 × 106 (or 0.125 × 106) splenocytes/well (Weaver and Barry, 2008; Seregin et al., 2010a). Ex vivo stimulation included the incubation of splenocytes in 100 μl of medium alone (unstimulated), medium containing HIV-Gag–specific H2Kd-restricted major immunodominant peptide (AMQMLKETI constructed by Genscript, Piscataway, NJ; 0.2 μg/well), HIV-Gag–specific H2Kb-restricted major immunodominant peptide QBI 304796 (EAMSQVTNSATIMMQ), other HIV-Gag–specific peptides [QBI 304753, QBI 304754 (both contain the AMQMLKETI sequence], QBI 304769, QBI 304779, QBI 304765, QBI 304723, QBI 304746, QBI 304800], Ad5-GFP vector, inactivated at 56°C for 45 min (100 vp/cell), HIV-Gag purified protein, GFP protein (nonspecific stimulation), or pool of three 15mer peptides from HIV-Gag library for 18–24 hr in a 37°C, 5% CO2 incubator. 15mer Gag-specific peptides, spanning the entire Gag sequence (overlapping by five amino acids at both N- and C-termini) were obtained from the NIH AIDS Reagent and Reference Program catalogue no. 8117, lot no. 9. A Ready-Set Go IFNγ mouse ELISpot kit was purchased from eBioscience (San Diego, CA). Staining of plates was completed per the manufacturer's protocol. Spots were counted and photographed by an automated ELISpot reader system (Cellular Technology, Cleveland, OH). CD8+ T cells were depleted from pooled splenocyte preparations using MACS beads and LS columns per the manufacturer's protocol (Miltenyi Biotec, Bergisch Gladbach, Germany). The % CD8- SFC (percent reduction) = SFC CD8- (depleted) / SFC CD8+(nondepleted) × 100 (where SFC represents spot-forming cells). Depletion was verified by FACS analysis using APC-CD8a and Pacific Blue-CD4 antibodies (BD Biosciences). FACS analyses revealed >96% depletion of CD8+ T cells (Appledorn et al., 2010).
Antibody titering assay
ELISA-based titering experiments were essentially completed as previously described (Hensley et al., 2007; Seregin et al., 2009a). In brief, 5 × 108 vp of Ad5 vector per well or 0.2 μg of recombinant Gag protein per well (each diluted in PBS) was used to coat wells of a 96-well plate overnight at 4°C. Plates were washed with PBS-Tween (0.05%) solution, and blocking buffer (3% bovine serum albumin in PBS) was added to each well and incubated for 1–3 hr at RT. For titering of total IgG antibodies, plasma from IV or IM injected mice was diluted 1:10 to 1:1,638,400 (Ad5) or 1:10 to 1:20,000 (Gag) in blocking buffer. Following dilution, plasma was added to the wells and incubated at RT for 1 hr. Wells were washed using PBS-Tween (0.05%), and horseradish peroxidase–conjugated rabbit anti-mouse antibody (Bio-Rad, Hercules, CA) was added at a 1:5,000 dilution in PBS-Tween. TMB (Sigma-Aldrich) substrate was added to each well, and the reaction was stopped with 2 N sulfuric acid. Plates were read at 450 nm in a microplate spectrophotometer.
Statistical analysis
For every experiment, pilot trials were performed with n = 3 per group. This allowed us to determine effect size and sample variance so that power analysis could be performed to correctly determine the number of subjects per group required to achieve a statistical power of >0.8 at the 95% confidence level. Statistically significant differences in adaptive immune responses (Gag-tetramer staining, intracellular cytokine staining) were determined using one-way ANOVA with a Student-Newman-Keuls post hoc test (p < 0.05). Furthermore, a two-way ANOVA with a Bonferroni post hoc test was used to analyze the levels of T-cell responses in splenocytes, derived from Ad-treated mice and stimulated with several different peptides, to determine significant differences (p < 0.05) between groups. For NAB assays, a two-tailed Student's t test was used to compare two groups of virus-injected animals (p < 0.05). Graphs in this article are presented as means of the average ± SD, unless otherwise specified. GraphPad Prism software was used for statistical analysis.
Results
Ad5-naive mice IV-injected with DAF-displaying Ads generate significantly reduced transgene-specific T-cell responses as well as Ad-NAB responses
It is well known that pathogen-induced innate immune responses can have a significant impact on the quality of subsequent adaptive immune responses to the pathogen (Gasque, 2004; Mollnes and Kirschfink, 2006; Kemper and Atkinson, 2007; Appledorn et al., 2008b). Equal levels of long-term expression of the transgene from the conventional Ad vector (Ad5-Gag) and the DAF-displaying Ad vector (DAF-Ad5-Gag) were confirmed previously (Seregin et al., 2010a) and have been further validated in the present study by quantitative western blot analysis on liver tissues of C57BL/6 mice, collected at 28 days post injection (dpi) from Ad5-Gag– or DAF-Ad5-Gag–injected mice (Supplementary Fig. S1; supplementary data are available online at www.liebertonline.com/hum). To address more fully the short-term transgene expression efficiency possessed by DAF-displaying Ad5-based vectors, we used several sets of mice, injected with Ad5-GFP or Ad5-GFP-DAF (Supplementary Table S1, Supplementary Fig. S2). We found similar levels of transduction of GFP into splenocytes between the two groups of Ad-injected mice in all immunization settings (IV, IM, preimmune mice IM). We also tested efficiency of GFP transgene expression in vitro and found no significant differences between Ad5-GFP and the Ad5-DAF (Supplementary Fig. S3). Upon confirming equal transductional efficiencies of conventional and DAF-displaying Ads, we set out to determine how minimization of the typical Ad vector–induced innate immune responses (using Ad capsids displaying complement inhibiting peptides) might impact upon subsequent adaptive immune responses to the vector, as well the transgene the vector expresses. For these experiments, we used a DAF-displaying Ad vector engineered to express the highly immunogenic, HIV-derived Gag antigen, DAF-Ad5-Gag, and compared its properties with a conventional Ad5 vector also expressing HIV-Gag. Long-term (i.e., 100–200 dpi) adaptive immune responses were studied to facilitate our ability to examine long-term effector memory (EM) and central memory (CM) T-cell responses.
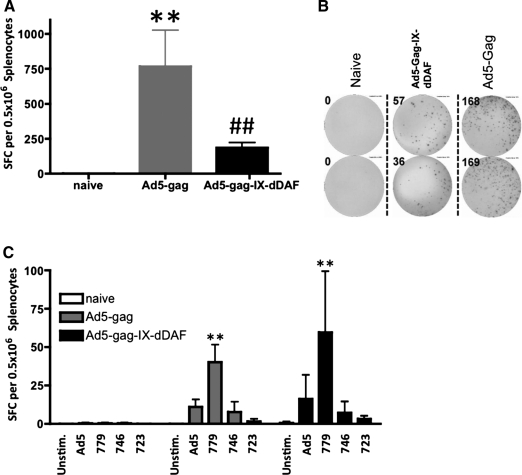
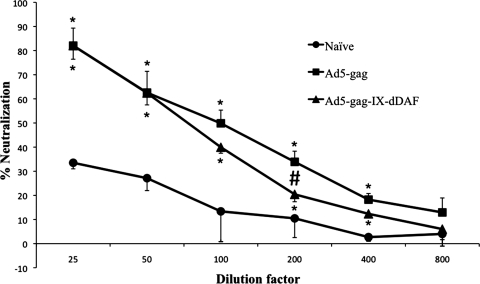
We found that DAF-Ad5-Gag IV–injected mice had significantly reduced numbers of IFNγ-secreting T cells in the spleen as compared with Ad5-Gag–injected mice (p < 0.001) when exposed to an immunodominant HIV-Gag–specific peptide at 200 dpi (Fig. 1A and B). Stimulation of splenocytes with other Gag-specific peptides or with Ad5 capsid proteins did not reveal any statistically significant differences between control or experimental Ad vector–treated groups of mice (Fig. 1C), a result that was likely due to the reduced immunogenicity of these compounds, as compared with the major immunodominant HIV-Gag peptide used in Fig. 1A and B. Importantly, plasma samples, collected at 105 dpi (15 weeks post injection) demonstrated a consistent trend of decreased Ad5 NAB titer (1:100 to 1:400 dilutions) generated by DAF-displaying Ads as compared with titers elicited by conventional Ad vectors, with statistically significant differences noted at the 1:200 plasma dilution (Fig. 2). Plasma samples, collected at 140 dpi (20 weeks post injection) followed exactly the same trend (data not shown). Significantly decreased NAB titers (as compared with control Ads) were also detected with DAF-displaying Ads, expressing an alternative transgene (GFP) (Supplementary Fig. S4).
FIG. 1.
Ad5 vectors with capsid-displaying retro-DAF complement inhibitor induce significantly diminished Ad-derived HIV-Gag–specific T-cell responses when injected IV. WT C57Bl/6 mice were IV injected (2 × 1011 vp/mouse) with Ad5-Gag or Ad5-Gag-IX-dDAF. At 200 dpi, splenocytes were harvested and processed as described in Materials and Methods. To test the magnitude of HIV-Gag–specific T-cell responses, splenocytes from Ad-treated (n = 4) and naive (n = 3) mice were stimulated ex vivo with either Gag-specific peptide 304796 (EAMSQVTNSATIMMQ) (A) or additional Gag-specific peptides and heat (56°C)-inactivated Ad5 (C). Representative wells from ELISpot stimulated with peptide 304796 (B) show SFCs from splenocytes derived from naïve and Ad5-Gag– and Ad5-Gag-IX-dDAF–injected mice, plated at 125,000 cells/well. Columns represent means ± SD. Statistical analysis was completed using two-way ANOVA with a Bonferroni post hoc test (stimulations × treatments); p < 0.05 was deemed a statistically significant difference. **p < 0.001, statistically different from naive mice; ##p < 0.001, statistically different from WT Ad5-Gag treatment group within the same stimulation group. SFCs, spot-forming cells.
FIG. 2.
Mice injected IV with the retro-DAF–displaying Ads generate significantly reduced titers of Ad NABs. Plasma from naive (n = 2), conventional Ad5 (n = 4, 2 × 1011 vp/mouse), or retro-DAF–displaying Ad5-treated (n = 4, 2 × 1011 vp/mouse) C57BL/6 mice was collected at 105 dpi, and Ad5-specific NABs were measured as detailed in Materials and Methods. The error bars represent ± SEM. Statistical analysis was completed using two-tailed Student's t test to compare two groups of virus-injected animals (#p < 0.05) or to compare each of Ad5-injected group with naive (*p < 0.05). The graph represents % neutralization at 1:200 dilution (means ± SEM).
DAF-displaying Ads trigger blunted cellular and humoral transgene (HIV-Gag)–specific adaptive immune responses when administered IM into Ad5-naive mice
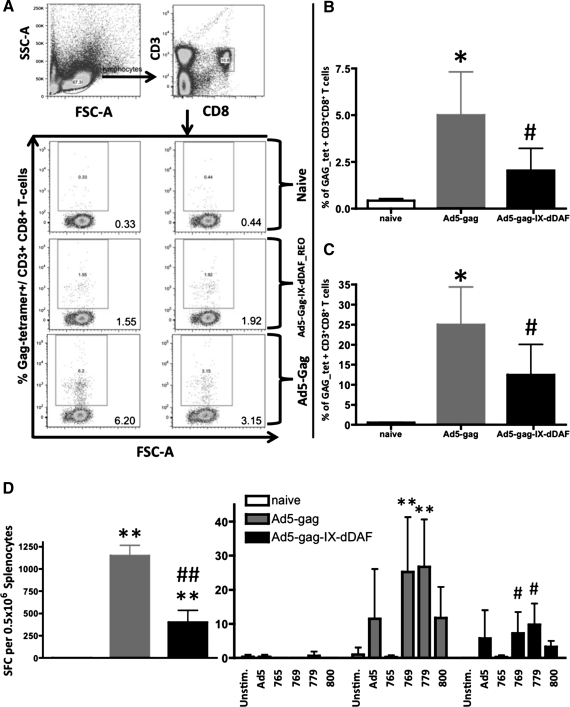
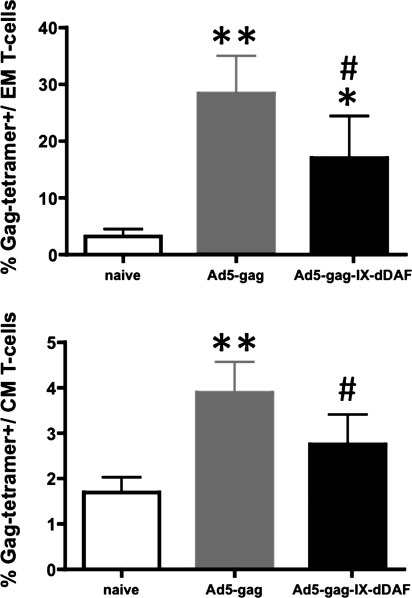
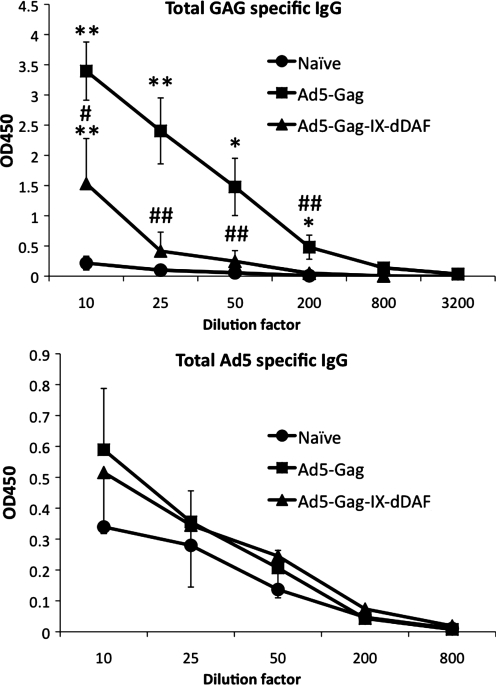
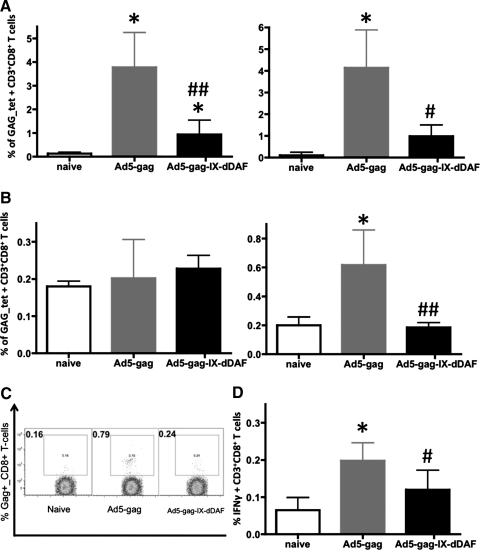
To investigate more fully the immunological effects of Ad capsid display of DAF on Ad vector–triggered adaptive immune responses, we also used IM administration procedures (Liu et al., 2009; Appledorn et al., 2010). We immunized BALB/c mice IM with DAF-Ad5-Gag or Ad5-Gag, and at 14 dpi, we collected spleen tissues and PBMCs. When the cells were derived from mice injected with the DAF-Ad5-Gag vector, we found significantly diminished populations of CD3+CD8+Gag-tetramer+ T cells in both of these compartments, as compared with similar analyses performed on identical cells derived from mice treated with a conventional Ad5 vector expressing the identical transgene (Fig. 3A–C). This result was consistent with the finding that splenocytes derived from DAF-Ad5-Gag–injected mice had a significantly reduced ability to secrete IFNγ on ex vivo stimulation with the HIV-Gag–derived, immunodominant, H2Kd-restricted peptide AMQMLKETI (Fig. 3D) and significantly reduced breadth (number of peptide-specific T-cell clones responding to GAG-derived peptides) of antigen-specific response upon stimulation with several less immunodominant peptides (Fig. 3D). Moreover, by tetramer staining, we also found that both EM (CD3+CD8+CD127+CD62Llow) and CM (CD3+CD8+CD127+CD62Lhigh) Gag-specific T-cell populations are significantly reduced in DAF-Ad5-Gag–injected mice as compared with Ad5-Gag–injected mice (Fig. 4). Plasma levels of total HIV-Gag–specific IgG (but not Ad5-specific IgG) were found to be significantly reduced in DAF-Ad5-Gag–injected mice as compared with control Ad5-Gag–treated mice (Fig. 5). The capsid composition of DAF-displaying Ads did not significantly alter binding of Ad-specific IgG antibodies, as confirmed by measurement of statistically identical anti-Ad5 IgG titers when Ad5-GAG or DAF-Ad5-Gag was used for ELISA plate coating (Supplementary Fig. S5).
FIG. 3.
DAF-displaying Ads trigger blunted cellular transgene (HIV-Gag)–specific adaptive immune responses when administered IM into Ad5-naive mice. WT Ad5-naive BALB/c mice were untreated (naive) or injected with Ad5-Gag or Ad5-Gag-IX-dDAF (108 vp/mouse, IM). At 14 dpi, splenocytes and PBMCs were harvested and processed as described in Materials and Methods. (A) Representative samples of Gag-tetramer staining of splenocytes, derived from each of three groups. Splenocytes (B) or PBMCs (C) derived from individual mice, were analyzed for the presence of CD3+CD8+Gag-tetramer+ T cells by flow cytometry. Columns represent means ± SD. Statistical analysis was completed using one-way ANOVA with a Student-Newman-Keuls post hoc test; p < 0.05 was deemed a statistically significant difference. *p < 0.05, statistically different from naive mice; #p < 0.05, statistically different from WT Ad5-Gag mice. (D) To test the magnitude and breadth of HIV-Gag–specific cellular responses, splenocytes from Ad-treated (n = 4) and naive (n = 3) BALB/c WT mice were stimulated ex vivo with either Gag-specific peptide AMQMLKETI or additional Gag-specific peptides and heat (56°C)-inactivated Ad5. Columns represent means ± SD. Statistical analysis was completed using two-way ANOVA with a Bonferroni post hoc test (stimulations × treatments); p < 0.05 was deemed a statistically significant difference. **p < 0.001, statistically different from naive mice; #p < 0.05, ##p < 0.001, statistically different from WT Ad5-Gag treatment group within the same stimulation group.
FIG. 4.
DAF-displaying Ads trigger significantly reduced populations of both EM and CM CD8+ T cells when administered IM into Ad5-naive mice. WT Ad5-naive BALB/c mice were untreated (naive) or injected with Ad5-Gag or Ad5-Gag-IX-dDAF (108 vp/mouse, IM). At 14 dpi, splenocytes were harvested, processed as described in Materials and Methods, and stained with the following antibodies: CD3-APC-Cy7, CD8-AlexaFluor700, CD127-PE-Cy7, CD62L-V450, and Gag-tetramer-PE. The percentage of Gag-tetramer positive EM (CD127+CD62Llow) and CM (CD127+CD62Lhigh) CD3+CD8+ T cells is shown. Columns represent means ± SD. Statistical analysis was completed using one-way ANOVA with a Student-Newman-Keuls post hoc test; p < 0.05 was deemed a statistically significant difference. *p < 0.05, **p < 0.01, statistically different from naive mice; #p < 0.05, statistically different from WT Ad5-Gag mice.
FIG. 5.
DAF-displaying Ads trigger reduced humoral transgene (HIV-Gag)–specific adaptive immune responses when administered IM into Ad5-naive mice. Plasma from naive (n = 3), conventional Ad5 (n = 4), or retro-DAF-displaying Ad5-treated (n = 4) BACB/c mice was collected at 14 dpi, and Ad5-specific or HIV-Gag–specific total IgG levels were measured as detailed in Materials and Methods. The error bars represent ± SD. Statistical analysis was completed using two-tailed Student's t test to compare two groups of virus-injected animals (#p < 0.05, ##p < 0.001) or to compare each of Ad5-injected group with naive (*p < 0.05, **p < 0.01). No significant Ad5-specific IgG was detected in any group. Significant (1:10) titers of Gag-specific IgG were detected in DAF-Ad5-Gag–treated mice; significant (up to 1:200) titers of specific IgG were detected in Ad5-Gag–treated mice.
DAF-displaying Ads induce blunted transgene (HIV-Gag)–specific T-cell responses when administered IM into Ad-immune mice
Importantly, pre-existing Ad5 immunity in a wide range of human populations may significantly limit the efficiency of Ad-based gene-transfer approaches (Sumida et al., 2004; Tang et al., 2006; Lasaro and Ertl, 2009). To test if DAF-displaying Ad5 vectors can improve the efficacy of Ad-mediated gene transfer in the face of pre-existing Ad5 immunity, we preimmunized BALB/c mice with Ad5-Null, and then injected the Ad-immune mice with control or DAF-displaying Ad vectors. Importantly, two immunizations with Ad5-Null induced Ad5 NAB titers that were >1/200, a level that closely parallels levels of pre-existing Ad5 immunity noted in human populations (Abbink et al., 2007; Gabitzsch et al., 2009). We found that at 14 and 28 dpi, DAF-Ad5-Gag–injected mice had significantly diminished populations of CD3+CD8+Gag-tetramer+ T cells present in PBMCs, as compared with Ad5-immune mice identically injected with the conventional Ad5-HIV-Gag vector (Fig. 6A). Splenocytes also derived from DAF-Ad5-Gag–injected mice had significantly reduced numbers of CD3+CD8+Gag-tetramer+ T cells present at 28 dpi (Fig. 6B and C), a result that also correlated with a significantly reduced IFNγ production by Gag-specific CD3+CD8+ T cells in these same mice (Fig. 6D). A second injection with Ad5-Gag appears to increase T-cell responses in splenocytes, but not in PBMCs; however, experimental staining procedures were performed at different days for groups of mice sacrificed at 14 and 28 dpi and, therefore, results obtained at these time points cannot be directly compared with each other.
FIG. 6.
Ad5 vectors with capsid-displaying retro-DAF complement inhibitor induce significantly diminished Ad-derived transgene-specific T-cell responses in Ad5-immune mice. WT BALB/c mice were made Ad5-immune by two 2-week-spaced IM injections with Ad5-Null (1010 vp/mouse), followed by one or two doses of either Ad5-Gag or Ad5-Gag-IX-dDAF (1010 vp/mouse, IM). At 14 dpi (one dose; left) or 28 dpi (two doses; right), splenocytes and PBMCs were harvested and processed as described in Materials and Methods. PBMCs (A) or splenocytes (B) derived from individual mice were analyzed for the presence of CD3+CD8+Gag-tetramer+ T cells by flow cytometry. Columns represent means ± SD. Statistical analysis was completed using one-way ANOVA with a Student-Newman-Keuls post hoc test; p < 0.05 was deemed a statistically significant difference. *p < 0.05, statistically different from naive mice; #p < 0.05, ##p < 0.01, statistically different from WT Ad5-Gag mice. (C) Representative samples of Gag-tetramer staining of splenocytes, derived from each of three groups (28 dpi). (D) For intracellular cytokine staining, splenocytes (n = 5) collected at 14 dpi were stimulated with AMQMLKETI Gag-specific peptide and stained as detailed in Materials and Methods. Columns represent means ± SD. Statistical analysis was completed using one-way ANOVA with a Student-Newman-Keuls post hoc test; p < 0.05 was deemed a statistically significant difference. *p < 0.05, statistically different from naive mice; #p < 0.05, statistically different from WT Ad5-Gag mice.
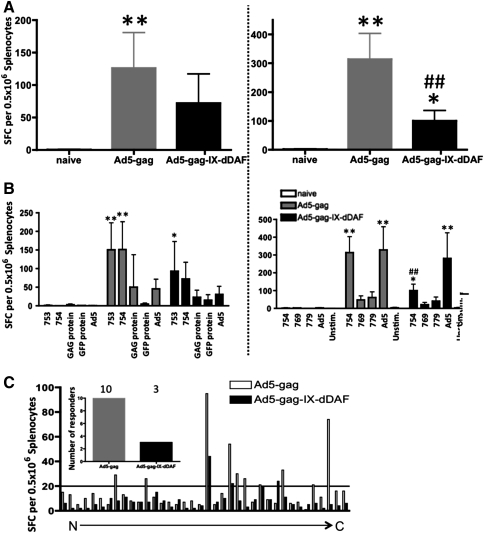
Moreover, as measured by IFNγ-specific ELISpot, splenocytes derived from DAF-Ad5-Gag–injected Ad5-immune mice contained significantly reduced numbers of Gag-specific T cells, as compared with Ad5-Gag–injected, Ad5-immune mice. This was quantified after ex vivo stimulation of splenocytes with the HIV-Gag–derived immunodominant H2Kd-restricted peptide AMQMLKETI (Fig. 7A). Recall responses to several other Gag-specific peptides, the full-length Gag-protein, and Ad5 capsid-specific T-cell responses were, however, found to be identical between the two groups of Ad-injected mice (Fig. 7B), a result that was confounded by the significantly reduced immunogenicity of these peptides relative to AMQMLKETI peptide.
FIG. 7.
Ad5 retro-DAF–displaying vectors induce transgene (HIV-gag)–specific T-cell responses with a dramatically reduced magnitude and breadth. To test the magnitude and breadth of HIV-Gag–specific T-cell responses, splenocytes from Ad-treated (n = 5) and naive (n = 2) BALB/c WT mice were collected at 14 dpi (one dose; left) and 28 dpi (two doses; right), stimulated ex vivo (IFNγ ELISpot) with either Gag-specific peptide AMQMLKETI (A) or additional peptides and proteins, including irrelevant GFP protein as nonspecific control (B). Columns represent means ± SD. Statistical analysis was completed using two-way ANOVA with a Bonferroni post hoc test (stimulations × treatments); p < 0.05 was deemed a statistically significant difference. *p < 0.05, **p < 0.01, statistically different from naive mice; ##p < 0.01, statistically different from WT Ad5-Gag treatment group within the same stimulation group. (C) To more fully test the breadth of HIV-Gag–specific T-cell responses, splenocytes collected at 28 dpi from Ad-treated (n = 5) and naive (n = 2) BALB/c WT mice were pooled and stimulated ex vivo with a pool of three 15mer peptides, spanning the complete HIV-Gag protein sequence (excluding peptides, used for individual stimulation). (Inset) Graph indicates the number of wells with >20 SFCs [arbitrary threshold (Appledorn et al., 2010)].
Therefore, to examine more fully if Ad5 vector display of DAF may significantly impact upon induction of transgene-specific cellular immune responses in Ad-immune hosts, we used a whole HIV-Gag peptide library. Specifically, splenocytes derived from Ad5-Gag– or DAF-Ad5-Gag–injected Ad5-immune mice were stimulated ex vivo with 38 different HIV-Gag–derived peptide pools, each pool consisting of three 15mer overlapping peptides that spanned the entire Gag protein (Fig. 7C) (Appledorn et al., 2010). We found that both the magnitude and breadth of transgene (HIV-Gag)–specific cellular responses were again reduced in Ad5-immune mice injected with DAF-displaying Ads as compared with Ad5-immune mice treated with the control Ad5-Gag vector (Fig. 7). Depletion of CD8+ T cells from the splenocytes confirmed that transgene-specific cellular responses were primarily CD8+ T cell mediated, as there was a 70% reduction of IFNγ SFCs detected in spots after ELISpot (data not shown).
Discussion
Ad type 5–based vectors possess several attractive features, including high transduction efficiencies for many different cell types, an easy and relatively inexpensive scalability to high titers, a large cloning capacity, and a long safety record in 400 human clinical trials to date (Seregin and Amalfitano, 2009; http://www.wiley.com/legacy/wileychi/genmed/clinical/), which makes them outstanding candidates for numerous gene-therapy applications. The most critical drawbacks of Ad vectors are primarily noted when they are administered at higher dosages; these drawbacks include (a) their high innate immunogenicity, (b) their ability to trigger acute toxicities, associated with the innate immune responses, and (c) their sometimes limited long-term efficiency due to induction of vector- and capsid-specific adaptive immune responses, especially in Ad-immune hosts (Cichon et al., 2001; Abbink et al., 2007; Hartman et al., 2008; Seregin et al., 2009a). Although these concerns are validly pertinent to any other gene-transfer platform (including viral- and DNA-based vectors) (Barry et al., 1999; Zaiss and Muruve, 2005; Follenzi et al., 2007; Hensley and Amalfitano, 2007; Mingozzi and High, 2007), in regard to Ad vectors, these safety and efficiency concerns have driven researchers to modify the vector to minimize induction of innate or adaptive immune responses without reducing their gene-transfer efficiencies (Palmer and Ng, 2005; Seregin and Amalfitano, 2009, 2010). It would be most desirable, therefore, to develop Ad vector modifications that can simultaneously achieve both reduction of innate immunogenicity, coupled with a decreased induction of capsid, and transgene-specific adaptive immune responses.
Our laboratory has focused considerable efforts on understanding the interactions of the Ad capsid with several arms of the innate immune system, including the Toll-like receptor (Hartman et al., 2008) and complement systems (Appledorn et al., 2008b; Seregin et al., 2010a,b). Upon confirming that a majority of Ad-triggered innate immune responses are dependent on Ad capsid interactions with the complement system (Kiang et al., 2006; Seregin et al., 2010a), we have developed novel Ad vector capsid-displaying specific complement inhibitors. These include capsid display of the short peptide COMPinh (Seregin et al., 2010b), as well as the human DAF protein displayed from the pIX protein in both the native and a retro-oriented fashion (Seregin et al., 2010a). These studies confirmed that Ad vector capsid display of the retro-oriented form of human DAF dramatically reduced complement activation and complement-dependent innate immune responses in vivo without reducing gene-transfer efficiency (Seregin et al., 2010a).
In this study, we have investigated the adaptive immune responses triggered by retro-DAF-displaying Ad vectors, expressing immunogenic transgenes. The selection of transgene was critical for our studies, as it is well known that first-generation Ad-derived transgene expression can be persistent (without triggering robust adaptive immune responses) if one or more of the following conditions are met: (a) the transgene is not recognized by the host as foreign (i.e., host expresses nonfunctional copy of the same gene), (b) hosts are immune-tolerant, (c) tissue-specific promoter was used to drive the expression of the transgene, and (d) the encoded transgene is not highly immunogenic for other reasons (Tripathy et al., 1994, 1996). To carefully distinguish the effects of DAF display on Ad vector–mediated induction of adaptive immune responses, we used a foreign, highly immunogenic HIV-derived transgene (Gag), expressed from the robust and ubiquitously active CMV promoter/enhancer element. This format allowed us to confirm in vivo that display of DAF directly from the Ad capsid significantly reduced humoral and cell-mediated adaptive immune responses (CMI), induced by both the Ad capsid and the transgene expressed by the DAF-displaying Ad. Specifically, we confirmed that systemically treated mice generate significantly reduced titers of Ad5-specific NAB, and blunted transgene-specific CMI responses, when injected with a DAF-displaying Ad vector as compared with a conventional Ad vector. Similarly, after IM injections, both humoral (IgG) and CMI (IFNγ ELISpot) antigen-specific immune responses were dramatically reduced in DAF-Ad5–injected Ad-naive mice. We also found that even though >70% of Ad vector–induced antigen-specific CMI responses are mediated by CD8+ T cells (depletion ELISpot), transgene-specific tetramer-staining experiments revealed that DAF-Ad5–injected mice have significantly diminished transgene-specific CD8+ T-cell responses in both Ad5-immune and Ad5-naive mice. Finally, both EM (CD3+CD8+CD127+CD62Llow) and CM (CD3+CD8+CD127+CD62Lhigh) transgene-specific T-cell populations were significantly diminished in DAF-Ad5-Gag–injected mice as compared with mice injected with a conventional Ad5 vector expressing the same target antigen.
Complement is known to serve as an important bridge between innate and adaptive immunity (Morgan et al., 2005; Mollnes and Kirschfink, 2006; Kemper and Atkinson, 2007). Lack of complement activation results in impaired humoral immune responses against viral infections (Suresh et al., 2003; Mehlhop et al., 2005). Restoration of C3 protein production in complement-deficient (C3-KO) mice (by infusion of donor macrophages) restores the adaptive immune responses to T-dependent antigens (Fischer et al., 1998). The complement system has been proven to mediate CD8+ T-cell responses to viral antigens (Fischer et al., 1998; Suresh et al., 2003; Mehlhop et al., 2005; Mehlhop and Diamond, 2006; Fang et al., 2007; Seregin et al., 2009a). Specifically, these effects can be direct (via interaction of C1q with C1qR on T cells, thereby promoting cytokine secretion) or indirect (by complement components acting to activate dendritic cells (DCs), which express a high number of complement receptors, i.e., CR3, C3aR, C5aR) (Morgan et al., 2005; Hawlisch and Kohl, 2006). C3-KO mice were described to have significantly delayed virus clearance, which correlated with reduced populations of virus-specific CD4+ and CD8+ T cells in these complement-deficient mice, as compared with mice (Morgan et al., 2005). In addition, Fang et al. (2007) confirmed an important role for the DAF protein in direct modulation (inhibition) of CD8+ T-cell adaptive immune responses during viral (lymphocytic choriomeningitis virus) infection and a specific role for DAF protein in reducing EM CD8+ T-cell responses.
Memory response is a hallmark of acquired immunity, where EM and CM T cells play an integral part. It is now thought that EM T cells are capable of migrating to damaged/infected peripheral tissues immediately (within hours) upon re-exposure to specific antigen and performing their effector functions (aided by large amounts of prestored perforin), whereas CM T cells home to lymphoid tissues, where they generate a robust recall response (proliferate and differentiate into EM T cell). EM and CM T cells can be distinguished by the presence/absence of immediate effector function and expression of a number of homing receptors, but both EM and CM T cells may be CD4+ or CD8+ (for a full description of memory T cells and their functions, see Sallusto et al., 2004).
Based on important roles of complement in modulating (promoting) robust adaptive immune responses, our results with the DAF-displaying Ads can likely be attributed to a specific diminution of the overall levels of complement activation typically induced by similar particle numbers of the native Ad capsid. Likely, the decreased production of C3-derived cleavage products (i.e., C3d), mediated by the DAF-displaying Ad, leads to reduced opsonization of the viral particle, which in turn leads to decreased DC activation and reduced adaptive immune responses to both the Ad capsid and the transgene expressed from the DAF-displaying Ad (Lutz and Jelezarova, 2006; Seregin et al., 2010a). Lack of complement activation by DAF-displaying Ads significantly reduces complement-dependent capsid- or transgene-specific CD8+ T-cell responses. Therefore, the lack of complement activation by DAF-displaying Ads mitigates the subsequent magnitude of T-cell clonal expansion upon antigen re-encounter, and/or augments elimination of Gag-specific CD8+ T cells during the contraction phase, likely due to reduced DC activation (Seregin et al., 2010a) and blunted primary CD8 responses. Both effects would eventuate in the induction of diminished numbers of both EM and CM antigen-specific CD8+ T cells (Kaufman et al., 2008; Liu et al., 2008), a result that we also confirmed in our studies. This utility, possessed by DAF-displaying Ads, coupled with significantly reduced generation of Ad NAB titers and mitigated CMI responses, offers an immense advantage for gene-therapy applications, which may require repeated administration of the vector.
In conclusion, the use of DAF-displaying Ad5 vectors can minimize the acute induction of innate immune responses (and thereby improve their safety profile). This feature directly correlates with their ability to diminish induction of capsid- and transgene-specific humoral and cellular adaptive immune responses. These features, coupled with preserved transduction efficiency, make DAF-displaying Ads an attractive platform for a variety of gene-therapy applications.
Supplementary Material
Acknowledgments
We wish to thank the Michigan State University Laboratory Animal support facility for its assistance in the humane care and maintenance of the animals utilized in this work. S.S.S. was supported by American Heart Association Midwest Affiliate Fellowship 0815660G. A.A. was supported by National Institutes of Health grants RO1DK-069884 and P01 CA078673, the Michigan State University Foundation, and the Osteopathic Heritage Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- Abbink P. Lemckert A.A. Ewald B.A., et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 2007;81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appledorn D.M. Kiang A. McBride A., et al. Wild-type adenoviruses from groups A–F evoke unique innate immune responses, of which HAd3 and SAd23 are partially complement dependent. Gene Ther. 2008a;15:885–901. doi: 10.1038/gt.2008.18. [DOI] [PubMed] [Google Scholar]
- Appledorn D.M. McBride A. Seregin S., et al. Complex interactions with several arms of the complement system dictate innate and humoral immunity to adenoviral vectors. Gene Ther. 2008b;15:1606–1617. doi: 10.1038/gt.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appledorn D.M. Aldhamen Y.A. Depas W., et al. A new adenovirus based vaccine vector expressing an Eimeria tenella derived TLR agonist improves cellular immune responses to an antigenic target. PLoS One. 2010;5:e9579. doi: 10.1371/journal.pone.0009579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry M.E. Pinto-Gonzalez D. Orson F.M., et al. Role of endogenous endonucleases and tissue site in transfection and CpG-mediated immune activation after naked DNA injection. Hum. Gene Ther. 1999;10:2461–2480. doi: 10.1089/10430349950016816. [DOI] [PubMed] [Google Scholar]
- Cichon G. Boeckh-Herwig S. Schmidt H.H., et al. Complement activation by recombinant adenoviruses. Gene Ther. 2001;8:1794–1800. doi: 10.1038/sj.gt.3301611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.S. Hodges B.L. Ding E.Y., et al. Liver toxicities typically induced by first-generation adenoviral vectors can be reduced by use of E1, E2b-deleted adenoviral vectors. Hum. Gene Ther. 2003;14:1715–1726. doi: 10.1089/104303403322611737. [DOI] [PubMed] [Google Scholar]
- Fang C. Miwa T. Shen H. Song W.C. Complement-dependent enhancement of CD8+ T cell immunity to lymphocytic choriomeningitis virus infection in decay-accelerating factor-deficient mice. J. Immunol. 2007;179:3178–3186. doi: 10.4049/jimmunol.179.5.3178. [DOI] [PubMed] [Google Scholar]
- Fischer M.B. Ma M. Hsu N.C. Carroll M.C. Local synthesis of C3 within the splenic lymphoid compartment can reconstitute the impaired immune response in C3-deficient mice. J. Immunol. 1998;160:2619–2625. [PubMed] [Google Scholar]
- Follenzi A. Santambrogio L. Annoni A. Immune responses to lentiviral vectors. Curr. Gene Ther. 2007;7:306–315. doi: 10.2174/156652307782151515. [DOI] [PubMed] [Google Scholar]
- Gabitzsch E.S. Xu Y. Yoshida L.H., et al. Novel adenovirus type 5 vaccine platform induces cellular immunity against HIV-1 Gag, Pol, Nef despite the presence of Ad5 immunity. Vaccine. 2009;27:6394–6398. doi: 10.1016/j.vaccine.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasque P. Complement: a unique innate immune sensor for danger signals. Mol. Immunol. 2004;41:1089–1098. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Hartman Z.C. Kiang A. Everett R.S., et al. Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses in vivo. J. Virol. 2007;81:1796–1812. doi: 10.1128/JVI.01936-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman Z.C. Appledorn D.M. Amalfitano A. Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 2008;132:1–14. doi: 10.1016/j.virusres.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawlisch H. Kohl J. Complement and Toll-like receptors: key regulators of adaptive immune responses. Mol. Immunol. 2006;43:13–21. doi: 10.1016/j.molimm.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Hensley S.E. Amalfitano A. Toll-like receptors impact on safety and efficacy of gene transfer vectors. Mol. Ther. 2007;15:1417–1422. doi: 10.1038/sj.mt.6300217. [DOI] [PubMed] [Google Scholar]
- Hensley S.E. Cun A.S. Giles-Davis W., et al. Type I interferon inhibits antibody responses induced by a chimpanzee adenovirus vector. Mol. Ther. 2007;15:393–403. doi: 10.1038/sj.mt.6300024. [DOI] [PubMed] [Google Scholar]
- Hodges B.L. Evans H.K. Everett R.S., et al. Adenovirus vectors with the 100K gene deleted and their potential for multiple gene therapy applications. J. Virol. 2001;75:5913–5920. doi: 10.1128/JVI.75.13.5913-5920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D.R. Liu J. Carville A., et al. Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination. J. Immunol. 2008;181:4188–4198. doi: 10.4049/jimmunol.181.6.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper C. Atkinson J.P. T-cell regulation: with complements from innate immunity. Nat. Rev. Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- Kiang A. Hartman Z.C. Everett R.S., et al. Multiple innate inflammatory responses induced after systemic adenovirus vector delivery depend on a functional complement system. Mol. Ther. 2006;14:588–598. doi: 10.1016/j.ymthe.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Lasaro M.O. Ertl H.C. New insights on adenovirus as vaccine vectors. Mol. Ther. 2009;177:1333–1339. doi: 10.1038/mt.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Ewald B.A. Lynch D.M., et al. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J. Virol. 2008;82:4844–4852. doi: 10.1128/JVI.02616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. O'Brien K.L. Lynch D.M., et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz H.U. Jelezarova E. Complement amplification revisited. Mol. Immunol. 2006;43:2–12. doi: 10.1016/j.molimm.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Mehlhop E. Diamond M.S. Protective immune responses against West Nile virus are primed by distinct complement activation pathways. J. Exp. Med. 2006;203:1371–1381. doi: 10.1084/jem.20052388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhop E. Whitby K. Oliphant T., et al. Complement activation is required for induction of a protective antibody response against West Nile virus infection. J. Virol. 2005;79:7466–7477. doi: 10.1128/JVI.79.12.7466-7477.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F. High K.A. Immune responses to AAV in clinical trials. Curr. Gene Ther. 2007;7:316–324. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- Mollnes T.E. Kirschfink M. Strategies of therapeutic complement inhibition. Mol. Immunol. 2006;43:107–121. doi: 10.1016/j.molimm.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Morgan B.P. Marchbank K.J. Longhi M.P., et al. Complement: central to innate immunity and bridging to adaptive responses. Immunol. Lett. 2005;97:171–179. doi: 10.1016/j.imlet.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Ng P. Graham F.L. Construction of first-generation adenoviral vectors. Methods Mol. Med. 2002;69:389–414. doi: 10.1385/1-59259-141-8:389. [DOI] [PubMed] [Google Scholar]
- Palmer D.J. Ng P. Helper-dependent adenoviral vectors for gene therapy. Hum. Gene Ther. 2005;16:1–16. doi: 10.1089/hum.2005.16.1. [DOI] [PubMed] [Google Scholar]
- Sallusto F. Geginat J. Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Schiedner G. Hertel S. Kochanek S. Efficient transformation of primary human amniocytes by E1 functions of Ad5: generation of new cell lines for adenoviral vector production. Hum. Gene Ther. 2000;11:2105–2116. doi: 10.1089/104303400750001417. [DOI] [PubMed] [Google Scholar]
- Seregin S.S. Amalfitano A. Overcoming pre-existing adenovirus immunity by genetic engineering of adenovirus-based vectors. Expert Opin. Biol. Ther. 2009;9:1521–1531. doi: 10.1517/14712590903307388. [DOI] [PubMed] [Google Scholar]
- Seregin S.S. Amalfitano A. Improving adenovirus based gene transfer: strategies to accomplish immune evasion. Viruses. 2010;2:2013–2036. doi: 10.3390/v2092013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seregin S.S. Aldhamen Y.A. Appledorn D.M., et al. CR1/2 is an important suppressor of adenovirus-induced innate immune responses and is required for induction of neutralizing antibodies. Gene Ther. 2009a;16:1245–1259. doi: 10.1038/gt.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seregin S.S. Appledorn D.M. McBride A.J., et al. Transient pretreatment with glucocorticoid ablates innate toxicity of systemically delivered adenoviral vectors without reducing efficacy. Mol. Ther. 2009b;17:685–696. doi: 10.1038/mt.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seregin S.S. Aldhamen Y.A. Appledorn D.M., et al. Adenovirus capsid-display of the retro-oriented human complement inhibitor DAF reduces Ad vector-triggered immune responses in vitro and in vivo. Blood. 2010a;116:1669–1677. doi: 10.1182/blood-2010-03-276949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seregin S.S. Hartman Z.C. Appledorn D.M., et al. Novel adenovirus vectors 'capsid-displaying' a human complement inhibitor. J. Innate Immun. 2010b;2:353–359. doi: 10.1159/000284368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida S.M. Truitt D.M. Kishko M.G., et al. Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors. J. Virol. 2004;78:2666–2673. doi: 10.1128/JVI.78.6.2666-2673.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh M. Molina H. Salvato M.S., et al. Complement component 3 is required for optimal expansion of CD8 T cells during a systemic viral infection. J. Immunol. 2003;170:788–794. doi: 10.4049/jimmunol.170.2.788. [DOI] [PubMed] [Google Scholar]
- Tang J. Olive M. Pulmanausahakul R., et al. Human CD8+ cytotoxic T cell responses to adenovirus capsid proteins. Virology. 2006;350:312–322. doi: 10.1016/j.virol.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Tian J. Xu Z. Smith J.S., et al. Adenovirus activates complement by distinctly different mechanisms in vitro and in vivo: indirect complement activation by virions in vivo. J. Virol. 2009;83:5648–5658. doi: 10.1128/JVI.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy S.K. Goldwasser E. Lu M.M., et al. Stable delivery of physiologic levels of recombinant erythropoietin to the systemic circulation by intramuscular injection of replication-defective adenovirus. Proc. Natl. Acad. Sci. U.S.A. 1994;91:11557–11561. doi: 10.1073/pnas.91.24.11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy S.K. Black H.B. Goldwasser E. Leiden J.M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat. Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- Weaver E.A. Barry M.A. Effects of shielding adenoviral vectors with polyethylene glycol (PEG) on vector-specific and vaccine-mediated immune responses. Hum. Gene Ther. 2008;19:1369–1382. doi: 10.1089/hum.2008.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolins N. Lozier J. Eggerman T.L., et al. Intravenous administration of replication-incompetent adenovirus to rhesus monkeys induces thrombocytopenia by increasing in vivo platelet clearance. Br. J. Haematol. 2003;123:903–905. doi: 10.1046/j.1365-2141.2003.04719.x. [DOI] [PubMed] [Google Scholar]
- Zaiss A.K. Muruve D.A. Immune responses to adeno-associated virus vectors. Curr. Gene Ther. 2005;5:323–331. doi: 10.2174/1566523054065039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.