Abstract
We previously demonstrated that Toll-like receptor/myeloid differentiation primary response gene 88 (MyD88) signaling is required for maximal innate and acquired [T helper cell type 1 (Th1)] immune responses following systemic administration of helper-dependent adenoviral vectors (HDAds). However, MyD88-deficient mice injected with HDAdLacZ exhibited only partial reduction of innate immune cytokine expression compared with wild-type mice, suggesting MyD88-independent pathways also respond to HDAds. We now show that NOD2, a nucleotide-binding and oligomerization domain (NOD)–like receptor known to detect muramyl dipeptides in bacterial peptidoglycans, also contributes to innate responses to HDAds, but not to humoral or Th1 immune responses. We established NOD2/MyD88 double-deficient mice that, when challenged with HDAds, showed a significant reduction of the innate response compared with mice deficient for either gene singly, suggesting that NOD2 signaling contributes to the innate response independently of MyD88 signaling following systemic administration of HDAds. In addition, NOD2-deficient mice exhibited significantly higher transgene expression than did wild-type mice at an early time point (before development of an acquired response), but not at a later time point (after development of an acquired response). These results indicate that the intracellular sensor NOD2 is required for innate responses to HDAds and can limit transgene expression during early phases of infection.
In this study, Suzuki and colleagues examine the role of the cytosolic sensor NOD2 (nucleotide-binding and oligomerization domain 2) in the innate immune response against helper-dependent adenovirus vectors (HDAd). The authors show that NOD2 deficiency in mice significantly reduces the induction of proinflammatory cytokines after systemic administration of HDAds in vivo. They also report that NOD2 is required for an innate response to HDAd independent of MyD88, and that the NOD2 signaling pathway inhibits transgene expression at early time points.
Introduction
Host immune responses represent the foremost obstacle limiting the clinical use of adenoviral gene replacement therapy. The development of helper-dependent adenoviral vectors (HDAds), devoid of all functional viral genes, has largely but not entirely overcome immune responses. Although HDAds have demonstrated long-term transgene expression and reduced immunogenicity compared with their first-generation vector counterparts in both small and large animal models (Mian et al., 2004; Toietta et al., 2005; McCormack et al., 2006; Cerullo et al., 2007a,b; Brunetti-Pierri et al., 2009), systemic administration of HDAd induces a rapid (within 6 hr) host immune response similar to that seen with first-generation adenoviral vectors (FGAds) (McCaffrey et al., 2008). This acute response is characterized by infiltration of monocytes and activated neutrophils, with a concomitant increase of secreted cytokines and chemokines (Muruve et al., 2004). Understanding the biology of early innate host responses to HDAd is essential for improving the safety and efficacy of adenoviral (Ad)-mediated gene therapy. This response involves complex cellular and humoral factors that are only partially understood.
Toll-like receptors (TLRs) are major components of pathogen recognition (Akira et al., 2006) and are emerging as significant players in Ad-induced acute toxicity (Basner-Tschakarjan et al., 2006; Cerullo et al., 2007b; Hensley and Amalfitano, 2007; Iacobelli-Martinez and Nemerow, 2007). The myeloid differentiation primary response gene 88 (MyD88) is critical for signaling from all TLRs except TLR3 (Akira et al., 2006) and is expressed in a variety of human and murine tissues at varying levels (Bonnert et al., 1997). TLR/MyD88 signaling is rapidly induced after stimulation with TLR ligands leading to the activation of pro-inflammatory genes and type I interferon (IFN) (Garcia-Sastre and Biron, 2006). Previously, we and others reported that TLR/MyD88 signaling is a major pathway for the induction of innate (Nociari et al., 2007; Yamaguchi et al., 2007) and acquired immune responses to Ad vector (Hartman et al., 2007; Suzuki et al., 2010b). However, our results showed that MyD88 deficiency leads to only a partial reduction in innate cytokine expression in response to HDAds, thereby underscoring the contribution of additional innate sensing pathways (Suzuki et al., 2010b).
The innate recognition of Ad by plasmacytoid dendritic cells mediated by TLRs depends on MyD88, whereas that by myeloid dendritic cells and macrophages is TLR-independent, possibly via cytosolic sensing of Ad (Nociari et al., 2007; Zhu et al., 2007). TLRs recognize microbes and/or microbial products at the cell surface and in the endosome, whereas nucleotide-binding and oligomerization domain (NOD)–like receptors (NLRs) and retinoid acid-inducible gene I (RIG-I)–like receptors (RLRs) detect microbial components in the cytosol (Meylan and Tschopp, 2006; Kanneganti et al., 2007). NLRs that have been assigned functions include NOD1 and NOD2, which sense the dipeptide γ-D-glutamyl-meso-diaminopimelic acid (iE-DAP) and muramyl dipeptide (MDP), respectively (O'Neill, 2008). Both are breakdown products of bacterial peptidoglycan. In addition, NACHT, LRR, and PYD domains–containing protein-3 (NALP3) senses multiple pathogens, pathogen products such as MDP, and products of damaged cells such as uric acid crystals and exogenous crystals such as asbestos (Dostert et al., 2008). NOD1 is ubiquitously expressed, whereas NOD2 and NALP3 are restricted to monocytes, macrophages, dendritic cells, and intestinal Paneth cells (Shaw et al., 2008). The best-known RLR is RIG-I, which senses viral RNA (Yoneyama et al., 2004).
In this study, we focused on the cytosolic sensor NOD2, which recognizes MDPs that are produced by both Gram-negative and Gram-positive bacteria (Girardin et al., 2003; Inohara et al., 2003). NOD2 is expressed in macrophages, which are major barriers in Ad-based gene transfer (Nociari et al., 2007; Smith et al., 2008), and in dendritic cells, which are important antigen-presenting cells (APCs) for initiating acquired immunity (Shaw et al., 2008). Once activated, NOD2 induces pro-inflammatory gene transcription through nuclear factor-κB (NFκB) independently of MyD88 (Kanneganti et al., 2007). In addition to the NFκB pathway, NOD2 stimulation activates the MAP kinases p38, ERK, and JNK (Park et al., 2007). These cascades are rapidly induced after stimulation with MDP, leading to activation of pro-inflammatory genes (Marina-Garcia et al., 2008).
Our experiments show that NOD2 deficiency significantly reduces the induction of pro-inflammatory cytokines after systemic administration of HDAds in vivo. We found that NOD2 is required for an innate response to HDAd independent of MyD88, and the NOD2 signaling pathway inhibits transgene expression at early time points. These observations suggest that cytoplasmic pathogen recognition receptors (e.g., NOD2) also contribute to the induction of innate immune response to HDAds in vivo after systemic administration of HDAds.
Materials and Methods
Adenoviral vectors and reagents
HDAd HD28E4 (HDAd0) and HDAd HD28E4LacZ constructs containing the β-galactosidase transgene driven by the cytomegalovirus (CMV) promoter (HDAdLacZ) were produced as described elsewhere (Suzuki et al., 2010a,b). Helper-virus contamination in this viral preparation was assessed by Southern blot and PhosphorImager analysis and was estimated to be less than 0.05% as described elsewhere (Zhu et al., 2007; Suzuki et al., 2010a).
MDP (Ac-muramyl-Ala-D-Glu-NH2) was purchased from Bachem (Torrance, CA). β-D-Galactoside galactohydrolase (β-Gal) was purchased from Worthington (Lakewood, NJ).
Preparation of bone marrow–derived macrophages (BMDMs)
Bone marrow cells were extracted from the femurs and tibiae of mice, and erythrocytes were eliminated by using lysis solution (QIAGEN, Valencia, CA). Bone marrow cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS) and 30% supernatant derived from a confluent mouse fibroblast cell line (L929 cell: ATCC, Manasas, VA) cultures (Suzuki et al., 2010b). At day 7, immature macrophages were collected. This procedure yields a pure population of macrophage colony-stimulating factor–dependent, adherent macrophages.
Mice and injections
MyD88–/– mice were provided by S. Akira (Department of Host Defense, Research Institute for Microbial Diseases, Osaka University, Osaka, Japan) (Adachi et al., 1998); NOD2–/– and C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Both MyD88- and NOD2-deficient (MyD88/NOD2–/–) mice were established as described below. All knockout mice are C57BL/6 background. All of the mice were housed under pathogen-free conditions; food and water were provided ad libitum. All mice used in experiments were males and between 7 and 10 weeks of age, and all experimental procedures were conducted in accordance with institutional guidelines for animal care and use. HDAd was diluted in sterile phosphate-buffered saline (PBS) and injected into the tail vein. The injections were performed with a total volume of 200 μl. Blood was collected retro-orbitally for analysis. Serum was frozen immediately and stored at −80°C until analysis. Upon sacrifice, the liver was harvested and kept at −80°C until analysis.
Generation of MyD88- and NOD2-deficient (MyD88/NOD2–/–) mouse
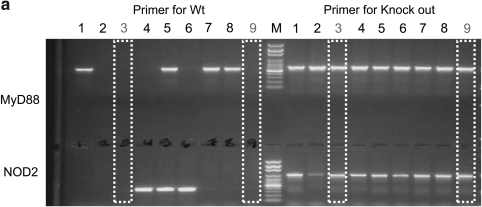
MyD88–/– and NOD2–/– mice were mated under pathogen-free conditions. Genotyping was performed by PCR using genomic DNA isolated from the tails of mice. DNA from each mouse tail was analyzed for MyD88 deficiency status by PCR (3 min at 94°C and then 35 cycles of 30 sec at 95°C, 1 min at 60°C, and 1 min at 72°C) with wild-type (WT) MyD88 gene-specific primers (F: 5′-TGGCATGCCTCCATCATAGTTAACC-3′ and R: 5′- GTCAGAAACAACCACCACCATGC-3′) and MyD88-deficient–specific primers (F: 5′-TGGCATGCCTCCATCATAGTTAACC-3′ and R: 5′-ATCGCCTTCTATCGCCTTCTTGACG-3′). NOD2 deficiency was also assessed by PCR (3 min at 94°C and then 35 cycles of 30 sec at 95°C, 1 min at 60°C, and 1 min at 72°C) using NOD2 gene–specific primers (F: 5′-ACAGAGATGCCGACACCATACTG-3′ and R: 5′-TGGAGAAGGTTGAAGAGCAGAGTC-3′) and NOD2-deficient–specific primers (F: 5′-TGACTGTGGCTAATGTCCTTTGTG-3′ and R: 5′-TTCTATCGCCTTCTTGACGAGTTC-3′). PCR products were electrophoresed on an agarose gel, and each gene or deficiency gene was confirmed (see Fig. 2a). After confirmation of both MyD88 and NOD2 deficiency by PCR (see Fig. 2a), mice were mated, and mice derived from deficiency parental mice were used for experiments in this study.
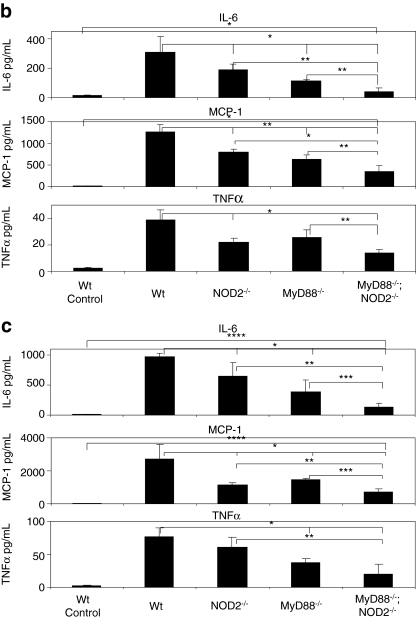
FIG. 2.
NOD2 signaling induces innate immune response to HDAds independently of MyD88 signaling in vivo. (a) Genotype of MyD88–/–;NOD2–/– double-knockout mice. DNA sample extracted from the tail of each mouse was used as a template in PCR as described in Materials and Methods, and PCR products were electrophoresed on an agarose gel. WT, NOD2–/–, MyD88–/–, and MyD88–/–;NOD2–/– mice were injected with 5×1012 vp/kg HDAd0 (b) or HDAdLacZ (c) into tail veins. Serum samples were collected 6 hr post injection. IL-6, MCP-1, and TNFα levels were measured by using BD cytokine multiplex bead array system. Data are presented as means±SD (n=4). The experiments were repeated with similar results. In (b), *p<0.02 and **p<0.001 for IL-6; *p<0.001 and **p<0.02 for MCP-1; *p<0.003 and **p<0.002 for TNFα. In (c), *p<0.04, **p<0.02, ***p<0.2, and ****p<0.001 for IL-6; *p<0.04, **p<0.003, ***p<0.02, and ****p<0.001 for MCP-1; *p<0.01 and **p<0.03 for TNFα.
Cytokine analysis
Mouse interleukin (IL)-6, IL-12p40, tumor necrosis factor-α (TNFα), monocyte chemotactic protein-1 (MCP-1), Kupffer cells (KCs), and RANTES in serum were assayed using the BD cytokine multiplex bead array system (BD Biosciences, San Jose, CA) and analyzed using a BD FACSArray instrument (BD Biosciences) according to the manufacturer's instructions (Cerullo et al., 2007a,b; Suzuki et al., 2010b).
Time course of β-Gal activity, vector genome DNA, and LacZ mRNA expression in liver of mice
Mice were injected with 5×1012 viral particles (vp)/kg HDAdLacZ as described above. At defined time points following injection, mice were euthanized by CO2 inhalation. β-Gal activity of each liver sample was measured using a β-galactosidase assay kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. Total DNA was extracted from liver using DNeasy Blood and Tissue Kit (QIAGEN), and total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA). RNA samples were treated with TURBO DNA-free Kit (Ambion, Austin, TX) and then reverse-transcribed into complementary DNA (cDNA) using the Superscript III first-strand cDNA synthesis system (Suzuki et al., 2010b). DNA and cDNA samples prepared from each liver were analyzed by quantitative real-time PCR (10 min at 95°C and then 45 cycles of 10 sec at 95°C, 7 sec at 60°C, and 30 sec at 72°C) using a Roche Light Cycler 1.1 and Roche master mix (Roche, Indianapolis, IN) with human stuffer gene-specific primers (F: 5′-TCTGAATAATTTTGTGTTACTCATAGCGCG-3′ and R: 5′-CCCATAAGCTCCTTTTAACTTGTTAAAGTC-3′) and LacZ gene-specific primers (F: 5′-ATACTGTCGTCGTCCCCTCAAACT-3′ and R: 5′-CCTCCAGATAACTGCCGTCACTC-3′). Vector copy numbers per microgram of total DNA were calculated using a standard curve generated by quantitative PCR with the original plasmid DNA of HDAdLacZ (pHDAd-LacZ) serially diluted over nine orders of magnitude.
Titering of antibodies to Ad viral particle and transgene product
ELISA-based titering experiments were conducted as previously described.(Suzuki et al., 2010b). In brief, 5×108 vp/well HDAds or 2 μg/well β-galactosidase protein (each diluted in PBS) were used to coat the wells of a 96-well plate overnight at 4°C. Plates were washed with PBS-Tween 20 (0.05%) (PBS-T) solution, and blocking buffer [3% bovine serum albumin (BSA) in PBS] was added to the wells and incubated at room temperature for 2 hr. For titering of total immunoglobulin G (IgG) antibodies, plasma was diluted 1/300 in sample dilution buffer (1% BSA in PBS), added to the wells, and incubated overnight at 4°C. Wells were washed with PBS-T, and horseradish peroxidase–conjugated rabbit anti-mouse IgG antibodies were added at a 1/3,000 dilution in PBS-T. Tetramethylbenzidine (Sigma-Aldrich, St. Louis, MO) substrate (100 μl) was added to each well, and the reaction was stopped with 50 μl of 2 N sulfuric acid. Plates were read at 450 nm in a microplate spectrophotometer.
IFNγ secretion from splenocyte culture
As a measure of cell-mediated immune response to HDAd, we assessed the expression of IFNγ produced by splenic T cells (Barcia et al., 2007). Three weeks after injection, spleens were isolated from each group of mice, and splenocytes at 5×106 cells/well were cultured with RPMI supplemented with 50 μM β-mercaptoethanol and 10% FBS in 24-well plates. Cultured splenocytes were added with RPMI medium containing 5×109 vp/well heat-inactivated HDAd0 (inactivated at 85°C for 15 min) or 10 μg/ml β-Gal and incubated at 37°C for 48 hr. The IFNγ concentration in the medium of each sample was measured using the BD cytokine multiplex bead array system (BD Biosciences) and analyzed using a BD FACSArray instrument (BD Biosciences) according to the manufacturer's instructions.
Analysis of T-cell population
Splenocytes cultured with heat-inactivated HDAd as described above were collected by centrifugation and blocked with Fc block (eBioscience, San Diego, CA). Cells were washed and stained with APC-anti-mouse CD8 and APC-Cy7-anti-mouse CD4 antibodies (eBioscience) according to the manufacturer's instructions. After washing, each sample was measured using the BD FACSArray instrument (BD Biosciences) and analyzed using FlowJo software (FlowJo, Ashland, OR) according to the manufacturer's instructions.
Quantitative PCR analysis and chromatin immunoprecipitation (ChIP) assay
WT and NOD2–/– BMDMs described above were seeded at subconfluency in 12-well plates for DNA and RNA extraction and 6-cm dishes for ChIP experiments. After 24 hr, BMDMs were infected with 5,000 vp/cell HDAdLacZ. Medium was replaced after 3 hr. Total RNA and total cellular DNA were extracted at 24 hr using TRIzol reagent (Invitrogen) and DNeasy Blood and Tissue Kit (QIAGEN). First-strand cDNA prepared from the RNA samples and DNA samples were subjected to quantitative real-time PCR analysis as described herein. ChIP PCR assay was performed using the ChIP assay kit (Millipore, Temecula, CA) with anti-K9-dimethylated H3 (clone mAbcam 1220), anti-K9-acetylated H3 antibodies (Abcam, Cambridge, MA) as previously described (Suzuki et al., 2007, 2010b). In brief, chromatin was cross-linked with 1% formaldehyde. The cells were washed and resuspended in sodium dodecyl sulfate lysis buffer and then sonicated. The soluble chromatin was precleared by incubation with protein A–agarose–salmon sperm DNA slurry. Antibodies were added to the precleared supernatant and immunoprecipitated overnight at 4°C. After washing, antibody-bound histone-DNA complex was eluted, and histone-DNA cross-links were reversed by heating to 65°C for 4 hr. The immunoprecipitated DNA from each sample was subjected to quantitative PCR analysis using the CMV promoter–specific primers (5′-GGGTGGTGACTCAATGGCCTTTAC-3′ and 5′-CCCACATTGACTTATATGCTTGCCAAC-3′) and β-Gal transgene–specific primers (5′-GCAGCATCAGGGGAAAACCTTATTTA-3′ and 5′-TCGCCACTTCAACATCAACGGTA-3′). Vector copy numbers per sample were calculated using a standard curve described above.
Statistical analysis
We analyzed data by t-test analysis of variance followed by Shapiro-Wilk's protected least significant difference test (SigmaPlot).
Results
NOD2 contributes to the acute inflammatory response triggered by systemic HDAd administration in vivo
We have previously demonstrated that TLRs/MyD88 signaling is important in the immune response to HDAds in vitro and in vivo (Cerullo et al., 2007b; Suzuki et al., 2010b). However, MyD88-deficient mice exhibited only partial reduction of the innate immune response. Therefore, to identify MyD88-independent molecular components involved in recognizing HDAd, we systemically injected NOD2–/– and WT C57BL6 mice with 5×1012 vp/kg HDAd0 or HDAdLacZ (Fig. 1a). There are no prokaryotic sequences in the vector DNA of HDAd0; therefore, we did not expect it to be strongly sensed by TLR9. TLR9–/– and MyD88–/– mice each showed a significant reduction in the innate immune response compared with WT mice after systemic administration of HDAdLacZ, suggesting that the TLR9/MyD88 pathway responds to bacterial β-Gal.
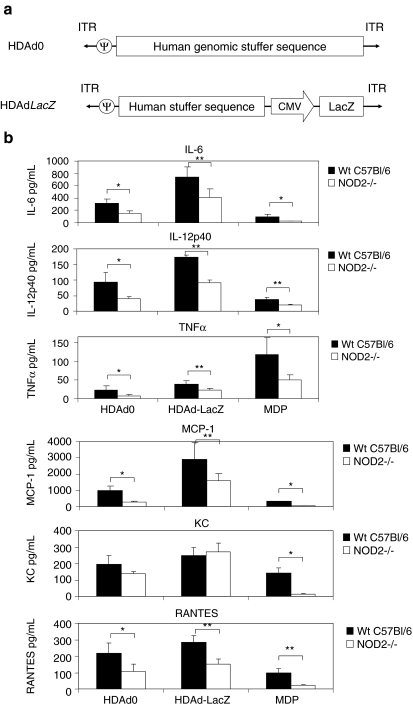
FIG. 1.
NOD2 attenuates the innate immune response to HDAds in vivo. (a) Schematic structure of the HDAd0 and HDAdLacZ vector genomes. (b) WT C57Bl/6 and NOD2–/– mice were injected with 5×1012 vp/kg HDAd0 or HDAdLacZ or 300 μg of MDP into tail veins. Serum samples were collected 6 hr post injection. Cytokine and chemokine levels were assayed. IL-6, IL-12p40, TNFα, MCP-1, KC, and RANTES levels were measured by using the BD cytokine multiplex bead array system. Data are presented as means±SD (n=5). The experiments were repeated with similar results. *p<0.005 and **p<0.01 for IL-6; *p<0.03 and **p<0.005 for IL-12p40; *p<0.04 and **p<0.006 for TNFα; *p<0.002 and **p<0.03 for MCP- 1; *p<0.001 for KCs; *p<0.03 and **p<0.002 for RANTES.
To test whether induction of TLR9/MyD88 signaling masks the effect of NOD2, we also compared the induction of the innate response between WT and NOD2–/– mice after systemic administration of HDAdLacZ. Six hours after injection, NOD2–/– mice injected with HDAd0 or HDAdLacZ showed a 50% reduction in IL-6, IL-12p40, TNFα, MCP-1, and RANTES compared with WT mice (Fig. 1b). NOD2–/– mice injected with MDP (known to be sensed by NOD2) also showed a significant reduction of these cytokine levels in blood, indicating that the NOD2-dependent signaling pathway contributes to the innate immune response at 6 hr. Although NOD2–/– mice injected with MDP showed a significant reduction in KC levels in serum compared with WT mice, no difference in KC levels was observed between WT and NOD2–/– mice injected with HDAds, suggesting that induction of some cytokines/chemokines to HDAds may be regulated mainly by a NOD2-independent pathway (Appledorn et al., 2008). It is noteworthy that cytokines produced by WT mice injected with HDAdLacZ significantly exceeded those of mice injected with HDAd0, in which both NOD2 and TLR9 are probably activated. These results suggest that NOD2 is required for full induction of the innate response to systemically administered HDAd, but the partial reduction still points to a NOD2-independent pathway as well.
Both MyD88- and NOD2-deficient mice showed a significant reduction in the innate response compared with mice deficient for either gene singly
A NOD2-dependent innate response to breakdown products of bacterial peptidoglycan is not required by MyD88 (Kanneganti et al., 2007). To ascertain that an innate response to HDAd via NOD2 signaling is also independent of the MyD88 signaling pathway, we established MyD88/NOD2 double-deficient (MyD88/NOD2–/–) mice by mating NOD2–/– and MyD88–/– mice (Fig. 2a). After establishment of MyD88/NOD2–/– mice, we first compared the induction of the innate response to HDAds among WT, NOD2–/–, MyD88–/–, and MyD88/NOD2–/– mice following systemic administration of 5×1012 vp/kg HDAd0 (Fig. 2b) or HDAdLacZ (Fig. 2c). MyD88–/–;NOD2–/– mice showed a significant reduction of IL-6, MCP-1, and TNFα compared with mice singly deficient for either gene and WT mice, suggesting that NOD2 signaling induces the acute immune response to HDAds independently of MyD88 signaling.
NOD2 is dispensable for the induction of acquired immune response to systemically administered HDAd
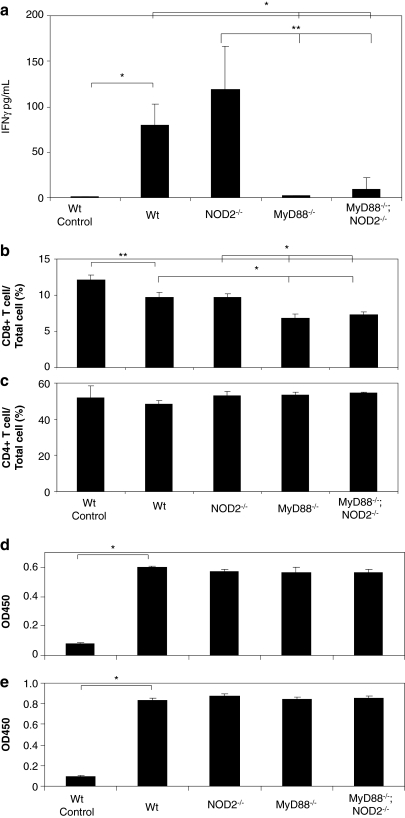
We previously showed that TLR/MyD88 signaling was important for the T helper cell type 1 (Th1) acquired immune responses (Suzuki et al., 2010b). To test if NOD2 also contributes to the development of acquired immune responses after infection by HDAd, WT, NOD2–/–, MyD88–/–, and MyD88–/–;NOD2–/– mice were primed with 5×1012 vp/kg HDAdLacZ, and splenocytes from these mice were cultured in vitro 3 weeks later. Splenocytes isolated from WT mice injected with PBS were used as naive mouse controls (indicated as “control” in the figures). Heat-inactivated HDAd was used as an antigenic stimulator. After 48 hr, the splenocyte culture medium was collected to measure the concentration of IFNγ, one of the major cytokines secreted from Th1 lymphocytes (Fig. 3a) (Niesner et al., 2008). There was very little production of IFNγ by heat-inactivated HDAd from splenocytes isolated from PBS-injected mice, indicating that any IFNγ production detected from splenocytes of HDAdLacZ-treated mice was indeed Ad-dependent. Although NOD2–/– mice showed a significant reduction of circulating pro-inflammatory cytokines at 6 hr post injection compared with WT mice (Fig. 1b), there was no difference of IFNγ secretion from isolated splenocytes between NOD2–/– and WT mice after systemic administration of HDAdLacZ. Splenocytes derived from MyD88–/– and MyD88–/–;NOD2–/– mice showed low induction of IFNγ, and there was no significant difference of IFNγ level between MyD88–/– and MyD88–/–;NOD2–/– mice.
FIG. 3.
NOD2 signaling has no effect on acquired response to HDAd in vivo. WT C57Bl/6, NOD2–/–, MyD88–/–, and MyD88–/–;NOD2–/– mice were injected with 5×1012 vp/kg HDAdLacZ into tail veins. Spleens from HDAd-injected groups of mice (n=4 for each group) were collected 3 weeks post injection. (a) Splenocytes were incubated with heat-inactivated Ad capsid protein for 48 hr. IFNγ in the medium was measured by using BD cytokine multiplex bead array system and analyzed. Data are presented as means±SD (n=4). *p<0.005 and **p<0.01. Splenocytes incubated with heat-inactivated Ad capsid protein were measured for the development of CD8+ T cells (b) and CD4+ T cells (c) by FACS analysis as described in Materials and Methods. *p<0.005 and **p<0.01. Serum samples were collected at 3 weeks from each group of mice (n=4) after systemic administration of HDAdLacZ (5×1012 vp/kg). The development of antibodies to viral particles (d) and β-Gal (e) was evaluated by using ELISA as described in Materials and Methods. Data are presented as means±SD (n=4). *p<0.005.
We also assessed CD4-positive (CD4+) and CD8-positive (CD8+) T-cell populations in cultured splenocytes by FACS analysis (Fig. 3b and c). Splenocytes from control WT mice demonstrated slightly higher numbers of CD8+ T cells compared with those injected with HDAdLacZ. However, control WT splenocytes showed a significantly lower induction of IFNγ compared with HDAdLacZ primed WT splenocytes (Fig. 3a), suggesting that CD8+ cells in control WT mice are predominately immature T cells. Splenocytes from primed NOD2–/– mice showed no difference in CD4+ and CD8+ T-cell populations compared with those from WT mice, suggesting that NOD2 does not limit acquired immunity to Ad vectors. Although MyD88–/– and MyD88–/–;NOD2–/– mice showed a significantly reduced population of CD8+ T cells compared with WT mice, there was no difference in numbers of CD4+ T cells in cultured splenocytes among these mice. This finding suggests that the MyD88 signaling pathway regulates the development of the CD8+ T cell (Th1), but is not required to develop the CD4+ T cell (Th2), at least at the acquired immune stage. There was also no difference in levels of adenovirus and β-Gal-specific IgGs at 3 weeks after priming (Fig. 3d and e), suggesting that the MyD88 signaling pathway does not limit the humoral response to Ad vectors or to the β-Gal (transgene product).
NOD2 limits transgene expression of HDAd in the liver before development of acquired response
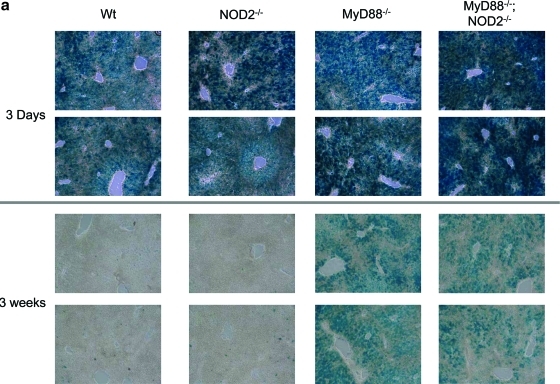
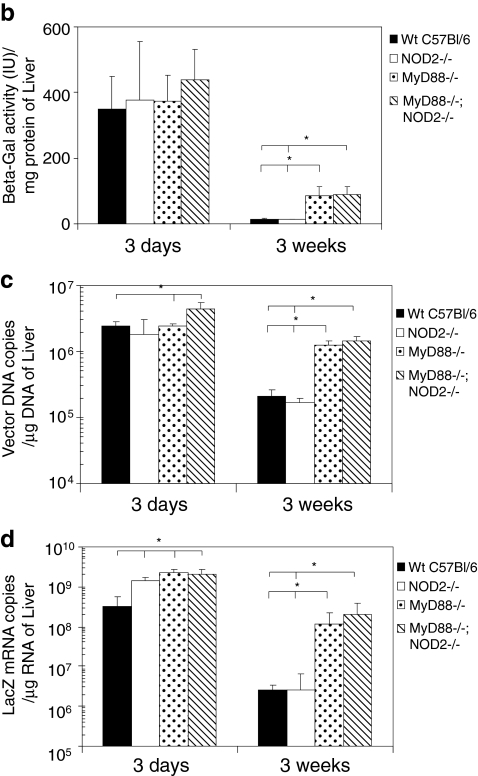
To determine whether innate and acquired immune responses limit transgene expression from HDAd, we evaluated β-Gal expression in the livers of WT, NOD2–/–, MyD88–/–, and MyD88–/–;NOD2–/– mice following systemic administration of HDAdLacZ. We performed X-Gal staining of these livers and measured β-Gal activity for each (Fig. 4a and b). There was no difference in β-Gal activity in livers of WT, NOD2–/–, MyD88–/–, and MyD88–/–;NOD2–/– mice at 3 days post injection. MyD88–/– and MyD88–/–;NOD2–/– mice, however, showed significantly higher β-Gal activity compared with WT and NOD2–/– mice at 3 weeks. This directly correlated with previously conducted X-Gal staining results (Fig. 4a). There was no difference seen in β-Gal activity between MyD88–/– and MyD88–/–;NOD2–/– mice, suggesting that transgene expression of HDAd following development of an acquired immune response (3 weeks) is regulated mainly by MyD88 signaling.
FIG. 4.
Time course of transgene expression in the livers of mice. Each group of mice was injected with 5×1012 vp/kg HDAdLacZ. (a) X-Gal staining of livers from WT, NOD2–/–, MyD88–/–, and MyD88–/–;NOD2–/– mice was performed both 3 days and 3 weeks post injection. The animals were sacrificed at 3 days and 3 weeks post injection, and β-Gal activity of each sample was measured at the same time points (b) (*p<0.001). The copy numbers of vector genome DNA (c) and β-Gal mRNA transcripts (d) in livers were also determined by real-time quantitative PCR (*p<0.002 for both c and d). Data are presented as means±SD (n=4). Color images available online at www.liebertonline.com/hum
We also quantified the vector genome and LacZ mRNA copy numbers in the livers of HDAdLacZ-injected mice at these time points (Fig. 4c and d). Although vector DNA levels were similar in WT, NOD2–/–, and MyD88–/– mice 3 days post injection, NOD2–/– and MyD88–/– mice showed significantly (approximately fivefold) higher LacZ mRNA levels compared with WT. These results suggest that NOD2 signaling may influence the regulation of transgene expression of HDAd at the mRNA level before host development of an acquired immune response.
Although MyD88–/– and MyD88–/–;NOD2–/– mice showed comparable populations of CD4+ T cells and total IgG to Ad particles and the β-Gal transgene product compared with WT mice (Fig. 3d and e), these deficient mice exhibited 10-fold higher levels of vector and 100-fold higher LacZ mRNA levels compared with WT mice at 3 weeks post injection. This suggests that transgene expression of HDAd is mainly suppressed by the Th1 adaptive immune response due to MyD88 signaling in WT mice. Both WT and NOD2–/– mice showed negligible LacZ activity after 3 weeks; there was no difference in vector and LacZ mRNA copy numbers between MyD88–/– and MyD88–/–;NOD2–/– mice, indicating that development of the acquired response to HDAd does not require NOD2 signaling.
NOD2 signaling partially contributes to transgene expression of HDAd at the mRNA level independent of epigenetic modification of vector DNA in vitro
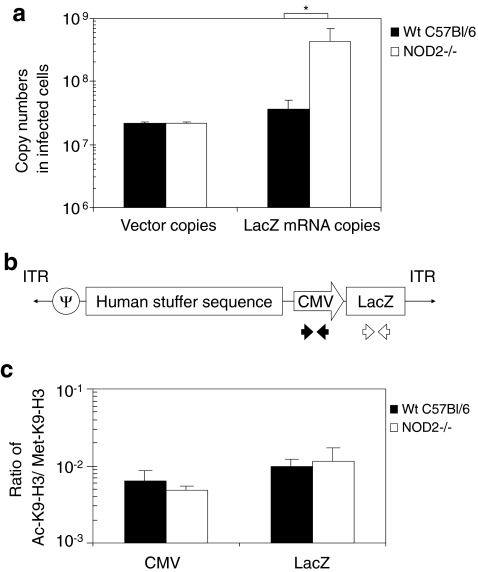
To test whether NOD2 signaling (induced by infection of HDAd) regulates transgene expression at the cellular level, we assessed transgene expression in primary WT and NOD2–/– mouse BMDMs that endogenously express NOD2 (Shaw et al., 2008). We isolated DNA and RNA from these HDAd-infected BMDMs at 24 hr and measured vector and LacZ mRNA copy number in infected BMDMs (Fig. 5a). Although HDAdLacZ-infected NOD2–/– BMDMs showed equivalent transduction efficacy (vector copy numbers) to WT BMDMs, LacZ mRNA copy numbers in transduced NOD2–/– BMDMs were approximately 10-fold higher than WT BMDMs. These in vivo and in vitro data suggested that NOD2-dependent innate immunity inhibits expression of β-Gal mRNA from HDAdLacZ. Our previous results indicated that vector DNA can be sensed and transcriptionally inactivated through MyD88-dependent epigenetic modifications (Suzuki et al., 2010b).
FIG. 5.
Transgene expression is partially regulated by NOD2 signaling independent of epigenetic modification in vitro. (a) BMDMs derived from WT or NOD2–/– mice were infected with 5,000 vp/cell HDAdLacZ for 3 hr. Cells were harvested at 24 hr and were evaluated by real-time quantitative PCR for vector genome DNA and β-Gal mRNA copy numbers. Data are presented as means ± SD (n = 4). The experiments were repeated with similar results. *p < 0.02. (b) Schematic structure of the HDAdLacZ vector genome and location of primer pair used for quantification of ChIP data using real-time quantitative PCR. (c) BMDMs derived from WT and NOD2–/– were infected with 5,000 vp/cell HDAdLacZ for 3 hr. Cells were harvested at 24 hr and ChIP experiments were performed as described in Materials and Methods. Ratio of vector DNA associated with Ac-K9-H3 to Met-K9-H3 was calculated. A higher ratio correlates with transcriptionally active loci. Data are presented as means ± SD (n = 5).
To determine whether NOD2 signaling also regulates transcription of vector transgenes by epigenetic modification of vector DNA chromatin, ChIP PCR analysis was performed. DNA samples were harvested at 24 hr from infected WT and NOD2–/– BMDMs, and immunoprecipitated with an antibody specific to histone H3, either dimethylated at lysine 9 (Met-K9-H3) or acetylated at lysine 9 (Ac-K9-H3). Met-K9-H3 is known to associate with transcriptionally inactive heterochromatic domains, whereas Ac-K9-H3 is known to associate with transcriptionally active euchromatic domains (Zhang and Reinberg, 2001). The immunoprecipitated DNA-chromatin complexes were evaluated by real-time PCR analysis using CMV promoter and LacZ transgene specific primers (Fig. 5b). The ratios of Ac-K9-H3–associated DNA to Met-K9-H3–associated DNA were calculated for each sample and plotted (Fig. 5c). Although LacZ mRNA copy numbers in transduced NOD2–/– BMDMs were approximately 10-fold higher than in WT BMDMs, there was no difference in the ratio of Ac-K9-H3–associated DNA to Met-K9-H3–associated DNA. This result suggests that NOD2 signaling transcriptionally inactivates transgene expression of HDAdLacZ independently of epigenetic modification.
Discussion
To address the inherent toxicity of early-generation Ad vectors, HDAds devoid of all viral coding sequences were developed (Parks et al., 1996). However, acute toxicity (similar to that of FGAds) after in vivo delivery continues to limit the clinical translation of HDAds (Brunetti-Pierri et al., 2004; Muruve et al., 2004; McCaffrey et al., 2008). Although several studies of Ad vectors have begun to characterize the mechanisms of the innate immune activation responsible for this acute toxic response (Muruve, 2004), the full context of the response is still unclear. Moreover, how these innate factors regulate the acquired immune response in the context of HDAd gene therapy remains unknown. The host innate immune response includes many cellular and humoral factors, and no single pathway accounts for the overall response to Ad-based vectors. By systematically identifying the specific host pathways that mediate the innate response, we may be able to develop combinatorial approaches that decrease overall toxicity, and thereby increase the maximal tolerated dose for any specific intervention.
Our previous studies with HDAds and FGAds demonstrated that TLR/MyD88 signaling is a major, but not the only, factor serving as a critical signaling pathway after systemic intravenous delivery of Ad (Cerullo et al., 2007b; Yamaguchi et al., 2007; Suzuki et al., 2010b). In this study, we characterized the role of the cytosolic sensor NOD2 in the immune response to HDAd in vivo by using genetic mouse models. Our studies indicate that NOD2 and TLR/MyD88 pathways cooperate independently but additively to stimulate innate immune responses to HDAds, suggesting that activation of the NOD2 signaling pathway by infection of HDAds follows the same mechanism as activation of NOD2 signaling by bacterial peptidoglycan, which does not require the MyD88 signaling pathway (Kanneganti et al., 2007). NOD2 senses bacterial peptidoglycan released during phagocytosis and transported into the cell by endocytosis (Lecat et al., 2010). Although endogenous NOD2 expression is restricted in myelomonocytic cells (e.g., macrophages), NOD2 expression is also up-regulated by pro-inflammatory stimuli in liver cells (e.g., hepatocytes) of mice and humans (Body-Malapel et al., 2008). After systemic administration, over 80% of Ad5-based vectors distribute in livers of mice and nonhuman primates (Sakurai et al., 2008). Ad5-based vectors are taken up by KCs in liver, and Ad-transduced KCs induce necrosis (Manickan et al., 2006). These results suggest that NOD2 in macrophages may sense HDAd components during phagocytosis, and induced NOD2 in hepatocytes via pro-inflammatory stimuli (to HDAds) may sense HDAd components released from necrotizing KCs by endocytosis. Other NLRs (e.g., NALP3) have been reported to sense bacterial and viral (influenza A) RNA similar to bacterial peptidoglycans and lead to production of IL-1 (Kanneganti et al., 2006a,b).
Recently, NALP3 and ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), components of the innate cytosolic molecular complex termed the inflammasome, were identified to induce the maturation of pro-IL-1β in macrophages after internalization of viral DNA derived from DNA viruses (e.g., adenovirus, herpes simplex virus) (Muruve et al., 2008). Although empty viral capsid of adenovirus was incapable of inducing IL-1β maturation, transfected viral and bacterial DNAs increase the secretion of IL-1β (Muruve et al., 2008). However, the mechanism of cytosolic DNA sensing by the NALP inflammasome complex is still unclear. Sabbah et al. reported that NOD2 is also required for responses to viral single-strand RNA (Sabbah et al., 2009). The results of Sabbah et al. and our current study support the idea that NOD2 recognizes viral components as well as bacterial molecular patterns. NOD2 has a nucleotide-binding domain identical to NALP3 (Mariathasan and Monack, 2007), suggesting that NOD2 may function to sense cytosolic DNA in a manner similar to NALP3. Further studies of how NOD2 detects HDAd components will be necessary to define the molecular mechanisms that induce the innate responses in vitro and in vivo.
We also assessed the effect of NOD2 on transgene expression in vitro and in vivo after HDAd infection. We found that NOD2 signaling may suppress transgene expression at the mRNA levels in HDAd-infected BMDMs as well as in vivo in the livers of mice before induction of an acquired immune response. Our previous results indicated that vector DNA can be sensed and transcriptionally inactivated through MyD88-dependent epigenetic modifications (Suzuki et al., 2010b). However, there was no difference in chromatin modification of vector DNA between WT BMDMs and NOD2–/– BMDMs. Recently, Dugan et al. found that NOD2 binds to 2′-5′-oligoadenylate synthetase type 2 (OAS2), a double-stranded RNA–binding protein involved in the pathway that activates RNase-L (Dugan et al., 2009). Overexpression of NOD2 led to enhanced RNase-L activity in cells, suggesting that NOD2 may accelerate the degradation of transgene mRNA derived from HDAd via activation of RNase-L in transduced cells instead of by chromatin modification. NOD2 expression is also up-regulated by pro-inflammatory stimuli in liver cells (e.g., hepatocytes) (Body-Malapel et al., 2008), suggesting that transgene mRNA derived from HDAd in hepatocytes may also be degraded by RNase-L that is activated by interaction of OAS2 with NOD2 during an HDAd-dependent pro-inflammatory response. Our current and previous results suggest that HDAd-transduced cells may accelerate silencing of transgene expression at the transcriptional level by both dependent and independent epigenetic modification of vector DNA (Suzuki et al., 2010b).
Our current study suggests that cytoplasmic sensor molecules also contribute to the induction of an innate immune response to Ad-based vectors. Recently, Chiu et al. found that cytosolic double-strand DNA derived from DNA viruses (e.g., adenovirus, herpes simplex virus) is converted into 5′-triphosphate RNA by RNA polymerase III (Pol III), and this 5′-triphosphate RNA induces type I IFN expression through the RIG-I pathway in transduced cells (Chiu et al., 2009). This raises questions about how cytoplasmic sensor molecules and mechanisms (i.e., NALP3, Pol III/RIG-I) contribute to innate immune responses and/or development of an acquired response to systemic administration of HDAd.
HDAd is the likely vehicle for clinical trials of Ad-based gene replacement therapy for inherited disorders. However, our results suggest that the use of HDAd still faces hurdles, especially induction of acute toxicity through pathogen-recognition receptors. We need to better understand the molecular mechanisms of how host cells recognize HDAd and induce innate responses to improve the therapeutic index of these vectors.
Acknowledgments
This work was support by the National Institutes of Health (grants R01DK56787 and R01HL87836 to B. Lee and grant K99HL098692 to M. Suzuki). The authors thank Ming-Ming Jiang for her expertise and assistance with histology for this study.
Author Disclosure Statement
No competing financial interests exist.
References
- Adachi O. Kawai T. Takeda K., et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Akira S. Uematsu S. Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Appledorn D.M. Patial S. McBride A., et al. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J. Immunol. 2008;181:2134–2144. doi: 10.4049/jimmunol.181.3.2134. [DOI] [PubMed] [Google Scholar]
- Barcia C. Jimenez-Dalmaroni M. Kroeger K.M., et al. One-year expression from high-capacity adenoviral vectors in the brains of animals with pre-existing anti-adenoviral immunity: clinical implications. Mol. Ther. 2007;15:2154–2163. doi: 10.1038/sj.mt.6300305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basner-Tschakarjan E. Gaffal E. O'Keeffe M., et al. Adenovirus efficiently transduces plasmacytoid dendritic cells resulting in TLR9-dependent maturation and IFN-alpha production. J. Gene Med. 2006;8:1300–1306. doi: 10.1002/jgm.964. [DOI] [PubMed] [Google Scholar]
- Body-Malapel M. Dharancy S. Berrebi D., et al. NOD2: a potential target for regulating liver injury. Lab. Invest. 2008;88:318–327. doi: 10.1038/labinvest.3700716. [DOI] [PubMed] [Google Scholar]
- Bonnert T.P. Garka K.E. Parnet P., et al. The cloning and characterization of human MyD88: a member of an IL-1 receptor related family. FEBS Lett. 1997;402:81–84. doi: 10.1016/s0014-5793(96)01506-2. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N. Palmer D.J. Beaudet A.L., et al. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum. Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N. Stapleton G.E. Law M., et al. Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates. Mol. Ther. 2009;17:327–333. doi: 10.1038/mt.2008.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerullo V. McCormack W. Seiler M., et al. Antigen-specific tolerance of human alpha1-antitrypsin induced by helper-dependent adenovirus. Hum. Gene Ther. 2007a;18:1215–1224. doi: 10.1089/hum.2006.036. [DOI] [PubMed] [Google Scholar]
- Cerullo V. Seiler M.P. Mane V., et al. Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol. Ther. 2007b;15:378–385. doi: 10.1038/sj.mt.6300031. [DOI] [PubMed] [Google Scholar]
- Chiu Y.H. MacMillan J.B. Chen Z.J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert C. Petrilli V. Van Bruggen R., et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan J.W. Albor A. David L., et al. Nucleotide oligomerization domain-2 interacts with 2′-5′-oligoadenylate synthetase type 2 and enhances RNase-L function in THP-1 cells. Mol. Immunol. 2009;47:560–566. doi: 10.1016/j.molimm.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sastre A. Biron C.A. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- Girardin S.E. Boneca I.G. Viala J., et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- Hartman Z.C. Kiang A. Everett R.S., et al. Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses in vivo. J. Virol. 2007;81:1796–1812. doi: 10.1128/JVI.01936-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley S.E. Amalfitano A. Toll-like receptors impact on safety and efficacy of gene transfer vectors. Mol. Ther. 2007;15:1417–1422. doi: 10.1038/sj.mt.6300217. [DOI] [PubMed] [Google Scholar]
- Iacobelli-Martinez M. Nemerow G.R. Preferential activation of Toll- like receptor nine by CD46-utilizing adenoviruses. J. Virol. 2007;81:1305–1312. doi: 10.1128/JVI.01926-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohara N. Ogura Y. Fontalba A., et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J. Biol. Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- Kanneganti T.D. Body-Malapel M. Amer A., et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J. Biol. Chem. 2006a;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- Kanneganti T.D. Ozoren N. Body-Malapel M., et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006b;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- Kanneganti T.D. Lamkanfi M. Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Lecat A. Piette J. Legrand-Poels S. The protein Nod2: an innate receptor more complex than previously assumed. Biochem. Pharmacol. 2010;80:2021–2031. doi: 10.1016/j.bcp.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Manickan E. Smith J.S. Tian J., et al. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol. Ther. 2006;13:108–117. doi: 10.1016/j.ymthe.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Mariathasan S. Monack D.M. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat. Rev. Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- Marina-Garcia N. Franchi L. Kim Y.G., et al. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2. J. Immunol. 2008;180:4050–4057. doi: 10.4049/jimmunol.180.6.4050. [DOI] [PubMed] [Google Scholar]
- McCaffrey A.P. Fawcett P. Nakai H., et al. The host response to adenovirus, helper-dependent adenovirus, and adeno-associated virus in mouse liver. Mol. Ther. 2008;16:931–941. doi: 10.1038/mt.2008.37. [DOI] [PubMed] [Google Scholar]
- McCormack W.M., Jr. Seiler M.P. Bertin T.K., et al. Helper-dependent adenoviral gene therapy mediates long-term correction of the clotting defect in the canine hemophilia A model. J. Thromb. Haemost. 2006;4:1218–1225. doi: 10.1111/j.1538-7836.2006.01901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E. Tschopp J. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol. Cell. 2006;22:561–569. doi: 10.1016/j.molcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Mian A. McCormack W.M., Jr. Mane V., et al. Long-term correction of ornithine transcarbamylase deficiency by WPRE-mediated overexpression using a helper-dependent adenovirus. Mol. Ther. 2004;10:492–499. doi: 10.1016/j.ymthe.2004.05.036. [DOI] [PubMed] [Google Scholar]
- Muruve D.A. The innate immune response to adenovirus vectors. Hum. Gene Ther. 2004;15:1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- Muruve D.A. Cotter M.J. Zaiss A.K., et al. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J. Virol. 2004;78:5966–5972. doi: 10.1128/JVI.78.11.5966-5972.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruve D.A. Petrilli V. Zaiss A.K., et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- Niesner U. Albrecht I. Janke M., et al. Autoregulation of Th1-mediated inflammation by twist1. J. Exp. Med. 2008;205:1889–1901. doi: 10.1084/jem.20072468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nociari M. Ocheretina O. Schoggins J.W. Falck-Pedersen E. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J. Virol. 2007;81:4145–4157. doi: 10.1128/JVI.02685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill L.A. When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity. 2008;29:12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Park J.H. Kim Y.G. McDonald C., et al. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J. Immunol. 2007;178:2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- Parks R.J. Chen L. Anton M., et al. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah A. Chang T.H. Harnack R., et al. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 2009;10:1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai F. Nakamura S. Akitomo K., et al. Transduction properties of adenovirus serotype 35 vectors after intravenous administration into nonhuman primates. Mol. Ther. 2008;16:726–733. doi: 10.1038/mt.2008.19. [DOI] [PubMed] [Google Scholar]
- Shaw M.H. Reimer T. Kim Y.G. Nunez G. NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr. Opin. Immunol. 2008;20:377–382. doi: 10.1016/j.coi.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.S. Xu Z. Byrnes A.P. A quantitative assay for measuring clearance of adenovirus vectors by Kupffer cells. J. Virol. Methods. 2008;147:54–60. doi: 10.1016/j.jviromet.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Suzuki M. Chiocca E.A. Saeki Y. Early STAT1 activation after systemic delivery of HSV amplicon vectors suppresses transcription of the vector-encoded transgene. Mol. Ther. 2007;15:2017–2026. doi: 10.1038/sj.mt.6300273. [DOI] [PubMed] [Google Scholar]
- Suzuki M. Cela R. Clarke C., et al. Large-scale production of high-quality helper-dependent adenoviral vectors using adherent cells in cell factories. Hum. Gene Ther. 2010a;21:120–126. doi: 10.1089/hum.2009.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M. Cerullo V. Bertin T.K., et al. MyD88-dependent silencing of transgene expression during the innate and adaptive immune response to helper-dependent adenovirus. Hum. Gene Ther. 2010b;21:325–336. doi: 10.1089/hum.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toietta G. Mane V.P. Norona W.S., et al. Lifelong elimination of hyperbilirubinemia in the Gunn rat with a single injection of helper-dependent adenoviral vector. Proc. Natl. Acad. Sci. U.S.A. 2005;102:3930–3935. doi: 10.1073/pnas.0500930102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T. Kawabata K. Koizumi N., et al. Role of MyD88 and TLR9 in the innate immune response elicited by serotype 5 adenoviral vectors. Hum. Gene Ther. 2007;18:753–762. doi: 10.1089/hum.2007.016. [DOI] [PubMed] [Google Scholar]
- Yoneyama M. Kikuchi M. Natsukawa T., et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 2001;15:2343–2360. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- Zhu J. Huang X. Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J. Virol. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]