Abstract
The cerebellum is a subcortical brain structure that is essential for learning and controlling movement. Recent work shows that the cerebellum also plays a role in certain perceptual abilities, beyond what would be expected secondary to poor movement control. This review covers these and other recent advances, focusing on how cerebellar damage affects human abilities ranging from sensory perception to movement control and motor learning.
Introduction
Much of what is known about the function of the cerebellum is based on lesion studies. Damage produces characteristic motor deficits—e.g. cerebellar patients are uncoordinated or `ataxic,' with poor limb, eye, and walking control. Yet, the cerebellum is connected to many cerebral areas beyond cerebral motor areas and brainstem structures [1]. As such, it plays a role in a broader range of behaviors. This review summarizes some of the newest insights into how damage of the cerebellum affects human sensory and motor behavior, and highlights important unknowns.
Moving
Cerebellar damage leads to poor movement control—for example, reaching motions become irregular, curved, with poor targeting and oscillatory corrective movements. It has been debated for many years what the cerebellum normally contributes to movement control, and how losing it that would lead to these particular abnormalities. Two of the more popular ideas are that it acts as timer to clock movement and other behaviors, or as an internal model of body dynamics that can be used to make predictions about movement. There is good evidence for both of these ideas, though the latter may be better supported by recent work.
Timing is an essential part of movement control. Here a “timer” is used to mean an autonomous, self-paced system that could be used to help measure any temporal features of the movement (e.g. timing of stopping, velocity). For example, in order to stop a reaching movement on target, one needs precise timing of muscle activity to decelerate the arm. There is a large body of literature showing that cerebellar patients have difficulty with this type of timing in movement and other behaviors, as reviewed in [2]. It is, however, difficult to distinguish a defunct timing mechanism from an impaired internal model—both could lead to poorly timed decelerations as given in the reaching example. An internal model would have to be able to provide appropriately timed information, but would not act as a clocking mechanism per se.
An internal model is used here to mean a stored set of rules or parameters in the brain that mimic physical systems like the body or objects in the environment [3]. This type of model, sometimes called a forward model, would theoretically allow the nervous system to evaluate and predict how a motor command will affect the body and environment. Such a model is important for movement control is because long sensory delays from the eyes or body make peripheral feedback too slow to be useful for online control [3]. If, however, the brain is able to make good predictions about the state of the body (i.e. its position and derivatives) using an internal model, then it could rely on a much faster “internal feedback” system to control movement, and in our example, stop a reaching movement on target. Here internal feedback is used to mean the predicted state of the body that is made by the internal model in the brain without peripheral feedback. This notion of internal feedback control is difficult to prove since most movements of the body take long enough for both internal and peripheral feedback to be used. Because of that, studies eye movements are often useful - they can be made much faster than limb movement and would therefore benefit from internal feedback.
A recent study clearly showed that internal feedback during saccades is used to steer them to a target [4]. Saccades are ideal to for studying internal feedback since they are fast and do not rely much on proprioceptive feedback from eye muscles. In this study, a saccade target was jumped vertically up or down during a horizontal saccade (i.e. cross axis). As subjects were repeatedly exposed to an error—i.e. the jump in the target—the saccade began to curve toward the target late in the movement - the interpretation was that the saccade used internal feedback from a predictive model to steer it to the target [4].
A follow-up study showed that cerebellar subjects had difficulty learning to steer the saccade from this type of endpoint error, as evidenced by the lack of curved paths late in the movement [5*]. In addition, cerebellar patients showed a specific deficit in using internal feedback late in a saccade to correct for variable motor initiation, whereas control subjects could easily do so. Importantly, the patients learning ability correlated with their ability to correct for errors in motor initiation. The interpretation was that the cerebellum normally corrects for variable motor commands via a motor learning process that utilizes internal feedback to steer the saccade.
There are other studies that have directly compared timing and prediction functions of the cerebellum. A recent neuroimaging study directly compared the neural substrates for timing control versus predictive state estimation using two tasks [6]. One task could be accomplished with a pure timing signal (i.e. push a button after a certain amount of time following a reach) and the other required prediction of the state of the body (i.e. push a button when a reaching arm is in a particular state). They found that cerebellar activations occurred only in the task where the prediction of state was used - there were no cerebellar activations exclusively related to the timing task [6]. We have also tested whether a timing or internal model hypothesis best explains results from a circle-drawing task, where individuals trace a circle with the hand and attempt to produce a desired tempo [7]. Cerebellar patients have deficits that were improved by slowing the movement— in other words, they could produce slower tempos normally. We surmised that slowing the movement reduced the need to rely on an internal model of limb dynamics. In contrast, the addition of an external visual timing signal to reduce the need for a clock-like timer paradoxically worsened timing deficits rather than mitigating them. We interpreted these combined results as evidence that the cerebellum is indeed functioning as an internal model and is needed to make appropriate predictions for movement initiation and termination, rather than a timer.
Sensing
Our current understanding is that damage to the cerebellum does not impair primary sensory functions—that is, basic tests of sensory perception are comparable to controls. In the somatosensory realm, proprioception detection thresholds are normal [8] and our group has found that cutaneous sense tested with monfilaments is normal. There is little or no information on hearing loss from cerebellar damage per se. Hearing loss can occur after stroke in the anterior inferior cerebellar artery distribution, but this is due to cochlear and not cerebellar damage [9]. Visual acuity is also thought to be unaffected, except by virtue of eye movement deficits such as nystagmus, which is an involuntary oscillating or beating movement of the eye.
Yet there are studies that show that cerebellar damage can cause perceptual deficits in more complex tasks. The best-studied example is visual motion perception [10**]. Individuals with cerebellar damage are impaired in tasks that require discrimination of the velocity of visual motion [11] and discrimination of the direction of visual motion embedded in noise [12–15]. Some studies demonstrate that the visual direction impairment is specific to individuals with midline, but not lateral cerebellar damage[12], and the deficit persists from acute to chronic lesions [13]. There are conflicting reports about whether visual position discrimination is affected [11, 14].
What role might the cerebellum play in perception of visual motion? One possibility is that it affects perception indirectly through its control of eye movements. Damage to the cerebellum almost always leads to oculomotor deficits, such as abnormal smooth pursuit, dysmetric saccades, misalignment of the eyes, and nystagmus during gaze holding [16]—these could easily affect many visual perceptual tasks. Yet, a recent study of cerebellar patients showed abnormal performance on visual perceptual tasks, despite the fact that eye movements were tracked and there were no differences from controls during critical fixation periods [10**]. Thus it has been suggested that eye movement abnormalities cannot be the sole explanation for the visual perceptual deficits described in cerebellar patients [10**, 12]. One would, however, suspect that there are many situations where eye movement deficits affect visual perception.
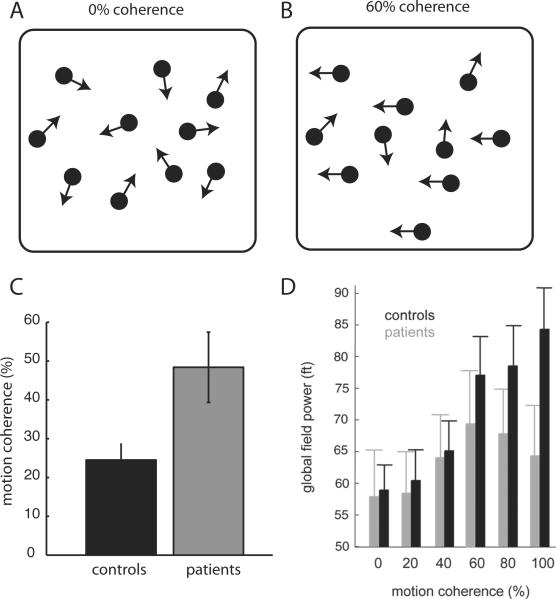
It is also possible that the cerebellum acts to optimize visual motion perception through connections with frontal and parietal cerebral regions. One idea is that the cerebellum makes sensory predictions about movement of the visual target [17]. This notion dovetails with ideas about human movement control, where the cerebellum is thought to make predictions about how motor commands will move the body [18]. In this manner, cerebellar circuits could act to improve the processing of other cerebral areas, particularly those that are involved in processing dynamic (i.e. time varying) information. A recent paper tested whether cerebellar damage alters motion perception through remote effects in cerebral target areas, which would be consistent with this idea [10**]. Using magnetoencephalography, they found that cerebellar damage results in reduced cerebral cortical responsiveness in a task of visual motion direction discrimination in different levels of noise (Figure 1). Cortical responsiveness directly related to the perceptual thresholds in individual subjects, suggesting that cerebellar damage affected visual perception through reduced function of target cerebral areas.
Figure 1.
Effect of cerebellar damage on visual motion discrimination and responses to visual motion in parieto-temporal cortex. A and B. Schematic of moving dots that show 0% coherent motion and 60% coherent motion. C. Perceptual thresholds for motion direction discrimination for controls and cerebellar patients. Cerebellar patients required higher percentages of coherent motion to discriminate direction as compared with controls. D. Global field power in the cerebrum, measured using magnetoencephalograpy, as a function of motion coherence for controls and cerebellar patients. Note that patients show a reduction in global field power, particularly at higher levels of motion coherence. Thus, cerebellar damage seemed to reduce cerebral responsiveness to this type of visual stimulus. Adapted from Handel et al. 2009 [10].
Neuroimaging studies also demonstrate that the cerebellum participates in visual discrimination of motion. A recent fMRI study of visual and auditory motion perception identified both distinct and overlapping cerebellar activations related to the task [19*]. The cerebellar activations also modulated with the difficulty of the task (i.e. the amount of motion signal versus noise in the display). Another neuroimaging study showed cerebellar activations only during a task that required prediction of the velocity, and not direction, of a visual cursor after it has been occluded [20]. This group used functional connectivity analysis to show a posterior lobe cerebellar activation that was linked to a fronto-parietal cerebral network. Results from both of these studies are consistent with the idea that the cerebellum provides a sensory prediction to improve cerebral function. The Reilly et al. study also suggests that cerebellar activity only relates to discriminations that require temporal processing, since direction discrimination did not activate the cerebellum [20].
These findings demonstrate that the cerebellum plays a role in perception of visual motion, though its exact contribution to this process, and even to motor function, is still not understood. One idea that should be more formally tested is that the cerebellum acts to make predictions related to the functions of different cerebral areas of the brain to optimize their abilities. In the case of sensory perception, it may predict upcoming sensory events. In the case of motor function, it may predict the movement outcome for given set of motor commands, or the appropriate motor command to produce a desired movement outcome (i.e. forward and inverse internal model, [21]). It is also important to understand whether the cerebellum is only needed to predict dynamic (i.e. time varying) phenomena. Finally, it should also be noted that much less is known about the effects of cerebellar damage on somatosensory or auditory perception. Does the cerebellum play the same role for these types of perceptual abilities? Cerebellar connections have not been mapped to somatosensory or auditory areas, though it clearly projects to association areas. Under the hypothesis of sensory prediction, one would expect it to contribute to perceptual processes for other modalities.
Learning
It is well accepted that cerebellar damage impairs an error-driven motor learning process referred to as adaptation. Adaptation of movement acts to account for a predictable new demand in the environment or body, and occurs on a time scale of minutes to hours. Errors are partially corrected from one movement to the next, and the corrections accumulate until the error is reduced. When the demand is removed, movement does not immediately revert back to the pre-adaptation state. Instead, it must be actively unlearned or de-adapted through a similar trial-by-trial process.
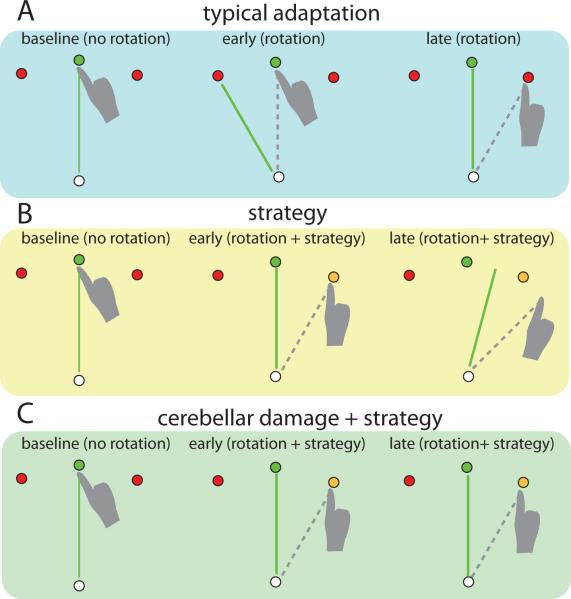
A specific type of error, called a sensory prediction error, drives cerebellum-dependent adaptation. This is defined as the difference between peripheral feedback about the end position of a movement and where the nervous system predicted it would be [3, 22]. It is dissociable from, for example, an error in reaching a target. One might reach and hit a target (i.e. no target error) but still have a sensory prediction error because they did not move where the brain predicted that that they would. This is nicely illustrated in a study of reaching in which an unseen hand was used to move a computer cursor to a target (Figure 2 [23]). When the cursor was made to move at a constant rotation (e.g. 30 degrees clockwise) relative to the hand, subjects initially missed the target by that amount. They subsequently adapt their reaching direction trial-by-trial in order to hit the target. However, if subjects are given an explicit instruction to aim at a target that is 30 degrees counter-clockwise from the cued target, they can immediately hit the desired target. Yet, despite having no target error there is still a sensory prediction error – that is, the cursor does not move to the predicted hand position. Repeated reaches show that even when using the strategy to hit the target, they adapt to reduce this sensory prediction error, which paradoxically moves them off target. This process is also cerebellum dependent, since people with cerebellar damage do not show this phenomenon (Figure 2 [24*]). Once they are given an explicit strategy, they stay on target and do not show any adaptation to the sensory prediction error [24*].
Figure 2.
Schematic showing adaptation to visual cursor rotation during a reaching task. A. Typical adaptation. A subject reaches to a visual target with a cursor representing their occluded hand. At baseline the cursor moves with the hand (green line) and the subject points to the instructed target (green). In early adaptation, the cursor is rotated 30 degrees counter clockwise and the subject misses the instructed target. In late adaptation, the subject has learned to reach 30 degrees clockwise (dotted line) in order to move the cursor to the green target. B. When a typical subject is given a strategy (i.e. aim at the yellow target) they hit the desired (green) target early in adaptation. Despite this explicit strategy, the subject still adapts to the mismatch between cursor position and hand position (i.e. sensory prediction error), as shown in late adaptation. This paradoxically causes them to miss the target. C. Individuals with cerebellar damage can use the explicit strategy during early adaptation, but do not learn from the sensory prediction error late in adaptation. Adapted from Mazzoni and Krakauer 2006 [23] and Taylor et al. 2010 [24].
The cerebellar deficit in adaptation occurs in most all types of motion, including: eye movements, reaching, hand movements, walking, and balancing. This means that patients cannot maintain optimal calibrations for movement control, which makes movements inaccurate, frustrating, and unsafe. Therefore it is important to understand what, if any, motor learning abilities are intact in these individuals, which could be potentially harnessed for rehabilitation.
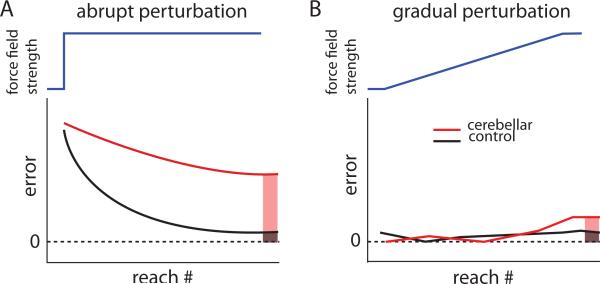
We recently found that cerebellar patients can improve adaptation of a reaching movement if a perturbation is introduced gradually over many movements, instead of abruptly all in one step (Figure 3 [25**]). Patients were studied adapting reaching while a force field pushed their hand off course in a perpendicular direction. When perturbing forces were abruptly stepped to full strength, patients with severe ataxia from cerebellar damage could not adapt. When the forces were gradually ramped up to full strength over many reaches, these same patients showed some ability to adapt and counteract the force. Thus, cerebellar patients learn better from small versus large errors [25**]. It is not known why this is the case—it may be that different brain regions are capable of adapting to smaller and more implicit errors, or that the damaged cerebellum can make small but not big adjustments. It is, however intriguing that such a simple manipulation of gradual introduction of a perturbation can improve their well-known motor adaptation deficit.
Figure 3.
Schematic showing adaptation to gradual versus abrupt force perturbations during reaching. A. An abrupt perturbation (blue line) given during reaching where a force field is turned on full strength in one step. Errors are high for both the control (black) and the cerebellar (red) groups early in adaptation, but after many reaches the control group adapts to reduce error. The cerebellar group does not adapt as much and their errors are greater at the end of adaptation compared with the controls (red versus black shaded). B. A gradual perturbation (blue line) ramps up slowly during adaptation. Both controls and cerebellar patients adapt to counter the gradual perturbation, showing low levels of error throughout. At the end of adaptation when the force field is at full strength, cerebellar subjects have learned nearly the same as controls (red versus black shaded). Thus, cerebellar patients adapt much more normally to gradual perturbations. Adapted from Criscimagna-Hemminger et al. 2010 [25].
Many questions remain about cerebellum-dependent motor learning. For example, since cerebellar damage disrupts some aspects of sensory perception, does this impact error dependent learning? It seems possible that faulty perception could lead to poorer detection of errors that normally drive adaptation. Is the cerebellum preferentially engaged for adapting to large errors that occur during big perturbations? This seems unlikely, though it is intriguing that the patients can adapt reaching movements when given small and gradual perturbations. What brain mechanisms are being used for gradual adaptation? Can other movement types, such as eye movements and walking, benefit from gradual adaptation? It will be important to understand if gradual training procedures can be leveraged to improve cerebellar patient movements for rehabilitation.
Summary
The cerebellum connects extensively with cerebral motor and non-motor areas [1] and appears to play a role in many types of behaviors. As such, cerebellar damage not only affects movement coordination, but also disrupts some perceptual abilities such as visual motion discrimination. A possible common function that could explain these deficits is that the cerebellum acts to make predictions for different cerebral areas of the brain to optimize their abilities-- it may help to predict optimal motor commands for movement control and upcoming sensory events for sensory perception [17]. Further work is needed to understand if this hypothesis can explain how cerebellar damage affects other behaviors, and how the cerebellum could perform this fundamental computation.
Highlights
-
>
We discuss how human cerebellar damage leads to behavioral and learning deficits.
-
>
Movement control and visual motion discrimination are both impaired.
-
>
Motor learning is impaired but can be improved with gradual training procedures.
Acknowledgements
Supported by NIH HD040289.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Ann Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- [2].Ivry RB, Spencer RM. The neural representation of time. Curr Opin Neurobiol. 2004;14:225–32. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- [3].Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–2. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- [4].Chen-Harris H, Joiner WM, Ethier V, Zee DS, Shadmehr R. Adaptive control of saccades via internal feedback. J Neurosci. 2008;28:2804–13. doi: 10.1523/JNEUROSCI.5300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5*].Xu-Wilson M, Chen-Harris H, Zee DS, Shadmehr R. Cerebellar contributions to adaptive control of saccades in humans. J Neurosci. 2009;29:12930–9. doi: 10.1523/JNEUROSCI.3115-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that the cerebellum is involved in controlling saccades using internal feedback about anticipated errors.
- [6].Diedrichsen J, Criscimagna-Hemminger SE, Shadmehr R. Dissociating timing and coordination as functions of the cerebellum. J Neurosci. 2007;27:6291–301. doi: 10.1523/JNEUROSCI.0061-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bo J, Block HJ, Clark JE, Bastian AJ. A cerebellar deficit in sensorimotor prediction explains movement timing variability. J Neurophysiol. 2008;100:2825–32. doi: 10.1152/jn.90221.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Maschke M, Gomez CM, Tuite PJ, Konczak J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain. 2003;126:2312–22. doi: 10.1093/brain/awg230. [DOI] [PubMed] [Google Scholar]
- [9].Lee H, Sohn SI, Jung DK, Cho YW, Lim JG, Yi SD, Lee SR, Sohn CH, Baloh RW. Sudden deafness and anterior inferior cerebellar artery infarction. Stroke. 2002;33:2807–12. doi: 10.1161/01.str.0000038692.17290.24. [DOI] [PubMed] [Google Scholar]
- [10**].Händel B, Thier P, Haarmeier T. Visual motion perception deficits due to cerebellar lesions are paralleled by specific changes in cerebro-cortical activity. J Neurosci. 2009;29:15126–33. doi: 10.1523/JNEUROSCI.3972-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study corroborates earlier studies showing that cerebellar damage impairs global visual motion discrimination. It adds important information showing that cerebellar damage impairs cerebral cortical function related to motion discrimination. Thus, the cerebellum may helping cerebral cortical mechanisms optimize this process.
- [11].Ivry RB, Diener HC. Impaired velocity perception in patients with lesions of the cerebellum. J Cogn Neurosci. 1991;3:355–66. doi: 10.1162/jocn.1991.3.4.355. [DOI] [PubMed] [Google Scholar]
- [12].Nawrot M, Rizzo M. Motion perception deficits from midline cerebellar lesions in human. Vision Res. 1995;35:723–31. doi: 10.1016/0042-6989(94)00168-l. [DOI] [PubMed] [Google Scholar]
- [13].Nawrot M, Rizzo M. Chronic motion perception deficits from midline cerebellar lesions in human. Vision Res. 1998;38:2219–24. doi: 10.1016/s0042-6989(97)00297-6. [DOI] [PubMed] [Google Scholar]
- [14].Thier P, Haarmeier T, Treue S, Barash S. Absence of a common functional denominator of visual disturbances in cerebellar disease. Brain. 1999;122:2133–46. doi: 10.1093/brain/122.11.2133. [DOI] [PubMed] [Google Scholar]
- [15].Jokisch D, Troje NF, Koch B, Schwarz M, Daum I. Differential involvement of the cerebellum in biological and coherent motion perception. Eur J Neurosci. 2005;21:3439–46. doi: 10.1111/j.1460-9568.2005.04145.x. [DOI] [PubMed] [Google Scholar]
- [16].Leigh RJ, Zee DS, editors. The neurology of eye movements. third edition Oxford University Press; 1999. [Google Scholar]
- [17].Paulin MG. The role of the cerebellum in motor control and perception. Brain Behav Evol. 1993;41:39–50. doi: 10.1159/000113822. [DOI] [PubMed] [Google Scholar]
- [18].Miall RC, Christensen LO, Cain O, Stanley J. Disruption of state estimation in the human lateral cerebellum. PLoS Biol. 2007;5:2733–2744. doi: 10.1371/journal.pbio.0050316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19*].Baumann O, Mattingley JB. Scaling of neural responses to visual and auditory motion in the human cerebellum. J Neurosci. 2010;30:4489–95. doi: 10.1523/JNEUROSCI.5661-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]; This fMRI study shows that the human cerebellum may be involved in processing auditory and visual motion. It suggests that portions of the cerebellum are involved in tracking of objects around the person, as hypothesized by [17].
- [20].O'Reilly JX, Mesulam MM, Nobre AC. The cerebellum predicts the timing of perceptual events. J Neurosci. 2008;28:2252–60. doi: 10.1523/JNEUROSCI.2742-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–47. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- [22].Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol. 2007;98:54–62. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- [23].Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci. 2006;26:3642–5. doi: 10.1523/JNEUROSCI.5317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24*].Taylor JA, Klemfuss NM, Ivry RB. An explicit strategy prevails when the cerebellum fails to compute movement errors. Cerebellum. 2010;9:580–586. doi: 10.1007/s12311-010-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that cerebellar patients can use an explicit strategy to compensate for a deficit in adaptation, but do not show the implicit adaptation driven by sensory prediction errors.
- [25**].Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol. 2010;103:2275–84. doi: 10.1152/jn.00822.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that cerebellar patients can adapt reaching movements to a force perturbation when it is introduced gradually rather than abruptly. It had important implications because it suggests that cerebellar patients may be able to learn through other adaptive mechanisms.