Abstract
A midbrain network interacts with the well-known frontoparietal forebrain network to select stimuli for gaze and spatial attention. The midbrain network, containing the superior colliculus (SC; optic tectum, OT, in non-mammalian vertebrates) and the isthmic nuclei, helps evaluate the relative priorities of competing stimuli and encodes them in a topographic map of space. Behavioral experiments in monkeys demonstrate an essential contribution of the SC to stimulus selection when the relative priorities of competing stimuli are similar. Neurophysiological results from the owl OT demonstrate a neural correlate of this essential contribution of the SC/OT. The multi-layered, spatiotopic organization of the midbrain network lends itself to the analysis and modeling of the mechanisms underlying stimulus selection for gaze and spatial attention.
Introduction
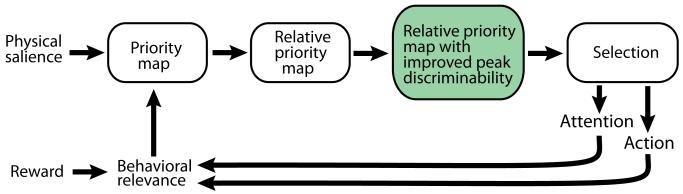
Animals are subjected to a constant and potentially overwhelming barrage of information from the environment. Their survival depends on the ability to correctly identify and process the most important information at every instant in time. The neural computations thought to achieve this goal center around three major steps: the evaluation of relative stimulus importance (“priority”), the selection of the location in the environment with the highest priority, and the deployment of attention or action to that location (Figure 1) [1-3]. These computational steps are carried out in overlapping populations of neurons [4].
Figure 1.
Computational steps that transform sensory inputs and internal goals into signals for driving spatial attention and action. In steps that are carried out by overlapping populations of neurons, the physical salience (strength) of stimuli is combined with their behavioral relevance to produce a topographic spatial representation (“map”) of the priority and relative priority of each stimulus. This map forms the basis for stimulus selection, which then drives subsequent shifts in attention or orienting action towards the selected stimulus. Shifts of attention and plans to orient to a stimulus both, in turn, influence the behavioral relevance of that stimulus. In green: a new step in the transformation, revealed by electrophysiological recordings in the barn owl OT and consistent with behavioral results following SC inactivation in monkeys and rats.
The midbrain and the forebrain contain networks involved in performing these computations [5]. The midbrain network contains the superior colliculus (SC; optic tectum, OT, in non-mammalian vertebrates) and the isthmic nuclei. Working together with the frontoparietal network in the forebrain [6-8], it evaluates the relative priorities of stimuli and maintains a topographic representation of those priorities [1,3,9]. This representation is updated rapidly, in real-time, as stimuli and the animal’s behavioral state change and is transmitted to other brain regions that control attention, analyze stimulus features, signal important events for reinforcement, and execute orienting movements [9-13].
Although both the midbrain and the forebrain networks are capable of carrying out most of these computational steps, recent evidence indicates that the midbrain network (specifically, the intermediate and deep layers of the SC) is necessary for selecting the highest priority stimulus when an animal encounters multiple stimuli of similar priorities. This essential role of the midbrain network in selecting the highest priority stimulus under difficult conditions adds to its well-established roles in multimodal sensorimotor integration, salience and priority evaluation, and gaze control [1,3,14,15].
Behavior: Role of the SC in stimulus selection
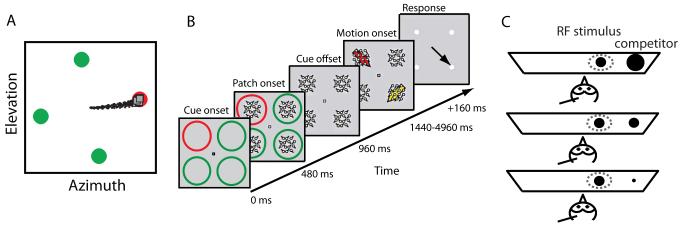
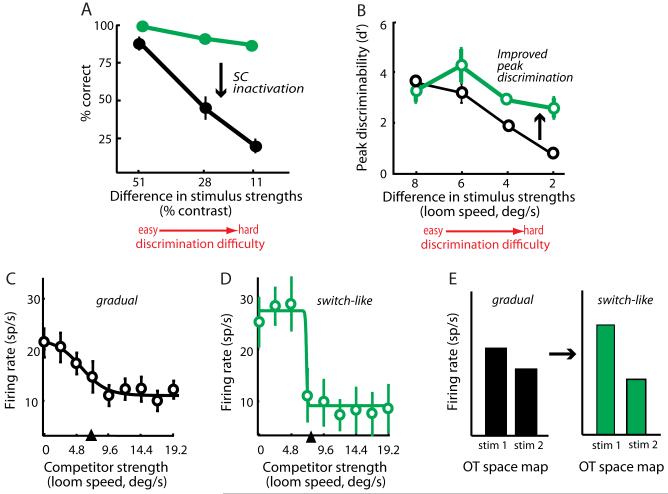
The necessity of the SC for stimulus selection was demonstrated in monkeys performing a color or contrast oddball search task (Figure 2A) [16]. Focal inactivation of the SC caused the monkey to select any of the four stimuli, rather than just the oddball stimulus, when the oddball stimulus was located in the inactivated portion of the SC space map. The impairment was specific to selection among multiple, competing stimuli and not due to visual or motor deficits. When the difference between the oddball and the other stimuli was large (easy task), the impairment resulting from SC inactivation was small (Figure 3A). However, when the difference among the stimuli was small (hard task) the impairment was profound (Figure 3A).
Figure 2.
Stimulus presentation protocols. A) Monkeys were trained to fixate on a central dot. The dot disappeared and an array of four dots appeared, of which one was an oddball. In one version of the task (shown here), the oddball stimulus had a different color from the others, and in another, it was of a different contrast than the others (Figure 3A). In both cases, the monkey was rewarded for making an eye saccade to the oddball stimulus. From [16]. B) Monkeys were cued by a red circle to attend to a particular location while maintaining fixation on a central dot. Patches of randomly moving dots appeared in each of the four locations, followed by a pulse of coherent motion at the cued location (target; red arrow) and in an orthogonal direction at the diagonally opposite location (task-relevant distracter; yellow arrow). The monkey was rewarded for reporting the direction of motion of the target with an eye movement in the corresponding direction (black arrow). Trials were also included in which the task-relevant distracter was replaced by a task-irrelevant distracter (patch of random motion). From [17]. C) Passive, untrained owls viewed a tangent screen. Simultaneous pairs of looming dots with different relative loom speeds (strengths) were presented for 250 ms. The strength of the stimulus inside the RF remained constant while the strength of a distant, competing stimulus (located outside of the classical RF, 30° to the side) was varied. The size of the dots represents the loom speeds of the stimuli. From [27].
Figure 3.
Selection deficits caused by SC inactivation in monkeys and improved peak discrimination by switch-like responses in the OT of owls. A) Effect of focal SC inactivation on behavioral performance by monkeys in a contrast, oddball task. The task was the same as described in Figure 2A, except that the oddball target was a brighter achromatic dot among three otherwise identical distracter dots. Discrimination difficulty was increased by decreasing the contrast difference between the target and distracter stimuli. Green data: selection performance before focal SC inactivation. Black data: selection performance after focal SC inactivation of the representation of the target stimulus. Downward black arrow indicates the decreased performance caused by SC inactivation. From [16]. B) Discriminability of the strongest (highest priority) stimulus by neuronal responses in the owl OT as a function of the difference in the relative strengths of competing stimuli (discrimination difficulty) [27]. Green data: neurons with switch-like responses. Black data: neurons with gradual responses. Peak discriminability, in each case, was based on a d’ analysis, comparing pooled responses from a condition in which the stronger stimulus was inside the RF and weaker stimulus was the competitor, with those from the mirror-symmetric condition. This was repeated for different values of relative stimulus strength. Upward black arrow indicates the improvement of discriminability provided by switch-like responses [27]. C) Gradual responses from an owl OT neuron measured with the protocol shown in Figure 2C. Responses to a stimulus inside the RF decreased gradually as the strength (loom speed) of a competing stimulus outside the RF increased. The arrowhead indicates the fixed strength of the RF stimulus. D) Switch-like responses from another owl OT neuron, measured with the protocol shown in Figure 2C. Conventions as in C. E) Improved peak discriminability by switch-like responses (green), compared to that by gradual responses (black), for a small relative difference between competing stimuli (2 deg/sec in B). The improved discriminability reflects both an increase in switch-like responses (relative to those of gradual responses) when the RF stimulus (stim 1) is stronger than the competitor (stim 2), and a decrease in switch-like responses (relative to those of gradual responses) when the RF stimulus is weaker. Modified from [27].
A similar effect of SC inactivation was demonstrated in monkeys performing a cued spatial attention task (Figure 2B) [17]. Focal SC inactivation caused the monkey to report the direction of the task-relevant distracter, rather than the direction of the cued stimulus, when the cued stimulus was located in the inactivated portion of the SC space map. The impairment was specific to selection among similar, competing stimuli and could not be explained by visual or motor deficits. When the difficulty of the task was reduced, by replacing the task-relevant distracter with a task-irrelevant distracter, SC inactivation resulted in almost no impairment of performance. Thus, as in the previous experiment, SC inactivation affects stimulus selection most when the difference among stimuli is small, while having little or no effect when the difference is large.
Other studies confirm the necessity of the SC in different types of stimulus selection tasks (monkeys: [18]; rats performing an odor discrimination task: [19]). The relationship between task difficulty and performance impairment following SC inactivation was observed in these tasks as well (but see [20]). In addition, focal electrical microstimulation applied to the SC results in a systematic improvement in the selection or discrimination of stimuli located in the activated portion of the SC space map, demonstrating the SC’s causal role in competitive stimulus selection [21-24].
In summary, these results reveal that an essential role of the SC is specific to the selection of one among many competing stimuli and that the importance of the SC increases with increasing difficulty of the selection task. We next describe neural correlates of the computational steps (Figure 1) that lead to the selection of the highest priority stimulus by the midbrain network.
Encoding stimulus priority
The identification of the highest priority stimulus begins with the evaluation of the priorities of stimuli (Figure 1). The key factors that contribute to evaluating the priority of a stimulus are its physical salience and its behavioral relevance. The SC/OT receives information about both of these factors. Most neurons in the SC/OT encode the physical salience (strength) of stimuli in their classical receptive fields (RFs). They respond vigorously to novel stimuli [25] and with increasing firing rates to increasing intensity or motion speed (strength) of the stimulus [26-29]. On the other hand, they exhibit little selectivity for stimulus features, such as color, orientation, motion direction, or sound frequency [17,26,30-34].
The behavioral relevance of a stimulus depends primarily on three factors (Figure 1). One factor is whether an animal associates the stimulus with rewarding or aversive consequences as a result of learning or innate predisposition. The influence of behavioral relevance in modulating SC/OT responses has been demonstrated by the finding that stimulus feature tuning can develop in SC neurons when the stimulus is associated with a reward [35]; reward conditioning dramatically increases SC/OT responses to the stimulus. Indeed, the strength of responses can be modulated in real-time by the size or probability of the reward [36,37]. Other factors that contribute to the representation of behavioral relevance in the SC are the selection of a stimulus for attention [38-40] or motor plans to orient gaze towards the location of the stimulus [41-44]. All three factors can increase SC/OT responses to a stimulus. In the midbrain network, the priorities of stimuli are encoded in terms of spike rates in a map of space, with higher firing rates corresponding to higher stimulus priority.
Encoding relative stimulus priority
The mechanism that computes relative stimulus priority is the global competitive surround. Studies in mammals and birds demonstrate that stimuli of any sensory modality, located far outside of the classical RF, can suppress the responses of SC/OT neurons to stimuli located inside their RFs [45-49]. A recent study of the owl OT characterized the spatial dependence of these competitive interactions in detail [50]. Another study explored the rules of relative priority representation in the OT [27]. Neuronal responses in the owl OT were recorded while systematically changing the relative priorities of competing stimuli; achieved by varying their relative strengths (salience) but not their behavioral relevance (Figure 2C) . The responses of most OT neurons were suppressed by the presence of the competing stimulus. About half of these neurons showed a gradual increase in response suppression as the strength of the competitor was increased, referred to as “gradual” suppression (Figure 3C). When these neurons were retested with a stronger stimulus inside the RF, the same gradual suppression was observed along with an overall increase in the neuronal firing rates. The results demonstrate that firing rates in the OT represent both the relative and the absolute stimulus strengths (priorities) of stimuli. The spatial map of relative stimulus priorities forms the basis for stimulus selection.
Stimulus selection
Selection of the highest priority stimulus involves an operation (a “decoder”) that compares firing rates across spatial locations in the relative priority map and determines the peak in the distribution of firing rates. A winner-take-all operation is one such decoder. It selects the location with the highest firing rate regardless of its difference from the next highest firing rate [2]. In contrast, the ideal observer analysis [3,51] is a probabilistic decoder. It selects the location with the highest firing rate with a probability that reflects how well the distributions of the highest and the next highest firing rates are separated. How the brain actually implements peak selection is not known. However, a recent study has shown that the probabilistic method correctly predicted the behavioral performance of monkeys from SC firing rates in a selection task on more trials than the winner-take-all method, with both methods performing equally well when the rate distributions were well separated [52].
The probabilistic method has been applied in several recent studies to decode SC/OT firing rates [27,28,35,53]. These studies have shown that the discriminability of the peak rate (probability of selection) decreases as the difference between the priorities of the competing stimuli decreases (for instance, Figure 3B, data in black; [27]). The behavioral observation that the normal decrease in selection performance with decreasing stimulus differences becomes far more severe following SC inactivation (Figure 3A, [16-19]) suggests that the SC/OT provides an additional computational step that improves stimulus discriminability prior to stimulus selection (Figure 1, box in green), as described next.
Improved peak discrimination in the relative priority map
We propose that one of the essential computational contributions of the midbrain network to stimulus selection is to improve the discriminability of the peak in the relative stimulus priority map in the SC/OT, specifically when the priorities of the competing stimuli are similar.
Neural correlates of such an essential computation (Figure 3B) were reported in the recent study that measured OT responses in owls to pairs of simultaneous stimuli (discussed previously, Figure 2C, [27]). Although many OT neurons exhibited gradual response suppression by a competing stimulus of increasing strength (Figure 3C), about 30% of the neurons exhibited “switch-like” suppression (Figure 3D): responses changed abruptly from a high level to a lower level as the strength of the competing stimulus increased, and this abrupt transition occurred when the strength of the competitor just exceeded that of the RF stimulus. When compared to gradual responses, switch-like responses improved the discriminability of the peak in the relative priority map specifically when the competing stimuli were similar (Figure 3B, data in green). This improvement resulted from an increase in firing rates to the strongest (highest priority) stimulus and a decrease in firing rates to the next strongest stimulus (Figure 3E; [27]). This computational step gave rise to a population-wide categorical representation of the strongest stimulus [54]. Interestingly, when a single stimulus was presented alone, there was no improvement in peak discriminability in the responses of switch-like versus gradual neurons [27,54], consistent with the little to no effect of SC inactivation on the selection of single stimuli. Thus, improved peak discriminability of the relative priority map is a neural correlate of an essential contribution of the midbrain network to competitive stimulus selection.
We next explore mechanisms that could underlie the construction of the relative priority map in the SC/OT and the improvement in its peak discriminability.
Mechanistic role of the isthmic nuclei
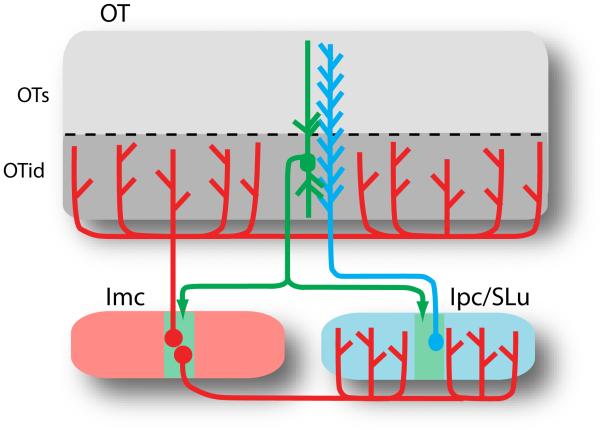
Globally projecting circuits, intrinsic to the SC/OT, that might support the generation of a relative priority map in the SC/OT ([27,50]) have been sought, but not found [55]. However, as recognized first by Sereno and Ulinski when studying the turtle midbrain [56], the unusual anatomy of the isthmic nuclei is perfectly suited to serve this function. The isthmic nuclei, located beneath the SC/OT in the lateral tegmentum, contain two major circuits. One circuit consists of a population of cholinergic neurons that are connected reciprocally and topographically with the SC/OT. In birds, these neurons are in two adjacent nuclei called the nucleus isthmi pars parvocellularis (Ipc) and the nucleus semilunaris (SLu) (Figure 4, blue [57]); in mammals, these neurons cluster in a single nucleus called the parabigeminal nucleus [58,59]. A second circuit, most thoroughly described in birds, consists of a population of GABAergic neurons that receive topographic input from the SC/OT and send projections back broadly to the space map in the SC/OT and to the cholinergic nuclei (Figure 4, red [60]). In birds, these neurons are in the nucleus isthmi pars magnocellularis (Imc); in mammals, the analogous neurons are thought to be in the lateral tegmental nucleus [59,61,62]
Figure 4.
The midbrain network for stimulus selection in birds. Schematic of cellular connections between the OT and the isthmic nuclei. OT neurons (green) send axons to cholinergic neurons (blue) in the Ipc and SLu and to GABAegic neurons (red) in the Imc. The OT is divided into superficial (OTs, layers 2-9) and intermediate and deep layers (OTid, layers 10-13) [5]. The green zones indicate the restricted termination zones for the OT neuron. Ipc neurons project back topographically to the OT, preferentially to the superficial layers; SLu neurons project back topographically, preferentially to the intermediate and deep layers, as well as to the thalamus and pretectum (not shown). Imc neurons project broadly to the OTid, Ipc and SLu. Abbreviations: Imc, nucleus isthmi pars magnocellularis; Ipc, nucleus isthmi pars parvocellularis; SLu, nucleus semilunaris.
The cholinergic isthmic circuit is thought to provide space-specific, feedback amplification of SC/OT responses, although this function has yet to be demonstrated directly. In owls, Ipc neurons are multimodal, sharply tuned for space, and respond according to the physical salience of stimuli [63]. In cats, the responses of PBN neurons are enhanced by motor plans for orienting eye movements [64]. Importantly, the responses of many Ipc neurons in owls are suppressed in a switch-like manner with increasing strength of a distant competing stimulus [65], suggesting that Ipc activity contributes, at least in part, to switch-like responses in the OT.
The GABAegic isthmic circuit is thought to mediate global competition in the SC/OT, although this function has yet to be demonstrated directly. In pigeons, Imc neurons are responsible for long-range inhibition in the Ipc [66]. Thus, the Imc is likely to mediate global competition in the OT both by regulating feedback amplification provided by the cholinergic isthmic circuit and by directly inhibiting OT neurons.
It has been proposed by many authors that the SC/OT, together with the isthmic circuits, form a module for the implementation of a winner-take-all evaluation of the most salient stimulus [56,66]. A detailed model of this module accounted for some, but not all, of the key aspects of switch-like responses in the OT [67], leaving open the question of the computational rules that implement improved peak discrimination in the midbrain network.
Conclusions and future directions
The role of the midbrain network in multimodal, sensorimotor integration and gaze control are well established. Its role in controlling attention is just being fully appreciated. One essential role of this network is to enable an animal to identify the highest priority stimulus among multiple stimuli with similar priorities. Switch-like response suppression when a competing stimulus becomes the strongest stimulus, as observed in the OT and in the cholinergic isthmic circuit in owls, provides a compelling neural correlate for this essential role.
It will be important to test whether switch-like response suppression and improved peak discriminability occur in the SC/OT of other species as well, particularly in monkeys. A behavioral protocol that will facilitate the observation of these phenomena is one that includes both the systematic variation of relative stimulus priorities of two or more simultaneous, task-relevant stimuli and a delayed response task (to allow for sufficient time for the neural feedback mechanisms of the isthmic circuits to exert their influence on SC/OT responses). In addition, further experimental and modeling investigations will be needed to understand the roles of the cholinergic and GABAergic isthmic circuits, both in the construction of the relative priority map and in improving peak discriminability in the OT. Equally important, the functional properties of the analogous circuits in mammals need to be explored in far greater detail.
Primate SC/OT is essential for selection when the difference among stimuli is small
Neural responses in owl SC/OT improve discriminability of the strongest stimulus
This improvement may be a neural correlate of the SC/OT’s contribution to behavior
The isthmic nuclei provide circuits that may mediate competitive selection
Acknowledgements
Funding from the Stanford University School of Medicine (Dean’s Postdoctoral Fellowship, SPM) and the NIH (R01 EY019179 to EIK) is gratefully acknowledged. We thank Phyllis Knudsen for preparing the figures. We also thank Ali Asadollahi, Astra Bryant, Cathy Dunn, Yvette Fisher, Alex Goddard, and Jason Schwarz for critically reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
• of special interest
 of outstanding interest
of outstanding interest
- 1.Fecteau JH, Munoz DP. Salience, relevance, and firing: a priority map for target selection. Trends Cogn Sci. 2006;10:382–390. doi: 10.1016/j.tics.2006.06.011. • This review introduced the concept of a “priority map” as a more accurate description of the information represented in the intermediate and deep layers of the SC.
- 2.Koch C, Ullman S. Shifts in selective visual attention: towards the underlying neural circuitry. Hum Neurobiol. 1985;4:219–227. [PubMed] [Google Scholar]
- 3.Krauzlis RJ, Liston D, Carello CD. Target selection and the superior colliculus: goals, choices and hypotheses. Vision Res. 2004;44:1445–1451. doi: 10.1016/j.visres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci. 33:269–298. doi: 10.1146/annurev.neuro.051508.135409. • This review presents a unified view of selection for attention and action and argues for an ethologically inspired parallel framework of neural information processing to explain real-time behavior.
- 5.Knudsen E. Control form below: The contribution of a midbrain network to spatial attention. European Journal of Neuroscience. doi: 10.1111/j.1460-9568.2011.07696.x. (in press) • This review described and compared the “midbrain network for stimulus selection” in mammals and birds.
- 6.Bisley JW. The neural basis of visual attention. J Physiol. 2010;589:49–57. doi: 10.1113/jphysiol.2010.192666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiller PH, Tehovnik EJ. Neural mechanisms underlying target selection with saccadic eye movements. Prog Brain Res. 2005;149:157–171. doi: 10.1016/S0079-6123(05)49012-3. [DOI] [PubMed] [Google Scholar]
- 9.Boehnke SE, Munoz DP. On the importance of the transient visual response in the superior colliculus. Curr Opin Neurobiol. 2008;18:544–551. doi: 10.1016/j.conb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Berman RA, Wurtz RH. Signals Conveyed in the Pulvinar Pathway from Superior Colliculus to Cortical Area MT. J Neurosci. 2011;31:373–384. doi: 10.1523/JNEUROSCI.4738-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaas JH, Lyon DC. Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain Res Rev. 2007;55:285–296. doi: 10.1016/j.brainresrev.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shipp S. The brain circuitry of attention. Trends Cogn Sci. 2004;8:223–230. doi: 10.1016/j.tics.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Reches A, Gutfreund Y. Auditory and multisensory responses in the tectofugal pathway of the barn owl. J Neurosci. 2009;29:9602–9613. doi: 10.1523/JNEUROSCI.6117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sparks DL. The brainstem control of saccadic eye movements. Nat Rev Neurosci. 2002;3:952–964. doi: 10.1038/nrn986. [DOI] [PubMed] [Google Scholar]
- 15.Stein BE, Stanford TR, Rowland BA. The neural basis of multisensory integration in the midbrain: Its organization and maturation. Hear Res. 2009 doi: 10.1016/j.heares.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
16.McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci. 2004;7:757–763. doi: 10.1038/nn1269.
 This was the first study to demonstrate a necessary role of the SC in target selection for saccades. The deficit resulting from focal SC inactivation required the presence of multiple stimuli and, in an oddball selection task, increased dramatically as the difficulty in discriminating the target from the distracters increased (hard tasks).
This was the first study to demonstrate a necessary role of the SC in target selection for saccades. The deficit resulting from focal SC inactivation required the presence of multiple stimuli and, in an oddball selection task, increased dramatically as the difficulty in discriminating the target from the distracters increased (hard tasks).
-
17.Lovejoy LP, Krauzlis RJ. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat Neurosci. 2010;13:261–266. doi: 10.1038/nn.2470.
 This study in monkeys showed, for the first time, a requirement for the SC in covert selection of stimuli for perceptual judgments. The deficit caused by focal SC inactivation was measured both with eye saccades or button presses and could not be explained by deficits in either the perceptual processing of stimuli or the execution of motor commands. The impairment decreased dramatically when the differences among stimuli increased (easy tasks). An important conclusion from this study is that the frontoparietal network for controlling attention is insufficient, on its own, to allocate spatial attention.
This study in monkeys showed, for the first time, a requirement for the SC in covert selection of stimuli for perceptual judgments. The deficit caused by focal SC inactivation was measured both with eye saccades or button presses and could not be explained by deficits in either the perceptual processing of stimuli or the execution of motor commands. The impairment decreased dramatically when the differences among stimuli increased (easy tasks). An important conclusion from this study is that the frontoparietal network for controlling attention is insufficient, on its own, to allocate spatial attention.
- 18.Nummela SU, Krauzlis RJ. Inactivation of primate superior colliculus biases target choice for smooth pursuit, saccades, and button press responses. J Neurophysiol. 2010;104:1538–1548. doi: 10.1152/jn.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felsen G, Mainen ZF. Neural substrates of sensory-guided locomotor decisions in the rat superior colliculus. Neuron. 2008;60:137–148. doi: 10.1016/j.neuron.2008.09.019. • This study demonstrated the necessity of the SC for sensory-guided locomotor choices, thereby establishing a general role for the SC in behavior involving spatial orienting. Inactivation of the SC in rats performing an odor discrimination task impaired the selection of the rewarded stimulus. The deficit in selection performance increased as stimulus discrimination difficulty increased.
- 20.McPeek RM. Reversal of a distractor effect on saccade target selection after superior colliculus inactivation. J Neurophysiol. 2008;99:2694–2702. doi: 10.1152/jn.00591.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carello CD, Krauzlis RJ. Manipulating intent: evidence for a causal role of the superior colliculus in target selection. Neuron. 2004;43:575–583. doi: 10.1016/j.neuron.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 22.Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci. 2004;24:11236–11243. doi: 10.1523/JNEUROSCI.3724-04.2004. • In monkeys performing a change-blindness task, the authors microstimulated in the intermediate layers of the SC and found an improvement in performance that paralleled the improvement obtained when the location of the upcoming change in a stimulus was previously cued. This paper was one of the first to show that subthreshold electrical microstimulation of the SC produces behavioral effects that are consistent with covert shifts of attention.
- 23.Muller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc Natl Acad Sci U S A. 2005;102:524–529. doi: 10.1073/pnas.0408311101. • The authors showed that in monkeys performing a motion direction discrimination task subthreshold electrical microstimulation of the SC improved the discrimination of stimuli specifically at the location encoded by the stimulated SC site. This is one of several studies showing that subthreshold electrical microstimulation of the SC produces behavioral effects that are consistent with covert shift of attention.
- 24.Cavanaugh J, Alvarez BD, Wurtz RH. Enhanced performance with brain stimulation: attentional shift or visual cue? J Neurosci. 2006;26:11347–11358. doi: 10.1523/JNEUROSCI.2376-06.2006. • This paper demonstrated that the behavioral effects of subthreshold electrical microstimulation of the intermediate layers of the SC in monkeys cannot be explained by the induction of a “phosphene,” an induced visual percept. The results have allowed for the effects of subthreshold electrical microstimulation of the SC to be interpreted as being due to shifts of attention.
- 25.Reches A, Gutfreund Y. Stimulus-specific adaptations in the gaze control system of the barn owl. J Neurosci. 2008;28:1523–1533. doi: 10.1523/JNEUROSCI.3785-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knudsen EI. Auditory properties of space-tuned units in owl’s optic tectum. J Neurophysiol. 1984;52:709–723. doi: 10.1152/jn.1984.52.4.709. [DOI] [PubMed] [Google Scholar]
-
27.Mysore SP, Asadollahi A, Knudsen EI. Signaling of the strongest stimulus in the owl optic tectum. J Neurosci. 2011;31:5186–5196. doi: 10.1523/JNEUROSCI.4592-10.2011.
 This study in barn owls discovered rules of relative priority analysis in the OT and demonstrated improvement in the representation of the highest priority stimulus in the OT. Responses from a subpopulation of OT neurons were suppressed in a switch-like manner when a distant competing stimulus exceeded the strength of the stimulus in the receptive field. Switch-like responses signaled the location of the strongest stimulus with high reliability, even when differences among stimuli were small.
This study in barn owls discovered rules of relative priority analysis in the OT and demonstrated improvement in the representation of the highest priority stimulus in the OT. Responses from a subpopulation of OT neurons were suppressed in a switch-like manner when a distant competing stimulus exceeded the strength of the stimulus in the receptive field. Switch-like responses signaled the location of the strongest stimulus with high reliability, even when differences among stimuli were small.
- 28.Ratcliff R, Hasegawa YT, Hasegawa RP, Smith PL, Segraves MA. Dual diffusion model for single-cell recording data from the superior colliculus in a brightness-discrimination task. J Neurophysiol. 2007;97:1756–1774. doi: 10.1152/jn.00393.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Updyke BV. Characteristics of unit responses in superior colliculus of the Cebus monkey. J Neurophysiol. 1974;37:896–909. doi: 10.1152/jn.1974.37.5.896. [DOI] [PubMed] [Google Scholar]
- 30.Li B, Wang L, Wang Y, Diao Y. Orientational and directional selectivities of visual neurons in the superior colliculus of the cat. Sci China C Life Sci. 1996;39:123–132. [PubMed] [Google Scholar]
- 31.Marrocco RT, Li RH. Monkey superior colliculus: properties of single cells and their afferent inputs. J Neurophysiol. 1977;40:844–860. doi: 10.1152/jn.1977.40.4.844. [DOI] [PubMed] [Google Scholar]
- 32.McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol. 2002;88:2019–2034. doi: 10.1152/jn.2002.88.4.2019. [DOI] [PubMed] [Google Scholar]
- 33.Ottes FP, Van Gisbergen JA, Eggermont JJ. Collicular involvement in a saccadic colour discrimination task. Exp Brain Res. 1987;66:465–478. doi: 10.1007/BF00270679. [DOI] [PubMed] [Google Scholar]
- 34.Wurtz RH, Albano JE. Visual-motor function of the primate superior colliculus. Annu Rev Neurosci. 1980;3:189–226. doi: 10.1146/annurev.ne.03.030180.001201. [DOI] [PubMed] [Google Scholar]
- 35.Horwitz GD, Newsome WT. Separate signals for target selection and movement specification in the superior colliculus. Science. 1999;284:1158–1161. doi: 10.1126/science.284.5417.1158. [DOI] [PubMed] [Google Scholar]
- 36.Ikeda T, Hikosaka O. Reward-dependent gain and bias of visual responses in primate superior colliculus. Neuron. 2003;39:693–700. doi: 10.1016/s0896-6273(03)00464-1. • This study shows that visual responses in the monkey SC are enhanced by the expectation of reward. The enhancement was either via a change in response gain or overall responsiveness. This study was the first to directly demonstrate the influence of reward on the encoding of stimulus priority in the SC.
- 37.Ikeda T, Hikosaka O. Positive and negative modulation of motor response in primate superior colliculus by reward expectation. J Neurophysiol. 2007;98:3163–3170. doi: 10.1152/jn.00975.2007. [DOI] [PubMed] [Google Scholar]
- 38.Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat Neurosci. 2004;7:56–64. doi: 10.1038/nn1169. [DOI] [PubMed] [Google Scholar]
- 39.Winkowski DE, Knudsen EI. Top-down control of multimodal sensitivity in the barn owl optic tectum. J Neurosci. 2007;27:13279–13291. doi: 10.1523/JNEUROSCI.3937-07.2007. • In this study, weak electrical microstimulation was applied to the forebrain gaze fields in barn owls, while recording auditory and visual responses in the intermediate and deep layers of the OT. Microstimulation induced space-specific, attention-like modulations of visual and auditory responses and revealed a role of top-down influences on the representation of stimulus priorities in the avian OT.
- 40.Winkowski DE, Knudsen EI. Distinct mechanisms for top-down control of neural gain and sensitivity in the owl optic tectum. Neuron. 2008;60:698–708. doi: 10.1016/j.neuron.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bell AH, Munoz DP. Activity in the superior colliculus reflects dynamic interactions between voluntary and involuntary influences on orienting behaviour. Eur J Neurosci. 2008;28:1654–1660. doi: 10.1111/j.1460-9568.2008.06393.x. [DOI] [PubMed] [Google Scholar]
- 42.Dorris MC, Olivier E, Munoz DP. Competitive integration of visual and preparatory signals in the superior colliculus during saccadic programming. J Neurosci. 2007;27:5053–5062. doi: 10.1523/JNEUROSCI.4212-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fecteau JH, Bell AH, Munoz DP. Neural correlates of the automatic and goal-driven biases in orienting spatial attention. J Neurophysiol. 2004;92:1728–1737. doi: 10.1152/jn.00184.2004. [DOI] [PubMed] [Google Scholar]
- 44.Shen K, Pare M. Neuronal activity in superior colliculus signals both stimulus identity and saccade goals during visual conjunction search. J Vis. 2007;7:15, 11–13. doi: 10.1167/7.5.15. [DOI] [PubMed] [Google Scholar]
- 45.Rizzolatti G, Camarda R, Grupp LA, Pisa M. Inhibitory effect of remote visual stimuli on visual responses of cat superior colliculus: spatial and temporal factors. J Neurophysiol. 1974;37:1262–1275. doi: 10.1152/jn.1974.37.6.1262. [DOI] [PubMed] [Google Scholar]
- 46.Schellart NA, Riemslag FC, Sperkreijse H. Center-surround organisation and interactions in receptive fields of goldfish tectal units. Vision Res. 1979;19:459–467. doi: 10.1016/0042-6989(79)90113-5. [DOI] [PubMed] [Google Scholar]
- 47.Frost BJ, Scilley PL, Wong SC. Moving background patterns reveal double-opponency of directionally specific pigeon tectal neurons. Experimental brain research. 1981;43:173–185. doi: 10.1007/BF00237761. [DOI] [PubMed] [Google Scholar]
- 48.Meredith MA, Stein BE. Spatial determinants of multisensory integration in cat superior colliculus neurons. J Neurophysiol. 1996;75:1843–1857. doi: 10.1152/jn.1996.75.5.1843. [DOI] [PubMed] [Google Scholar]
- 49.Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci. 1998;18:7519–7534. doi: 10.1523/JNEUROSCI.18-18-07519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mysore SP, Asadollahi A, Knudsen EI. Global inhibition and stimulus competition in the owl optic tectum. J Neurosci. 2010;30:1727–1738. doi: 10.1523/JNEUROSCI.3740-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim B, Basso MA. A probabilistic strategy for understanding action selection. J Neurosci. 2010;30:2340–2355. doi: 10.1523/JNEUROSCI.1730-09.2010. • This study compared the effectiveness of three models in predicting behavioral performance of monkeys in a color oddball selection task from the activities of SC neurons encoding the locations of the four competing stimuli. The models compared were, a Bayesian model, a population vector average model, and a winner-take-all model. The results demonstrated that the probabilistic Bayesian model, that considers the differences in firing rates among the competing locations, best accounted for the data.
- 53.Kim B, Basso MA. Saccade target selection in the superior colliculus: a signal detection theory approach. J Neurosci. 2008;28:2991–3007. doi: 10.1523/JNEUROSCI.5424-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
54.Mysore SP, Knudsen EI. Flexible categorization of relative stimulus strength by the optic tectum. J. Neurosci. doi: 10.1523/JNEUROSCI.5425-10.2011. (in press)
 This study in the owl OT is the first to demonstrate explicit and flexible categorization of stimuli by a neural network based on relative stimulus strength or salience. It demonstrates that the ensemble neural code in the OT/SC could mediate bottom-up stimulus selection for gaze and attention.
This study in the owl OT is the first to demonstrate explicit and flexible categorization of stimuli by a neural network based on relative stimulus strength or salience. It demonstrates that the ensemble neural code in the OT/SC could mediate bottom-up stimulus selection for gaze and attention.
- 55.Isa T, Hall WC. Exploring the superior colliculus in vitro. J Neurophysiol. 2009;102:2581–2593. doi: 10.1152/jn.00498.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sereno MI, Ulinski PS. Caudal topographic nucleus isthmi and the rostral nontopographic nucleus isthmi in the turtle, Pseudemys scripta. J Comp Neurol. 1987;261:319–346. doi: 10.1002/cne.902610302. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Luksch H, Brecha NC, Karten HJ. Columnar projections from the cholinergic nucleus isthmi to the optic tectum in chicks (Gallus gallus): a possible substrate for synchronizing tectal channels. J Comp Neurol. 2006;494:7–35. doi: 10.1002/cne.20821. [DOI] [PubMed] [Google Scholar]
- 58.Motts SD, Slusarczyk AS, Sowick CS, Schofield BR. Distribution of cholinergic cells in guinea pig brainstem. Neuroscience. 2008;154:186–195. doi: 10.1016/j.neuroscience.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Graybiel AM. A satellite system of the superior colliculus: the parabigeminal nucleus and its projections to the superficial collicular layers. Brain Res. 1978;145:365–374. doi: 10.1016/0006-8993(78)90870-3. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Major DE, Karten HJ. Morphology and connections of nucleus isthmi pars magnocellularis in chicks (Gallus gallus) J Comp Neurol. 2004;469:275–297. doi: 10.1002/cne.11007. [DOI] [PubMed] [Google Scholar]
- 61.Appell PP, Behan M. Sources of subcortical GABAergic projections to the superior colliculus in the cat. J Comp Neurol. 1990;302:143–158. doi: 10.1002/cne.903020111. [DOI] [PubMed] [Google Scholar]
- 62.Jiang ZD, King AJ, Moore DR. Topographic organization of projection from the parabigeminal nucleus to the superior colliculus in the ferret revealed with fluorescent latex microspheres. Brain Res. 1996;743:217–232. doi: 10.1016/s0006-8993(96)01042-6. [DOI] [PubMed] [Google Scholar]
- 63.Maczko KA, Knudsen PF, Knudsen EI. Auditory and visual space maps in the cholinergic nucleus isthmi pars parvocellularis of the barn owl. J Neurosci. 2006;26:12799–12806. doi: 10.1523/JNEUROSCI.3946-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cui H, Malpeli JG. Activity in the parabigeminal nucleus during eye movements directed at moving and stationary targets. J Neurophysiol. 2003;89:3128–3142. doi: 10.1152/jn.01067.2002. [DOI] [PubMed] [Google Scholar]
- 65.Asadollahi A, Mysore SP, Knudsen EI. Stimulus-driven competition in a cholinergic midbrain nucleus. Nat Neurosci. doi: 10.1038/nn.2573. in press. • This study in owls was the first to demonstrate switch-like responses in the midbrain network that encode the strongest stimulus among competing stimuli with high resolution. The cholinergic midbrain nucleus, the Ipc, is connected topographically and recurrently with the OT. Thus, this study reveals a possible mechanism for generating switch-like responses in the OT.
- 66.Marin G, Salas C, Sentis E, Rojas X, Letelier JC, Mpodozis J. A cholinergic gating mechanism controlled by competitive interactions in the optic tectum of the pigeon. J Neurosci. 2007;27:8112–8121. doi: 10.1523/JNEUROSCI.1420-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lai D, Brandt SF, Luksch H, Wessel R. Recurrent Antitopographic Inhibition Mediates Competitive Stimulus Selection in an Attention Network. J Neurophysiol. 2010 doi: 10.1152/jn.00673.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]