Abstract
We report the ability of simple glycoside donors to drastically shift the equilibria of glycosyltransferase-catalyzed reactions, transforming NDP-sugar formation from an endo- to an exothermic process. To demonstrate the utility of this thermodynamic adaptability, we highlight the glycosyltransferase-catalyzed synthesis of 22 sugar nucleotides from simple aromatic sugar donors as well as the corresponding in situ formation of sugar nucleotides as a driving force in context of glycosyltransferase-catalyzed reactions for small molecule glycodiversification. These simple aromatic donors also enabled the first general colorimetric assay for glycosyltransfer, applicable to drug discovery, protein engineering, and other fundamental sugar nucleotide-dependent investigations. This study directly challenges the general notion that NDP-sugars are ‘high-energy’ sugar donors when taken out of their traditional biological context.
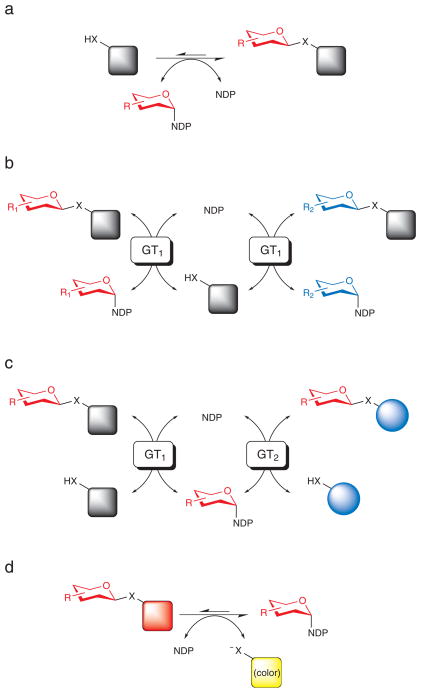
Glycosyltransferases (GTs) constitute a predominant enzyme superfamily responsible for the attachment of carbohydrate moieties to a wide array of acceptors that include nucleic acids, polysaccharides, proteins, lipids, carbohydrates and medicinally relevant secondary metabolites (1, 2). The majority of GTs are LeLoir (sugar nucleotide-dependent) enzymes and utilize nucleoside diphosphate sugars (NDP-sugars) as donors for glycosidic bond formation (Fig. 1a). Recent studies have revealed certain GT-catalyzed reactions from bacterial secondary metabolism to be reversible, presenting new GT-catalyzed methods for NDP-sugar synthesis as well as the GT-catalyzed exchange (Fig. 1b) or transfer (Fig. 1c) of sugars attached to both simple glycoside sugar donors(3, 4) and complex natural product glycosides including glycopeptides, enediynes(5), macrolides(6), macrolactams(7), (iso)flavonoids(8) and polyenes(9). Of the few examples which employed ‘activated’ sugar donors (e.g., glycosyl halides or aromatic glycosides) for GT-catalyzed transglycosylation(3, 4), only indirect evidence for the intermediacy of sugar nucleotides was provided. Among GT-catalyzed ‘reverse’ reactions where NDP-sugar formation has been confirmed, NDP-sugar formation was thermodynamically disfavored (i.e., with NDP-sugar as product, Keq < 1)(5, 10, 11) and typically required a 10- to 100-fold excess of NDP for sugar nucleotide production. In an effort to address the severe thermodynamic limitations of GT-catalyzed reactions run in reverse, herein we report the use of specific activated aromatic glycosides as donors in such reactions dramatically alters the equilibrium of GT-catalyzed reactions and thereby enables a variety of novel transformations (Fig. 1d) including: i) an unique platform for the efficient enzymatic syntheses of novel NDP-sugars, ii) a coupled GT-catalyzed platform for the differential glycosylation of small molecules (including natural products and synthetic drugs/targets), and iii) a colorimetric readout upon glycosyltransfer amenable to high throughput formats for glycodiversification and glycoengineering which can be coupled to nearly any downstream sugar nucleotide-utilizing enzyme.
Fig. 1.
Representative GT-catalyzed reactions. a) Classical GT-catalyzed transformation wherein the sugar, presented in the form of a sugar nucleotide donor, is conjugated to an acceptor target of interest to provide a thermodynamically-favored glycoside product. b) A GT-catalyzed ‘sugar exchange’ reaction. In this reaction, a small amount of NDP is used to ‘prime’ the removal of the endogenous sugar appendage of a target complex natural product thus enabling the exchange of a native sugar for an endogenous sugar supplied in vast excess as a sugar nucleotide. c) A GT-catalyzed ‘aglycon exchange’ reaction where the sugar from one complex natural product is excised (using excess NDP) and subsequently attached to a structurally distinct target aglycon. d) The present study demonstrates the use of simple activated glycosides to dramatically shift the thermodynamics of GT-catalyzed reactions and thereby drive GT-catalyzed NDP-sugar synthesis, sugar exchange/and or aglycon exchange reactions while also offering a convenient colorimetric screen for glycosylation.
RESULTS
Screening of β-D-glucoside donors
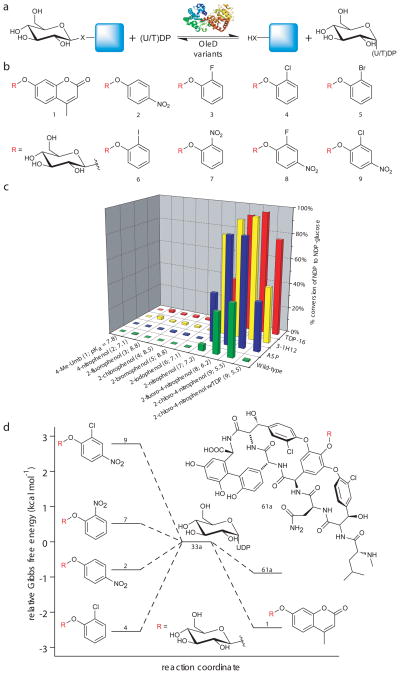
The availability of a GT capable of utilizing a wide array of both simple aromatic acceptors and sugar nucleotides set the stage for this systematic study. With the aid of a crystal structure(12), previous directed evolution and engineering of an inverting macrolide-inactivating GT (OleD) from S. antibioticus identified several highly permissive variants for both sugar nucleotides (14 known sugar substrates) and acceptors (>70 structurally diverse known substrates) in the context of the forward reaction(13–18). Utilizing the aglycons recognized in forward reactions as a template, a set of 32 putative β-D-glucopyranosides donors (1–32, Supplementary Results, Supplementary Fig. 2) were synthesized (Supplementary Methods) and tested against a series of OleD variants in reverse reactions for production of UDP-α-D-glucose (UDP-Glc, 33a) in the presence of UDP (Fig. 2a). The syntheses of these putative donors required 1–3 steps (37% average overall yield) and, in all but one case (19), provided the desired β-anomer exclusively. Of the 32 putative donors evaluated, 9 (1–9) led to UDP-Glc (33a) formation with all variants examined (Fig. 2b). This systematic analysis revealed a clear correlation between the leaving group ability of the sugar donor and the production of desired sugar nucleotide wherein the combination of OleD variant TDP-16 (containing the mutations P67T/S132F/A242L/Q268V)(17) and 2-chloro-4-nitrophenyl β-D-glucopyranoside (9) provided the best overall yields of UDP-Glc (33a) or TDP-Glc (33b) (Fig. 2c). Using this preferred donor, maximal turnover was observed at pH 7.0–8.5 (Supplementary Fig. 3), a range consistent with the previously reported pH-rate profile for the wild-type OleD in the forward direction(10). NDP-sugar formation was also observed in the presence of ADP and GDP, albeit with much lower efficiency than with UDP or TDP (Supplementary Fig. 4 and 5). Thus, four of the five standard nucleotide moieties utilized by all LeLoir GTs (including not only natural product GTs but also those which catalyze the formation of glycoproteins(19–21), oligosaccharides(21–25), glycolipids(26), glycoconjugates(1), etc.) are accessible via this method. To demonstrate preparative scale and provide material for full characterization, this reaction was conducted with a 1:1 molar ratio of glucoside donor to NDP using 9 mg of UDP or TDP to provide 6.9 (55% isolated yield) and 7.7 mg (61% isolated yield), of UDP-Glc (33a) and TDP-Glc (33b) (Supplementary Methods and Supplementary Results). While the few previously reported GT-catalyzed transglycosylation reactions using ‘activated’ sugar donors (e.g., glycosyl halides or aromatic glycosides) required NDP or NMP for transglycosylation(3, 4), the present study is the first to establish a robust GT-based platform for the syntheses of NDP-sugars, including an array of uniquely-functionalized non-metabolic sugar nucleotides. Under saturating donor 9, kinetic analysis (Supplementary Methods) revealed the kcat/Km of TDP-16 to be improved by a factor of 25 or 315 (varied UDP or TDP, respectively) compared to wild-type OleD (Supplementary Table 1), consistent with this mutant’s enhanced proficiency toward TDP-sugars(17). Equilibrium constants (Keq,pH8.5) were also determined for 1, 2, 4, 7, and 9 and utilized to calculate the corresponding Gibbs free energy according to equation (1) (Supplementary Methods, Supplementary Fig. 8 and Supplementary Table 2).
Fig. 2.
Evaluation of putative donors for sugar nucleotide synthesis. (a) General reaction scheme. (b) Structures of the β-D-glucopyranoside donors which led to (U/T)DP-glucose formation. (c) Percent conversion of (U/T)DP to (U/T)DP-glucose with various donors (n ≥ 2, standard deviation ≤ 5%). Reactions contained 2.1 μM (10 μg) OleD variant, 1 mM of (U/T)DP, and 1 mM of aromatic donor (1–9) in Tris-HCl buffer (50 mM, pH 8.5) with a final volume of 100 μl. After one hour at 25°C, reactions were flash frozen and analyzed by HPLC (Supplementary Methods). The pKa for each corresponding donor aglycon is highlighted in parentheses. (d) Plot depicting the relative Gibbs free energy of selected donors/acceptors in relation to 33a. Small glycoside donors display large shifts in relative free energy, transforming formation of UDP-Glc (33a) from an endo- to an exothermic process. The ΔG°pH8.5 for 1, 2, 4, 7, and 9 with UDP in Tris-HCl buffer (50 mM, pH 8.5) at 298K relative to 33a were determined in this study (Supplementary Methods). The ΔG° for 61a was previously determined (at pH 9.0 and 310K)(5).
| (1) |
In agreement with the typically observed thermodynamics for these reactions(5, 10, 11), the 4-, 1-, or 2- (ΔG°pH8.5 = +2.55, +2.44, and +0.92 kcal mol−1, respectively) UDP-Glc transformations were endothermic. In stark contrast, 7- or 9- (ΔG°pH8.5 = −0.52 and −2.78 kcal mol−1, respectively) UDP-Glc transformations were notably exothermic (Fig. 2d) and thereby correspond to a dramatic shift of GT-catalyzed reaction Keq which markedly favors NDP-sugar formation.
Screening of 2-chloro-4-nitrophenyl glycoside donors
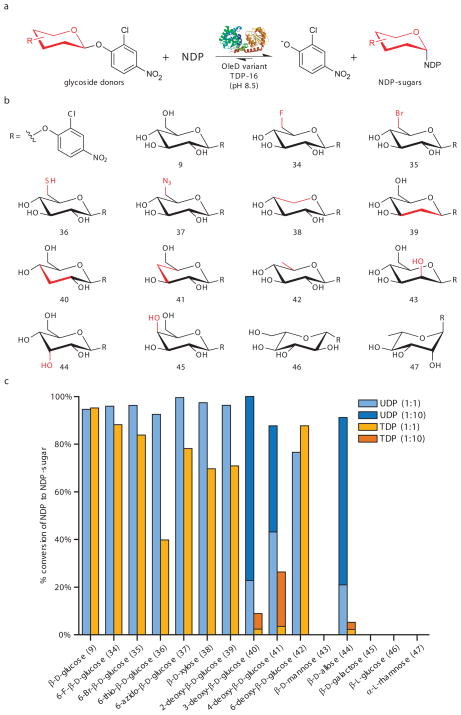
To further assess the utility of this reaction toward novel sugar nucleotide synthesis, 15 additional 2-chloro-4-nitrophenyl glycosides (34–47) were synthesized (Supplementary Methods) and evaluated for production of the corresponding sugar nucleotides in presence of UDP and TDP (Fig. 3a–b). This set of putative donors represents a series of uniquely functionalized gluco-configured sugars as well as corresponding epimers (C2, C3, C4), deoxy (C2, C3, C4 and C6) analogues and even L-sugars. The syntheses of these putative donors required 2–7 steps (35% average overall yield) and, in all cases, exclusively provided the desired anomeric stereochemistry (Supplementary Methods). Of the 15 glycoside donors evaluated (9, 34–47) with TDP-16, 11 (9, 34–42, 44) resulted in the formation of the desired sugar nucleotide with both UDP and TDP (Fig. 3c). With a 1:1 molar ratio of (U/T)DP to glycoside donor in these 11 reactions, an average conversion of 66% was observed, once again highlighting the thermodynamic driving force provided by the aromatic sugar donor. For a small subset of donors (40, 41, 44), a shift of the ratio to 1:10 of UDP to glycoside donor drastically increased yields of UDP-sugars (54a, 55a, 57a) to >85%, while yields of TDP-sugars (54b, 55b, 57b) remained low (<25%) (Fig. 3c). Distinct from the prior observation of extensive donor hydrolysis when attempting to use reactive p-nitrophenol glycoside donors for GT-catalyzed transglycosylation reactions(3), the current study revealed little or no background donor hydrolysis. Also in notable contrast to the prior use of GT-catalyzed reactions for the synthesis of single sugar nucleotides (wherein a molar ratio of up to 1:100 sugar donor to NDP was required and, in all cases, <50% desired sugar nucleotide product was observed)(5–7, 9, 11), this study reveals a truly useful synthetic transformation (wherein a 1:1 molar ratio of sugar donor to UDP provides >70% yield desired sugar nucleotide product for 8 out of 11 examples examined). This study revealed TDP-16 to tolerate deoxygenation at C2, C3, C4 or C6; C3 epimerization (as related to D-Glc); and an array of novel functionality at C6. While the C2 and C4 epimers did not turnover in this pilot study, the production of 2-chloro-4-nitrophenolate in this reaction offers a convenient high throughput screen as a basis for rapidly expanding the sugar scope of this reaction (as subsequently discussed). In the context of sugar nucleotide synthesis, this GT-catalyzed method offers a noteworthy alternative to both conventional chemoenzymatic approaches (requiring 1 to 11 enzymes with typical overall yields from unprotected sugars ranging from 10% to 35%)(27–32) and multi-step chemical syntheses (requiring 3–11 total steps for coupling sugar-1-phosphates and activated NMPs with typical overall yields from peracetylated sugars ranging from 9–66%)(33, 34). As a specific comparison, the previously reported chemical synthesis of 48b (TDP-6-deoxy-6-fluoro-α-D-glucose) from peracetylated 6-fluoro-D-glucose required 4 chemical steps including 9% yield for the final morpholidate coupling reaction,(33) while the present study reports 4 steps (3 chemical, 1 enzymatic) and 46% overall yield from the same starting material.
Fig. 3.
The synthesis of sugar nucleotides from 2-chloro-4-nitrophenyl glucosides. (a) General reaction scheme. (b) Structures of 2-chloro-4-nitrophenyl glycoside donors evaluated for D-sugars within this series, the differences between each member and the native OleD sugar substrate (β-D-glucose) are highlighted in red. (c) Maximum observed percent conversion of (U/T)DP to (U/T)DP-glucose within a 21 hour time course assay for each donor (n ≥ 2, standard deviation ≤ 5%). Standard reactions contained 7 μM TDP-16, 1 mM (U/T)DP, and 1 mM of 2-chloro-4-nitrophenyl glycoside donor (9, 34–47) in Tris-HCl buffer (50 mM, pH 8.5) with a final volume of 300 μl. Over 21 hours at 25°C, aliquots taken at various times were flash frozen and analyzed by HPLC (Supplementary Methods). For reactions with UDP yielding <45% conversion under standard conditions (40, 41, 43–47), identical assays using 10-fold less (U/T)DP (0.1 mM) were also conducted and, where relevant, the percent conversions for the modified reactions are represented by the darker colors. HPLC chromatograms, full time course data, and product characterization are presented in Supplementary Fig. 9–12 and Supplementary Table 3. In all cases where both the α- and β-anomers were examined as donors, only the β-anomer was found to be a substrate (Supplementary Methods and Supplementary Results).
Single GT coupled reactions
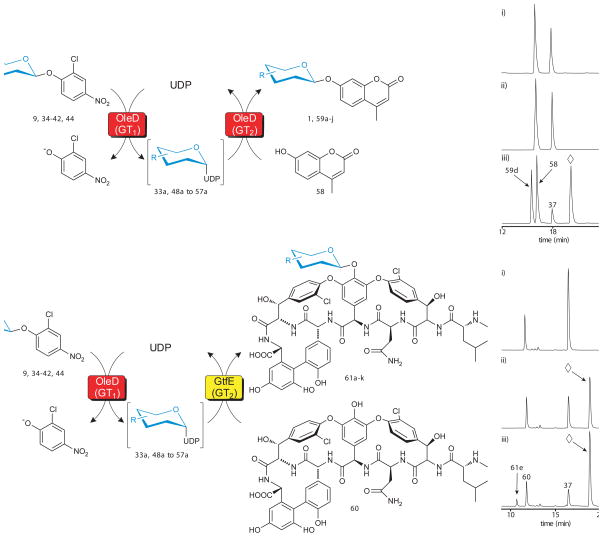
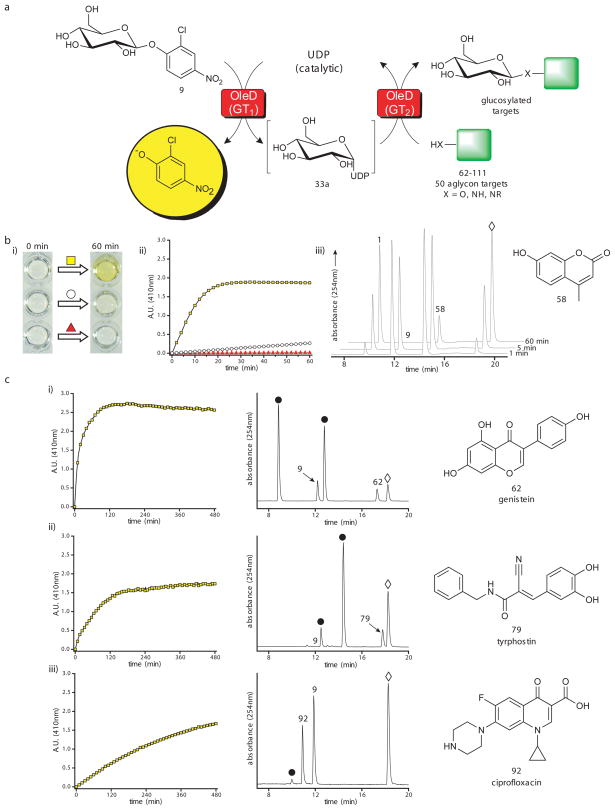
The previously reported promiscuity of OleD variants in forward reactions(13–18), coupled with the newly demonstrated ability to synthesize large numbers of NDP-sugars in situ, raised the question of whether TDP-16 could enable a one-pot transglycosylation wherein the sugar nucleotide formed from 9 (via the ‘reverse reaction’) could serve as a donor for a subsequent glycoside-forming reaction (via a simultaneous ‘forward reaction’) (Fig. 4a). Such single and double enzyme ‘aglycon exchange’ reactions have been previously reported in the context of complex natural products(5–7, 9, 11), but again, the Keq of such reactions has restricted their general utility. To assess the potential of a single GT-catalyzed transglycosylation, a series of model reactions, each containing the aglycon acceptor 4-methylumbelliferone (58; 1 mM), one member of the 2-chloro-4-nitrophenyl glycoside donor series (9, 34–42, 44; 1mM), UDP (1 mM) and OleD variant TDP-16 (11 μM), revealed all 11 expected products (1, 59a–59j) with an average yield of 45% (Supplementary Fig. 13–14 and Supplementary Table 4). For comparison, the yield of 1 in the single GT coupled reaction was 62% (n=1), while the average yield of 1 via a standard OleD catalyzed forward reaction (using 1 equivalent of UDP-Glc donor) was 60% ± 3% (n=3). Given the established ability of OleD variants to glycosylate a wide array of structurally-diverse small molecules, drugs and natural products(13–18), the extension of this OleD-catalyzed single pot transglycosylation (or ‘aglycon exchange’) reaction is anticipated to offer a variety of opportunities for the glycodiversification of bioactive molecules including a number of clinically-approved drugs and complex natural products.
Fig. 4.
Evaluation of 2-chloro-4-nitrophenyl glycosides as sugar donors in coupled GT-catalyzed transglycosylation reactions. (a) The scheme for a single enzyme (TDP-16) coupled system with 4-methylumbelliferone (58) as the final acceptor (left) and a representative HPLC analysis (right) using the donor for 6-azido-6-deoxy-D-glucose (37). Reactions contained 1 mM glycoside donor, 1 mM 58, 1 mM UDP, and 11 μM TDP-16 in a total volume of 100 μl with Tris-HCl buffer (50 mM, pH 8.5) at 25°C for 24 hour and were subsequently analyzed by HPLC (Supplementary Methods). For the representative reaction: (i) control reaction lacking TDP-16; (ii) control reaction lacking UDP; (iii) full reaction where 37 is donor, 58 is acceptor, 59d is desired product and ⋄ represents 2-chloro-4-nitrophenolate. (b) The scheme for a double enzyme (TDP-16 and GtfE) coupled system with vancomycin aglycon (60) as the final acceptor (left) and a representative HPLC analysis (right) using the donor for 6-azido-6-deoxy-D-glucose (37). Reactions contained 1 mM glycoside donor, 0.1 mM 60, 1 mM UDP, 11 μM TDP-16, and 11 μM GtfE in a total volume of 100 μl with Tris-HCl buffer (50 mM, pH 8.5) at 25°C for 24 hour and were subsequently analyzed by HPLC (Supplementary Methods). For the representative reaction: (i) control reaction lacking TDP-16; (ii) control reaction lacking GtfE; (iii) full reaction where 37 is donor, 60 is acceptor, 61e is desired product and ⋄ represents 2-chloro-4-nitrophenolate. Sample preparation and HPLC parameters, along with chromatograms (Supplementary Fig. 14 and 17), conversion rates, and mass characterization (Supplementary Table 4 and 5) for all products are presented in supporting online material.
Dual GT coupled reactions
To further probe the potential of in situ NDP-sugars from synthetic donors to ultimately serve as donors for GTs other than OleD variants, a series of dual GT-catalyzed model reactions were performed. For this set of model reactions, GtfE was selected because of its known NDP-sugar promiscuity(35, 36) as well as the clinical potential of glycodiversified vancomycin analogues(37, 38). Typical reactions for this assessment contained a NDP-sugar generating component consisting of a 2-chloro-4-nitrophenyl glycoside donor (9, 34–42 or 44; 1 mM), UDP (1 mM) and OleD variant TDP-16 (11 μM) coupled to a glycoside-forming component with vancomycin aglycon (60; 0.1 mM) and the vancomycin aglycon glucosyltransferase GtfE (11 μM). Remarkably, this series of dual GT-catalyzed transglycosylation (or ‘aglycon exchange’) reactions also led to the formation of all 11 expected products (61a–61k) with an average yield of 36% (Fig. 4b, Supplementary Fig. 16–17 and Supplementary Table 5). As a comparison, the yield of 61a in the dual-GT coupled reaction was 53% (n=1), while the average yield of 61a via a standard GtfE catalyzed forward reaction (using 10 equivalents of native substrate UDP-Glc) was 53% ± 0.5% (n = 3). Thus, this convenient 2-chloro-4-nitrophenyl glycoside donor-driven coupled format presents synthetically useful novel sugar nucleotides in situ without the need for the tedious a priori sugar nucleotide synthesis and/or purification. Furthermore, the formation of the colorimetric product 2-chloro-4-nitrophenolate (λmax = 398 nm; ε410 = 2.4 × 104 M−1 cm−1; pH 8.5) upon GT-catalyzed glycoside formation in these single or dual GT-catalyzed coupled reactions also offers a unique opportunity for high throughput screening as described below.
High throughput assay for glycosyltransfer
The 2-chloro-4-nitrophenolate released during the course of GT-catalyzed NDP-sugar formation directly, or in the context of the coupled reactions formats presented, can be followed spectrophotometrically at 410 nm in real-time (Fig. 5). The ability to do so presents one of the first truly general continuous GT assays as the colorimetric read-out directly correlates to NDP-sugar usage in such reactions and thereby avoids the need for additional manipulations or specialized probes commonly associated with conventional assays for glycosidic bond formation(39–41). To demonstrate this approach, a set of 50 (62–111) medicinally relevant compounds were screened with the single GT-catalyzed reaction in a high throughput format. Specifically, each 100μl reaction in the 96-well plate contained the sugar donor 9 (0.5 mM), a putative aglycon acceptor (0.5 mM), a catalytic amount of UDP (5 μM) and TDP-16 (11 μM) and reaction progress was monitored at 410 nm over 480 min (Fig. 5a–b and Supplementary Methods). Notably, the use of UDP as a limiting reagent within this coupled system reduces the potential for the various types of inhibition commonly observed in forward GT-catalyzed reactions with NDP and NDP analogues(1, 10). Based upon this cumulative rapid analysis, 43 compounds (62–103) led to a positive response (designated as three standard deviations above the mean for control reactions), 37 of which (62–93, 96, 97, 99, 101, 103) were subsequently confirmed by HPLC and/or LC/MS to lead to products consistent with glucoside formation (Fig. 5c, Supplementary Fig. 21, Supplementary Table 6). This study highlights a high throughput assay to identify novel acceptors that can be glycosylated by a given GT and greatly expands upon the use of simple ‘activated’ glycosides as sugar donors in coupled reactions. Additionally, the demonstrated ability to couple this assay to essentially any downstream sugar-utilizing enzyme/process is also anticipated to have a broad range of fundamental applications including screens for GT inhibitors, GT engineering/evolution (toward utilization of novel NDPs, glycoside donors, and/or acceptors)(39, 40, 42–44) and/or engineering/evolution/investigations of additional NDP-sugar utilizing enzymes(19–26, 45, 46).
Fig. 5.
Utilizing a colorimetric screen for glycosyl transfer. (a) Scheme for colorimetric screen using the single enzyme (TDP-16) coupled format. (b) Evaluation of the colorimetric assay with 58 as the final acceptor. The reactions contained 0.5 mM 9 as donor, 0.5 mM 58 as acceptor, 5 μM UDP, and 11μM TDP-16 in a final total volume of 100 μl with Tris-HCl buffer (50 mM, pH 8.5) in a 96-well plate incubated at 25°C for one hour. (i) Qualitative color change after one hour for the full reaction (yellow square), a control lacking the final acceptor 58 (white circle), and a control lacking UDP (red triangle). (ii) Δ410 nm over one hour for the full reaction (yellow squares), a control lacking the final acceptor 58 (white circles), and a control reaction lacking UDP (red triangles). (iii) HPLC chromatograms of full reaction at 1, 5, and 60 min where 1 is desired product, 9 is the donor, 58 is the target aglycon and ⋄ represents 2-chloro-4-nitrophenolate. (c) The absorbance data and HPLC chromatograms of three representative hits [(i) 62 (genistein), (ii) 79 (tyrphostin), or (iii) 92 (ciprofloxacin)] from the broad 50 compound panel screen using the single enzyme (TDP-16) coupled format. In HPLC chromatograms 9 indicates donor; 62, 79 or 92 represent target aglycon; ⋄ indicates 2-chloro-4-nitrophenolate; and ● depicts glucosylated product(s). For the overall results of the 50 compound screen, additional representative absorbance plots and chromatograms, and combined HPLC and LC/MS characterization, see Supplementary Fig. 19–21 and Supplementary Table 6.
DISCUSSION
This study directly challenges the general notion that NDP-sugars are ‘high-energy’ sugar donors when taken out of their traditional biological context, revealing the equilibria of GT-catalyzed reactions to be highly substrate-dependent and adaptable. The flexibility of the GT thermodynamic landscape, in turn, enabled general NDP-sugar syntheses, in situ formation of NDP-sugars to drive coupled LeLoir GT-catalyzed reactions for glycoconjugate formation, and the first general high throughput colorimetric assay for glycosyltransfer. Given the power of the screen presented, these preliminary data suggest both the ability to enable the rapid optimization (via directed evolution) of new OleD prodigy for nearly any desired NDP/sugar pair as well as the ability to couple this screen to nearly any downstream sugar-utilizing for engineering/evolution or biochemical analysis. While substrates providing the greatest thermodynamic advantage may not always provide an equivalent kinetic advantage, this study also highlights the merit of optimizing enzyme-catalyzed reactions based upon thermodynamic constraints. We anticipate future attempts to exploit and/or engineer other novel enzyme-catalyzed reactions may benefit from similar considerations.
METHODS
Protein expression and purification
Expression and purification of all enzymes are described in Supplementary Methods.
Initial β-D-glucoside screening
Reactions containing 2.1 μM (10 μg) of purified OleD variant, 1 mM of UDP or TDP, and 1 mM of β-D-glucopyranoside (1–32) in Tris-HCl (50 mM, pH 8.5) with a final volume of 100 μl were incubated at room temperature for 1 hour. Samples were frozen in a bath of dry ice and acetone and stored at −20°C. Following, samples were thawed at 4°C and filtered through a MultiScreen Filter Plate (Millipore, Billerica, MA, USA) according to manufacturer’s instructions and evaluated for formation of UDP- (33a) or TDP-α-D-glucose (33b) by analytical reverse-phase HPLC with a 250 mm × 4.6 mm Gemini-NX 5μ C18 column (Phenomenex, Torrance, CA, USA) using a linear gradient of 0% to 15% CH3CN (solvent B) over 15 minutes (solvent A = aqueous 50 mM triethylammonium acetate buffer [Sigma-Aldrich, St. Louis, MO, USA], flow rate = 1 ml min−1, with detection monitored at 254 nm).
2-chloro-4-nitrophenyl glycoside screening
Reactions containing 7.0 μM (100 μg) of OleD variant TDP-16, 1 mM or 0.1 mM of (U/T)DP, and 1 mM of glycoside member (9, 34–47) in 50 mM Tris (pH 8.5) with a final volume of 300 μl were incubated at room temperature. Aliquots were removed at various time points, mixed with an equal volume of ddH2O, frozen in a bath of dry ice and acetone, and stored at −20°C. Following, samples were thawed at 4°C and filtered through a MultiScreen Filter Plate (Millipore, Billerica, MA, USA) according to manufacturer’s instructions. Samples were evaluated for formation of NDP-sugar by analytical reverse-phase HPLC with a 250 mm × 4.6 mm Gemini-NX 5μ C18 column (Phenomenex, Torrance, CA, USA) using a linear gradient of 0% to 50% CH3CN (solvent B) over 25 minutes (solvent A = 50 mM PO4−2, 5 mM tetrabutylammonium bisulfate, 2% acetonitrile [pH adjusted to 6.0 with KOH]; flow rate = 1 ml min−1; A254 nm). In the case of 51a and 50b formation, percent conversion was calculated from peak height due to co-elution of NDP and product. Time course data, chromatographs, and characterization of each NDP-sugar product are described in Supplementary Methods and Supplementary Results. Screening of the α-anomer of 38 yielded no turnover in any reactions (see Supplementary Methods for synthesis and characterization), demonstrating that TDP-16 is only capable of recognizing the β-anomers of D-sugars.
Evaluation of single enzyme coupled system
Reactions containing 10.5 μM (50 μg) of purified OleD variant TDP-16, 1 mM of UDP, 1 mM 4-methylumbelliferone (58) and 1 mM of 2-chloro-4-nitrophenyl glycoside (9, 34–42, or 44) in Tris-HCl buffer (50 mM, pH 8.5) at a final volume of 100 μl were incubated in a 30°C water bath for 24 hours. Samples were subsequently mixed with an equal volume of MeOH, centrifuged at 10,000 g for 30 min at 0°C, and the supernatant removed for analysis. The clarified reaction mixtures were analyzed by analytical reverse-phase HPLC with a Gemini-NX C-18 (5 μm, 250 × 4.6 mm) column (Phenomenex, Torrance, California, USA) with a gradient of 10% B to 75% B over 20 min, 75% B to 95% B over 1 min, 95% B for 5 min, 95% B to 10% B over 3 min, 10% B for 6 min (A = dH2O with 0.1% TFA; B = acetonitrile; flow rate = 1 mL min−1) and detection monitored at 254 nm. Fractions corresponding to the desired products were collected, frozen, lyophilized, dissolved in 1:1 acetonitrile/water to a final concentration of 1 μg mL−1, and submitted for mass analysis. Product structures, HPLC chromatograms, calculated conversions, and mass characterization are presented in Supplementary Fig. 13–14 and Supplementary Table 4.
Evaluation of dual enzyme coupled system
All reactions were performed in a final volume of 100 μl Tris-HCl buffer (50 mM, pH 8.5) with 10.8 μM (50 μg) purified GtfE, 1 mM vancomycin aglycon (60) and 1 mM of 2-chloro-4-nitrophenyl glycoside (9, 34–42, or 44). Reactions with 35–38, 40–41, or 44 as donor contained 10.5 μM (50 μg) OleD variant TDP-16 and 1 mM UDP. Reactions with 9, 34, or 39 as donor contained 1.1 μM (5 μg) OleD variant TDP-16 and 1 μM UDP. A reaction with 42 as donor contained 0.1 μM (0.5 μg) OleD variant TDP-16 and 0.001 mM UDP. All components of the reaction(s) were added at time equals zero hours. Reactions were then incubated in a 30°C water bath for 24 hours. Samples were then prepared and analyzed as described for the single enzyme coupled reactions (see above). Product structures, HPLC chromatograms, calculated conversions, and mass characterization are presented in Supplementary Fig. 16–17 and Supplementary Table 5.
Evaluation of single enzyme coupled system for drug screening
Reactions containing 10.5 μM (50 μg) OleD variant TDP-16, 5 μM of UDP, 0.5 mM final acceptor (58 [as a positive control] and 62–111; see Supplementary Fig. 18), and 0.5 mM 9 in Tris-HCl buffer (50 mM, pH 8.5) with a final volume of 100 μl were prepared in a 96 well flat bottom Bacti plate (0.4 mL well−1; Nagle Nunc International, Rochester, NY, USA). Absorbance measurements were recorded every 2 min at 410 nm for 8 hours on a FLUOstar Optima plate reader (BMG, Durham, NC, USA) with the plate shaken within the reader for 5 seconds before collection of each time point. Reactions containing final acceptor were run at n = 1, control reactions lacking final acceptor were run at n = 6 and control reactions lacking both final acceptor and UDP were run at n = 3. At 8 hours, reactions were filtered through a MultiScreen Filter Plate with a 10 kDa molecular weight cut-off (Millipore, Billerica, MA, USA) according to manufacturer’s instructions at 4°C, frozen at −20°C, and thawed for additional analysis (see Supplementary Methods and Supplementary Results). Data analysis is described in Supplementary Methods.
Supplementary Material
Acknowledgments
This manuscript is dedicated to the late Prof. C. Richard Hutchinson for his pioneering contributions to engineered natural product glycosylation. We thank the School of Pharmacy Analytical Instrumentation Center for analytical support and Dr. Gavin J. Williams for materials and helpful discussion. R.W.G. is an AFPE Pre-Doctoral Fellow. J.S.T is a UW HI Romnes Fellow and holds the Laura and Edward Kremers Chair in Natural Products. This work was supported by NIH AI52218.
Footnotes
Author contributions
R.W.G., P.P.P. and J.S.T. contributed to the experimental design. R.W.G., P.P.P. and W.J.C. performed all experimental work. R.W.G., P.P.P. and J.S.T. wrote and edited the manuscript.
Competing financial interests
The authors report competing interests. J.S.T. is a co-founder of Centrose (Madison, WI, USA).
Additional information
Supplementary information and chemical compound information is available online at http://www.nature.com/naturechemicalbiology/. Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Varki A, et al., editors. Essentials of Glycobiology. 2. Cold Spring Harbor; New York: 2009. [PubMed] [Google Scholar]
- 2.Thibodeaux CJ, Melancon CE, III, Liu H. Unusual sugar biosynthesis and natural product glycodiversification. Nature. 2007;446:1008–1016. doi: 10.1038/nature05814. [DOI] [PubMed] [Google Scholar]
- 3.Lairson LL, Wakarchuk WW, Withers SG. Alternative donor substrates for inverting and retaining glycosyltransferases. ChemComm. 2007:365–367. doi: 10.1039/b614636h. [DOI] [PubMed] [Google Scholar]
- 4.Lougheed B, Ly HD, Wakarchuk WW, Withers SG. Glycosyl fluorides as substrates for nucleotide phosphosugar-dependent glycosyltransferases. J Biol Chem. 1999;274:37717–37722. doi: 10.1074/jbc.274.53.37717. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C, et al. Exploiting the reversibility of natural product glycosyltransferase-catalyzed reactions. Science. 2006;313:1291–1294. doi: 10.1126/science.1130028. [DOI] [PubMed] [Google Scholar]
- 6.Zhang C, Albermann C, Fu X, Thorson JS. The in vitro characterization of the iterative avermectin glycosyltransferase AveBI reveals reaction reversibility and sugar nucleotide flexibility. J Am Chem Soc. 2006;128:16420–16421. doi: 10.1021/ja065950k. [DOI] [PubMed] [Google Scholar]
- 7.Minami A, Kakinuma K, Eguchi T. Algycon switch approach toward unnatural glycosides from natural glycoside with glycosyltransferase VinC. Tet Lett. 2005;46:6187–6190. doi: 10.1021/ja042848j. [DOI] [PubMed] [Google Scholar]
- 8.Modolo LV, Escamilla-Treviño LL, Dixon RA, Wang X. Single amino acid mutations of Medicago glycosyltransferase UGT85H2 enhance activity and impart reversibility. FEBS Lett. 2009;583:2131–2135. doi: 10.1016/j.febslet.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C, Moretti R, Jiang J, Thorson JS. The in vitro characterization of polyene glycosyltransferases AmphDI and NysDI. ChemBioChem. 2008;9:2506–2514. doi: 10.1002/cbic.200800349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quirós LM, Carbajo RJ, Braña AF, Salas JA. Glycosylation of macrolide antibiotics: purification and kinetic studies of a macrolide glycosyltransferase from Streptomyces antibioticus. J Biol Chem. 2000;275:11713–11720. doi: 10.1074/jbc.275.16.11713. [DOI] [PubMed] [Google Scholar]
- 11.Okada T, et al. Bidirectional N-acetylglucosamine transfer mediated by β-1,4-N-acetylglucosaminyltransferase III. Glycobiol. 2009;19:368–374. doi: 10.1093/glycob/cwn145. [DOI] [PubMed] [Google Scholar]
- 12.Bolam DN, et al. The crystal structure of two macrolide glycosyltransferases provides a blueprint for host cell antibiotic immunity. Proc Natl Acad Sci USA. 2007;104:5336–5341. doi: 10.1073/pnas.0607897104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams GJ, Zhang C, Thorson JS. Expanding the promiscuity of a natural-product glycosyltransferase by directed evolution. Nat Chem Biol. 2007;3:657–662. doi: 10.1038/nchembio.2007.28. [DOI] [PubMed] [Google Scholar]
- 14.Williams GJ, Goff RD, Zhang C, Thorson JS. Optimizing glycosyltransferase specificity via “hot spot” saturation mutagenesis presents a catalyst for novobiocin glycorandomization. Chem Biol. 2008;15:393–401. doi: 10.1016/j.chembiol.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams GJ, Thorson JS. A high-throughput fluorescence-based glycosyltransferase screen and its application in directed evolution. Nat Protocols. 2008;3:357–362. doi: 10.1038/nprot.2007.538. [DOI] [PubMed] [Google Scholar]
- 16.Gantt RW, Goff RD, Williams GJ, Thorson JS. Probing the aglycon promiscuity of an engineered glycosyltransferase. Angew Chem Int Ed. 2008;47:8889–8892. doi: 10.1002/anie.200803508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams GJ, Yang J, Zhang C, Thorson JS. Recombinant E. coli prototype strains for in vivo glycorandomization. ACS Chem Biol. 2011;6:95–100. doi: 10.1021/cb100267k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang M, et al. Probing the breadth of macrolide glycosyltransferases: in vitro remodeling of a polyketide antibiotic creates active bacterial uptake and enhances potency. J Am Chem Soc. 2005;127:9336–9337. doi: 10.1021/ja051482n. [DOI] [PubMed] [Google Scholar]
- 19.Rexach JE, Clark PM, Hsieh-Wilson LC. Chemical approaches to understanding O-GlcNAc glycosylation in the brain. Nat Chem Biol. 2008;4:97–106. doi: 10.1038/nchembio.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakabe K, Hart GW. O-GlcNAc Transferase regulates mitotic chromatin dynamics. J Biol Chem. 2010;285:34460–34468. doi: 10.1074/jbc.M110.158170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanson S, Best M, Bryan MC, Wong C. Chemoenzymatic synthesis of oligosaccharides and glycoproteins. Trends Biochem Sci. 2007;29:656–663. doi: 10.1016/j.tibs.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Weigel PH, DeAngelis PL. Hyaluronan synthases: a decade-plus of novel glycosyltransferases. J Biol Chem. 2007;282:36777–36781. doi: 10.1074/jbc.R700036200. [DOI] [PubMed] [Google Scholar]
- 23.Bojarová P, et al. Synthesis of LacdiNAc-terminated glyconjugates by mutant galactosyltransferase – a way to new glycodrugs and materials. Glycobiol. 2009;19:509–517. doi: 10.1093/glycob/cwp010. [DOI] [PubMed] [Google Scholar]
- 24.Liu R, et al. Chemoenzymatic design of heparin sulfate oligosaccharides. J Biol Chem. 2010;285:34240–34249. doi: 10.1074/jbc.M110.159152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau K, et al. Highly efficient chemoenzymatic synthesis of β1-4-linked galasctosides with promiscuous bacterial β1-4-galactosyltransferases. Chem Commun. 2010;46:6066–6068. doi: 10.1039/c0cc01381a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maccioni HJF, Quiroga R, Ferrari ML. Cellular and molecular biology of glycosphingolipid glycosylation. J Neurochem. 2011;117:589–602. doi: 10.1111/j.1471-4159.2011.07232.x. [DOI] [PubMed] [Google Scholar]
- 27.Rupprath C, Schumacher T, Elling L. Nucleotide deoxysugars: essential tools for the glycosylation engineering of novel bioactive compounds. Curr Med Chem. 2005;12:1637–1675. doi: 10.2174/0929867054367167. [DOI] [PubMed] [Google Scholar]
- 28.Thibodeaux CJ, Melançon CE, III, Liu H. Natural-product sugar biosynthesis and enzymatic glycodiversification. Angew Chem Int Ed. 2008;47:9814–9859. doi: 10.1002/anie.200801204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Hoffmeister D, Liu L, Fu X, Thorson JS. Natural product glycorandomization. Bioorg Med Chem. 2004;12:1577–1584. doi: 10.1016/j.bmc.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi H, Liu Y, Liu H. A two-stage one-pot enzymatic synthesis of TDP-L-mycarose from thymidine and glucose-1-phosphate. J Am Chem Soc. 2006;128:1432–1433. doi: 10.1021/ja0562144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rupprath C, Kopp M, Hirtz D, Muller R, Elling L. An enzyme module system for in situ regeneration of deoxythymidine 5′-diphosphate (dTDP)-activated deoxy sugars. Adv Synth Catal. 2007;349:1489–1496. [Google Scholar]
- 32.Timmons SC, Mosher RH, Knowles SA, Jakeman DL. Org Lett. 2007;9:857–860. doi: 10.1021/ol0630853. [DOI] [PubMed] [Google Scholar]
- 33.Wagner GK, Pesnot T, Field RA. A survey of chemical methods for sugar-nucleotide synthesis. Nat Prod Rep. 2009;26:1172–1174. doi: 10.1039/b909621n. [DOI] [PubMed] [Google Scholar]
- 34.Ko H, et al. Molecular recognition in the P2Y14 receptor: probing the structurally permissive terminal sugar moiety of uridine-5′-diphosphoglucose. Bioorg Med Chem. 2009;17:5298–5311. doi: 10.1016/j.bmc.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Losey HC, et al. Incorporation of glucose analogs by GtfE and GtfD from the vancomycin biosynthetic pathway to generate variant glycopeptides. Chem Biol. 2002;9:1305–1314. doi: 10.1016/s1074-5521(02)00270-3. [DOI] [PubMed] [Google Scholar]
- 36.Fu X, et al. Antibiotic optimization via in vitro glycorandomization. Nat Biotech. 2003;21:1467–1469. doi: 10.1038/nbt909. [DOI] [PubMed] [Google Scholar]
- 37.Kahne D, Leimkuhler C, Lu W, Walsh C. Glycopeptide and lipoglycopeptide antibiotics. Chem Rev. 2005;105:425–448. doi: 10.1021/cr030103a. [DOI] [PubMed] [Google Scholar]
- 38.Schitter G, Wrodnigg TM. Update on carbohydrate-containing antibacterial agents. Expert Opin Drug Discov. 2009;4:315–356. doi: 10.1517/17460440902778725. [DOI] [PubMed] [Google Scholar]
- 39.Wagner GK, Pesnot T. Glycosyltransferases and their assays. ChemBioChem. 2010;11:1939–1949. doi: 10.1002/cbic.201000201. [DOI] [PubMed] [Google Scholar]
- 40.Kittl R, Withers SG. New approaches to enzymatic glycoside synthesis through directed evolution. Carbohydr Res. 2010;345:1272–1279. doi: 10.1016/j.carres.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Pesnot T, Palcic MM, Wagner GK. A novel fluorescent probe for retaining galactosyltransferases. ChemBioChem. 2010;11:1392–1398. doi: 10.1002/cbic.201000013. [DOI] [PubMed] [Google Scholar]
- 42.Romero PA, Arnold FH. Exploring protein fitness landscapes by directed evolution. Nature Rev Mol Cell Biol. 2009;10:866–876. doi: 10.1038/nrm2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams GJ, Gantt RW, Thorson JS. The impact of enzyme engineering upon natural product glycodiversfication. Curr Opin Chem Biol. 2008;12:556–564. doi: 10.1016/j.cbpa.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hancock SM, Vaughan MD, Withers SG. Engineering of glycosidases and glycosyltransferases. Curr Opin Chem Biol. 2006;10:509–519. doi: 10.1016/j.cbpa.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 45.Zhang B, et al. Golgi nucleotide sugar transporter modulates cell wall biosynthesis and plant growth in rice. Proc Natl Acad Sci USA. 2011;108:5110–5115. doi: 10.1073/pnas.1016144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dickmanns A, et al. Structural basis for the broad substrate range of the UDP-sugar pyrophosphorylase from Leishmania major. J Mol Biol. 2011;405:461–478. doi: 10.1016/j.jmb.2010.10.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.