Abstract
Aims
Glucagon-like peptide-1 (GLP-1) is an incretin hormone that induces glucose-dependent insulin secretion and may have neurotrophic properties. Our aim was to identify the presence and activity of GLP-1 receptors (GLP-1R) in peripheral nerve and to assess the impact of GLP-1R agonists on diabetes-induced nerve disorders.
Materials and methods
Tissues were collected from streptozotocin-diabetic rats. GLP-1R function was assessed by incubating tissues from normal and diabetic rats with GLP-1R agonists and antagonists and measuring induction of ERK1/2 phosphorylation by western blot. Streptozotocin-diabetic mice were also treated with the GLP-1R agonist exenatide for 8 weeks to assess the impact of GLP-1R signaling on peripheral nerve function and structure.
Results
GLP-1R protein was detected in rat dorsal root ganglia and the neurons and Schwann cells of the sciatic nerve. Protein levels were not affect by streptozotocin-induced diabetes. GLP-1R agonists did not signal via ERK1/2 in sciatic nerve of normal rats. However, GLP-1R agonists significantly increased pERK1/2 levels in sciatic nerves from diabetic rats, indicating that GLP-1Rs are functional in this tissue. Exenatide treatment did not affect blood sugar, insulin levels or paw thermal response latencies in either control or diabetic mice. However, the reductions of motor nerve conduction velocity and paw intraepidermal fibre density seen in diabetic mice were attenuated by exenatide treatment.
Conclusions
These data demonstrate that the peripheral nerve of diabetic rodents exhibits functional GLP-1R and suggest that GLP-1R-mediated ERK-signaling in sciatic nerve of diabetic rodents may protect large motor fibre function and small C fibre structure by a mechanism independent of glycaemic control.
Keywords: Diabetes, Exenatide, Extracellular signal-regulated kinase, Glucagon-like peptide-1, Glucagon-like peptide-1 receptor, Mice, Neuropathy, Pancreas, Peripheral nerve, Rat
Introduction
Glucagon-like peptide-1 (GLP-1) is a glucose-dependent insulinotropic hormone produced by cleavage of pro-glucagon in intestinal L cells [1]. Exendin-4 is a GLP-1 analog derived from the saliva of the gila monster (Heloderma suspectum) that shares 53% homology with GLP-1, is equipotent to GLP-1 at the GLP-1 receptor (GLP-1R) and is resistant to degradation by dipeptidyl peptidase IV (DPP IV). Exenatide, the chemical name for synthetic exendin-4, is approved in the US and in the European Union to improve glycaemic control in patients with type 2 diabetes, based on its abilities to enhance glucose-dependent insulin secretion from pancreatic β cells, to suppress glucagon secretion and to slow gastric emptying [2, 3]. GLP-1 is also effective at regulating glycaemic control in newly-diagnosed type 1 diabetic patients with residual β cells [4, 5] and reducing blood glucose levels in type 1 diabetic patients independent of effects on residual β cells, via inhibiting gastric emptying, inhibiting glucagon secretion and promoting satiety [6, 7].
The receptor for GLP-1 is a 7-membrane-spanning G protein-coupled receptor from the receptor family that includes the glucagon, vasoactive intestinal peptide (VIP), secretin, and calcitonin receptors. In pancreatic β cells, GLP-1 binding to GLP-1R activates adenylyl cyclase with subsequent involvement of cAMP, protein kinase A and the nuclear transcription activator CREB, which in turn promotes pro-insulin gene transcription and increases insulin biosynthesis. In addition to its insulinotropic property, GLP-1 possesses biological actions as a trophic factor for pancreatic β cells and has insulin-like effects on tissues other than the pancreas, including adipose tissue [8], skeletal muscle [9] and liver [10]. In rat liver cells, GLP-1 activates PI3K/PKB and MAP kinase pathways [11, 12]. The effect of GLP-1 on pancreatic β cells and assorted other organs have been extensively reviewed [13, 14].
Aside from the organs mentioned above, there is also evidence that GLP-1 has effects on the nervous system, including having anti-apoptotic properties in rat hippocampal neurons [15–17]. These observations have promoted increasing interest in the potential for GLP-1 to protect the central nervous system from neurodegenerative diseases [18–20]. GLP-1 also promotes neurite outgrowth in PC12 cells, but little is known about GLP-1 biology in peripheral nerve, other than the promising observation that GLP-1 prevented conduction slowing in a model of pyridoxine-induced peripheral neuropathy [21]. As exenatide is now in clinical use by patients with diabetes, many of whom are likely to either already have peripheral neuropathy or will go on to develop this complication, we were interested in determining whether peripheral nerve possesses functional GLP-1 receptors, the impact of diabetes on any such receptors and whether exenatide treatment could have either detrimental or protective effects on peripheral nerve function that were independent of glucose regulation.
Methods
Animals
Ex-vivo studies were performed using adult female Sprague-Dawley rats in order to allow collection of sufficient amounts of nerve tissue for assay. In vivo studies were performed using adult Swiss Webster mice (both Harlan Industries, San Diego CA, USA). Rats were housed 2–3 per cage and mice 4–5 per cage with free access to food and water and maintained in a vivarium approved by the American Association for the Accreditation of Laboratory Animal Care. All animal studies were carried out according to protocols approved by the Institutional Animal Care and Use Committee of the University of California San Diego.
Drugs
GLP-1 7–36 amide (human), the GLP-1R antagonist exendin 9–39 and the GLP-1R agonist exenatide were purchased from Sigma. GLP-1 7–36 amide was dissolved in deionized water containing 20 μg/ml of the DPP IV inhibitor valine pyrrolidide (Novo Nordisk, Denmark) to limit degradation of GLP-1 7–36.
Induction of diabetes
Insulin-deficient diabetes was induced following an overnight fast by a single i.p. injection of streptozotocin (STZ, Sigma) dissolved in 0.9% sterile saline at 50 mg/kg for rats and 180 mg/kg for mice. Hyperglycaemia was confirmed using a strip-operated reflectance meter (One Touch Ultra, Lifescan Inc., Miltipas, CA, USA) in a blood sample obtained by tail prick four days after STZ injection and also in another sample collected at the conclusion of the study. Only STZ-injected animals with a blood glucose level above 270 mg/dl were included as diabetic. Plasma insulin levels were measured at the end of the study using a rat/mouse insulin ELISA (Millipore, St. Charles, MO, USA).
GLP-1 incubation
Rats were sacrificed after 4–8 weeks of diabetes by isoflurane anesthesia followed by decapitation. The pancreas, dorsal root ganglia (DRG) and endoneurial preparations of mid-thigh sciatic nerve were dissected and immediately placed in ice-cold oxygenated modified Krebs buffer (125 mM NaCl pH7.35, 3 mM KCl, 1.2 mM MgCl2.6H2O, 2.5 mM CaCl2.2 H2O, 1.2 mM NaH2PO4.H2O, 25 mM NaHCO3, 5mM glucose). Tissues were placed into an incubation chamber containing oxygenated modified Krebs buffer plus either no GLP-1 or GLP-1 at 0.3, 3.3 or 33 μg/ml. The incubations were performed under constant oxygenation at 37°C for 5, 30 and 120 min. For experiments requiring a GLP-1 antagonist, exendin 9–39 was added to the incubation baths at a concentration of 10 μg/ml. At the end of incubation, tissues were rinsed and homogenized in buffer (50 mM Tris-HCl pH7.4, 150 mM NaCl, 0.5% Triton X, 1 mM EDTA, protease inhibitor cocktail). Homogenates were then centrifuged at 13,000g for 30 min and supernatants were stored in aliquots at −80°.
Western blotting
Ten to 20 μg of total protein extracted from rat pancreas, sciatic nerves and DRG and mouse sciatic nerves were separated on 12% SDS-PAGE Tris-glycine gels (Novex, Invitrogen, Carlsbad, CA, USA) and immunoblotted on nitrocellulose. Blots were incubated at room temperature with anti-phospho p44–42 MAP kinase antibodies (Cell Signaling, Danvers, MA, USA) at a dilution of 1/1000, with anti-GLP1-R (Alpha Diagnostic International, San Antonio, TX, USA) at a dilution of 1/1000 or with anti-actin antibodies (Sigma) at a dilution of 1/2000. Membranes were then incubated with horseradish peroxidase-linked goat anti-rabbit or anti-mouse secondary antibody (1/10,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washes, blots were developed using an ECL Western-blotting protocol (Enhanced Chemiluminescence, Amersham Pharmacia Biotech, UK). For sequential analysis of membranes, previously bound antibodies were removed with stripping buffer (0.2 M Glycine, pH2.5, 0.05% Tween 20) for 45 min at 60°C. Quantification of immunoreactivity was performed by densitometric scanning using Quantity One software (BioRad, San Diego, CA, USA). For each tissue, band intensities were normalized by calculating the ratio of the intensity of the band corresponding to pERK or GLP1-R to the intensity of the band corresponding to ERK or actin. To allow grouping of samples run on different gels, ERK- or actin-normalized densitometric measures of band intensity were then expressed as a percentage of the group mean of all samples from control rats or mice present on the same gel. For the ex vivo experiments, data obtained after GLP-1 incubation were normalized to data from paired tissue samples incubated in the same conditions but in the absence of GLP-1 and run on the same blot. This allowed the effect of incubation conditions to be accounted for. The limited amount of sciatic nerve available from each mouse meant that we loaded variable amounts of total protein to each lane to maximize signal. This lead to variable actin signal per lane but did not impede our capacity to normalize other proteins to the amount of actin present for any given sample.
In vivo study
Saline vehicle or exenatide were delivered at a rate of 0.35 pmoles.kg−1.min−1 by Alzet osmotic mini-pumps (0.25μl/h) implanted subcutaneously between the scapulae. This dose was selected based on prior publications showing efficacy in protecting against other forms of nerve injury [15, 21]. Pumps were replaced after 4 weeks of diabetes and the new pumps were left in place for an additional 4 weeks. After 8 weeks of treatment, paw thermal responses and nerve conduction velocity were measured as described below and mice were then sacrificed by isoflurane anesthesia followed by decapitation. Sciatic nerves were dissected and immediately prepared as described above.
Thermal response latency
Mice were placed in an observation chamber on top of the thermal testing apparatus (UARD, San Diego, CA, USA) and paw withdrawal latencies to the heat source placed below the center of the hind paw were recorded as previously described [22].
Nerve conduction velocity
Under isoflurane anesthesia, core and nerve temperature were maintained at 37°C using a heating pad and lamps. The sciatic nerve was stimulated (5V, 0.05 ms pulse, square wave) with needle electrodes at the sciatic notch or ankle. Evoked early (M waves) responses were recorded from interosseous muscles of the foot with fine needle electrodes, amplified and displayed on an oscilloscope. The difference in response latencies of the M wave after stimulation at the sciatic notch and ankle was recorded as the time required for the motor nerve conduction between the stimulation sites. The distance between stimulation sites was measured on the surface of the fully extended hind limb and divided by the difference in M wave latencies to calculate sciatic motor nerve conduction velocity (MNCV). Measurements were made in triplicate and the median value used to represent MNCV for each animal.
Immunohistochemistry
Mouse sciatic nerve and plantar foot skin were immersion fixed for 24 h in 4% paraformaldehyde in 0.1 M phosphate buffer. Samples were embedded in paraffin and cut at a thickness of 6 μm. Sections were collected onto glass slides and incubated with an antibody against GLP-1R (1/50, Abcam, Cambridge, MA, USA) to visualize GLP1-R in the sciatic nerve or the pan-neuronal marker PGP9.5 (1:1000, Biogenesis Ltd., UK) to visualize innervation of the skin. Sections were then incubated with biotinylated anti-rabbit secondary antibody following the Avidin:Biotinylated enzyme Complex method (Vecstatain ABC kit, Vector Laboratories, Burlingame, CA, USA). Immunoreactive products were visualized with Vector NovaRed chromogen (Vector Laboratories, Burlingame, CA, USA). To standardize immunostaining for quantification of intraepidermal nerve fibres (IENF) two sections of skin, each from a separate, randomly selected animal, were placed on each slide and all slides were processed as a single batch. IENF immunoreactive profiles were counted using a light microscope (400×magnification), with slides coded to obscure group and animal identity. Epidermal length was determined by tracing the line of the dermal:epidermal junction and recording its length in millimeters. The number of IENF profiles was normalized to epidermal length [23].
Statistical analysis
Data are expressed as group mean ± sem. Differences between groups were analyzed using unpaired t test when comparing control and diabetic groups (ex-vivo studies) or by 1-way ANOVA followed by Dunnett's post hoc test for multi-group comparisons (in vivo studies).
Results
Diabetes
All STZ-injected rats that provided tissue for the ex vivo studies were hyperglycaemic at death (blood glucose for control rats: 141 ± 7 mg/dl and for diabetic rats: 586 ± 9 mg/dl). Mice injected with STZ also exhibited insulinopenia and hyperglycemia at sacrifice (Table 1). Treatment with exenatide did not alter body weight, blood sugar or plasma insulin levels in either control or diabetic mice (Table 1). Occasional deaths occurred in both diabetic groups of mice during the study, with no indication of toxicity related to exenatide treatment (2/10 and 2/14 for diabetic + saline and diabetic + exenatide, respectively).
Table 1.
Body weight, plasma glucose and insulin levels of control and diabetic mice treated with saline or exenatide for 8 weeks.
| Control + saline | Control + Exenatide | Diabetic + saline | Diabetic + Exenatide | |
|---|---|---|---|---|
| Initial weight (g) | 24.2 ± 0.8 | 24.3 ± 0.4 | 25.4 ± 0.3 | 25.1 ± 0.6 |
| Final weight (g) | 26.7 ± 0.9 | 26.8 ± 0.4 | 26.4 ± 0.5 | 25.8 ± 0.5 |
| Glucose (mg/dl) | 249.6 ± 16.2** | 228.3 ± 9.2** | 559.3 ± 30.7 | 575.8 ± 14.3 |
| Insulin (ng/ml) | 1.08 ± 0.28* | 1.20 ± 0.20** | 0.27 ± 0.05 | 0.16 ± 0.03 |
| N= | 8 | 8 | 8 | 12 |
Data are group mean ± SEM
p<0.05
p<0.01 vs diabetic + saline by one-way ANOVA followed by Dunnett's post hoc test.
GLP-1 receptor
GLP-1R protein was detected in pancreas, DRG and sciatic nerve of rats (Figure 1). Expression levels appeared higher in pancreas than in sciatic nerve (see Figure 2), whereas levels in the DRG and sciatic nerve were similar. Densitometric quantification indicated that GLP-1R protein was significantly (p<0.001) reduced in pancreas from diabetic rats (Figure 1A) but unchanged in DRG or sciatic nerve of diabetic rats compared to tissue from control rats (Figure 1B–1D). GLP-1R-immunoreactivity was most notable in Schwann cells of the mouse sciatic nerve, with clear evidence of signet ring profiles indicating localization to Schwann cell cytoplasm. Some axonal staining was also observed (Figure 2).
Figure 1.
Levels of GLP-1 receptor protein in pancreas (A) L4-L5 DRG (B) and sciatic nerve (C) (n=16) from control (white bars) and diabetic (black bars) rats. Data are mean + SEM of n=9–16/group and represent the ratio of the intensity of bands corresponding to GLP-1R and actin. ***p<0.001 by unpaired Student t test. Representative Western blots (D) for GLP-1 receptor and actin in sciatic nerve and DRG from control and diabetic rats.
Figure 2.
GLP1-R immunoreactivity in mouse sciatic nerve. GLP1-R immunoreactivity is present in axons (A and B, arrowheads) and also in Schwann cell cytoplasm, where it is evident as perinuclear staining (brown reaction product surrounding the hematoxylin-counterstained Schwann cell nuclei) and signet ring profiles when Schwann cell nuclei are not in the plane of section (arrows in B). Note the lack of staining in the blood vessel or its cellular components subjacent to the epineurium (asterix in A). Inset in A: specificity of immunostaining demonstrated by omission of primary antibody. Inset in B: positive control showing GLP1-R immunoreactivity in an islet of Langerhans of the pancreas. Bar = 40 μm.
Ex vivo preparation of isolated tissue
Basal levels of ERK1/2 phosphorylation
ERK1/2 and phospho-ERK1/2 were detected in pancreas, DRG and sciatic nerve from control and diabetic rats (Figure 3A). Diabetes did not affect the phosphorylation of ERK1 or ERK2, when normalized against actin or their respective total ERK, in either rat pancreas (Figure 3B) or sciatic nerve (Figure 3D). Phosphorylation of ERK1 and ERK2, when normalized against actin or their respective total ERK, was significantly (p<0.05) increased in DRG from diabetic rats compared to controls (Figure 3C).
Figure 3.
Representative western blots (A) for pERK1/2 and ERK1/2 in pancreas, sciatic nerve and DRG from control (labeled C) and diabetic (labeled D) rats. Levels of basal phopshorylation of ERK1 and ERK2 in pancreas (B) L4-L5 DRG (C) and sciatic nerve (D) from control (white bars) and diabetic (black bars) rats. Data are mean + SEM of n=9–13/group and represent the ratio of the intensity of bands corresponding to pERK1 and ERK1 or pERK2 and ERK2. *p<0.05 by unpaired Student t test.
Effect of GLP-1 on ERK1/2 phosphorylation
Data resulting from incubation of pancreas or sciatic nerve endoneurial preparations with GLP-1 are presented as the relative increase above values in equivalent tissues present on the same blot that were incubated for the same time without GLP-1 present during incubation (shown as the dotted line at 100%, Figures 4, 5 and 6).
Figure 4.
Phosphorylation levels of ERK1 (A) and ERK2 (B) in pancreas from control (white bars) and diabetic (black bars) rats after 120 min incubation with variable concentrations of GLP-1. Data are mean + SEM of n=5–9/group and represent the ratio of the intensity of bands corresponding to pERK1 and ERK1 or pERK2 and ERK2. *p<0.05 by unpaired Student t test for each concentration of GLP-1. Blots represent pERK1/2 and ERK1/2 in rat pancreas after incubation with GLP-1 at 0, 0.3, 3.3 or 33 μg/ml for control and diabetic rats.
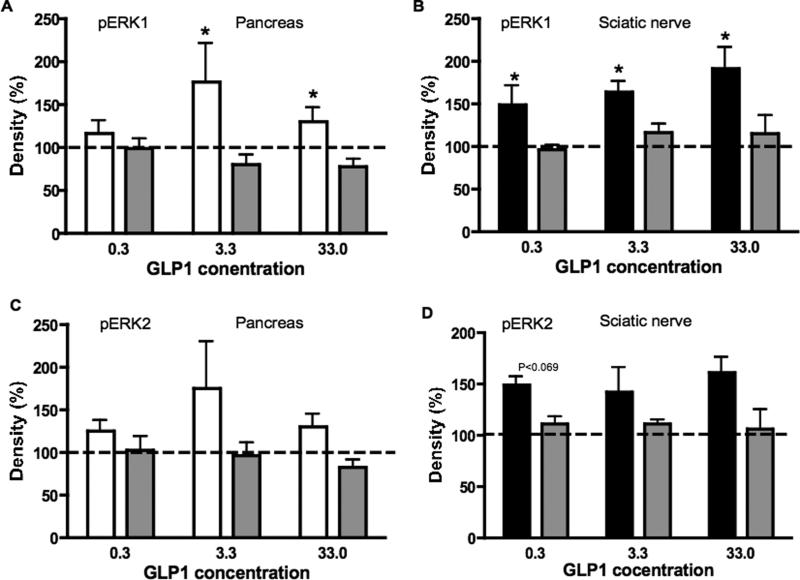
Figure 5.
Phosphorylation levels of ERK1 (A) and ERK2 (B) in sciatic nerve from control (white bars) and diabetic (black bars) rats after 5 min incubation with variable concentrations of GLP-1. Data are mean + SEM of n=6–10/group and represent the ratio of the intensity of bands corresponding to pERK1 and ERK1 or pERK2 and ERK2. *p<0.05, **p<0.01 by unpaired Student t test for each concentration of GLP-1. Blots represent pERK1/2 and ERK1/2 in rat sciatic nerve after incubation with GLP-1 at 0, 0.3, 3.3 or 33 μg/ml for control and diabetic rats.
Figure 6.
Effect of exendin 9–39 on ERK1 phosphorylation in pancreas from control rats (A) and sciatic nerve from diabetic rats (B) incubated with variable concentrations of GLP-1, the effect of exendin 9–39 on ERK2 phosphorylation in pancreas from control rats (C) and sciatic nerve from diabetic rats (D) incubated with variable concentrations of GLP-1. White bars represent control tissue incubated with GLP-1, black bars diabetic tissue incubated with GLP-1 and grey bars tissue incubated with GLP-1+ Ex 9–39. Data are mean + SEM of n=4–7/group and represent the ratio of the intensity of bands corresponding to pERK1 and ERK1. *p<0.05, by unpaired Student t test for each concentration of GLP-1.
In pancreatic tissue isolated from control rats, incubation with GLP-1 for either 5 or 30 min had no effect on phosphorylation of ERK1 or ERK2 at any concentration used. However, incubation with GLP-1 for 120 min induced significant (p<0.05) increases of ERK1 phosphorylation at the 3.3 and 33 μg/ml concentrations (Figure 4A). Similar increases were seen for ERK2 (Figure 4B). No increases were detected in pancreas from diabetic rats at any time point or GLP-1 concentration (Figure 4A, B).
GLP-1 did not alter phosphorylation of ERK1 and ERK2 in isolated sciatic nerve from control rats at any concentration (0.3, 3.3, 33.0 μg/ml) or duration of exposure (5, 30, 120 min) studied (5 min exposure shown in Figure 5A, B). In sciatic nerve from diabetic rats, GLP-1 induced a significant (p<0.05) phosphorylation of ERK1 and ERK2 that was detected after 5 min of incubation at all 3 concentrations studied (Figure 5A, B). Longer (30 or 120 min) incubations with GLP-1 did not induce an increase of phosphorylation of ERK1 or ERK2 at any concentration studied (data not shown).
Inhibition of GLP-1 effect by exendin 9–39
To confirm that the effect of GLP-1 on the pancreas from control rats after 120 min and on sciatic nerve from diabetic rats after 5 min incubation was the result of activation of the GLP-1R, similar experiments to those described above were performed in the presence of exendin 9–39, an antagonist of the GLP-1R. The presence of exendin 9–39 in the incubation bath prevented the otherwise significant (p<0.05) increase in phosphorylation of ERK1 in the pancreas following 120 min of incubation with GLP-1 (Figure 6A) and in phosphorylation of ERK1 in sciatic nerve from diabetic rats after 5 min incubation with GLP-1 (Figure 6B). Similar findings were noted for ERK2 (Figure 6C, D).
In vivo study
GLP-1 receptor protein levels
GLP-1R protein levels were not significantly different in the sciatic nerves from saline-treated control and diabetic mice or in control mice receiving exenatide (Figure 7). GLP-1R protein was significantly (p<0.01) increased in sciatic nerve from diabetic mice receiving exenatide for 8 weeks when compared to levels in diabetic mice receiving saline and a similar trend was noted in exenatide treated control mice.
Figure 7.
Levels of GLP-1 receptor protein in mouse sciatic nerve after 8 weeks of treatment with exenatide or saline. Data are mean + SEM and represent the ratio of the intensity of bands corresponding to GLP-1R and actin. **p<0.01 vs diabetic + saline by one-way ANOVA followed by Dunnett's post hoc test. Blots represent GLP1-R and actin in mouse sciatic nerve for control (C) and diabetic (D) mice treated with saline (S) or exenatide (Ex) for 8 weeks.
Phosphorylation of ERK1/2
ERK1 and ERK2 phosphorylation was similar in sciatic nerves from control and diabetic mice receiving saline and in nerves from control mice receiving exenatide (Figure 8A, B). However, ERK1 and ERK2 phosphorylation were both significantly (p<0.01) increased in sciatic nerve from diabetic mice receiving exenatide when compared to diabetic mice receiving saline (Figure 8A, B).
Figure 8.
Phosphorylation levels of ERK1 (A) and ERK2 (B) in sciatic nerve from mice treated with saline or exenatide for 8 weeks. Data are mean + SEM and represent the ratio of the intensity of bands corresponding to pERK1 and ERK1 or pERK2 and ERK2. **p<0.01, vs diabetic + saline by one-way ANOVA followed by Dunnett's post hoc test. Blots represent pERK1/2 and ERK1/2 in mouse sciatic nerve for control (C) and diabetic (D) mice treated with saline (S) or exenatide (Ex) for 8 weeks.
Nerve function
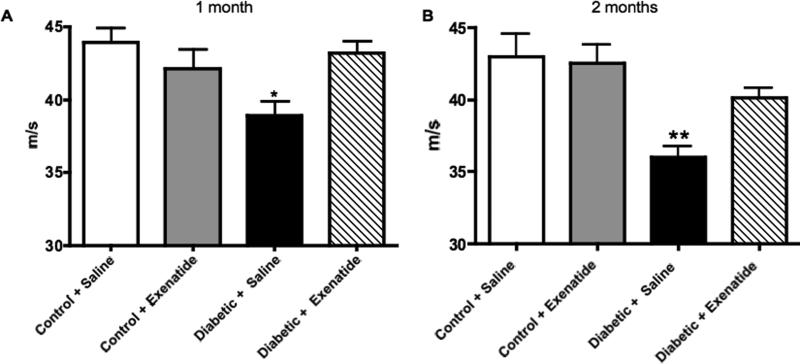
MNCV was significantly reduced after 1 month (p<0.05) and at the end of the study (p<0.01) in diabetic mice receiving saline when compared to saline-treated control mice (Figure 9A, B). Treatment with exenatide had no effect on MNCV of control mice but prevented the decrease observed in diabetic mice so that values were not significantly different from controls. Paw thermal response latencies were significantly (p<0.01) increased in diabetic mice receiving saline when compared to saline-treated control mice (Figure 10A). Treatment with exenatide had no significant effect on thermal latencies in either control or diabetic mice.
Figure 9.
Motor nerve conduction velocity of mouse sciatic nerve after 4 weeks (A) and 8 weeks (B) of treatment with saline or exenatide. Data are mean + SEM. *p<0.05, **p<0.01 vs control + saline by one-way ANOVA followed by Dunnett's post hoc test.
Figure 10.
Thermal responses (A) of the mouse hind paw and IENF immunoreactive profiles (B) after 8 weeks of treatment with saline or exenatide. Data are mean + SEM. *p<0.05, **p<0.01 vs control + saline by one-way ANOVA followed by Dunnett's post hoc test. Representative image (C) of mouse foot skin from control + saline. Arrows indicate IENF profiles. Bar: 40 μm.
Intraepidermal nerve fibre profiles
IENF profile number was significantly (p<0.05) decreased in diabetic mice receiving saline when compared to saline-treated control mice (Figure 10B, C). Treatment with exenatide had no significant effect on IENF profile number in control mice but prevented the decrease observed in diabetic mice, so that they were not significantly different from controls.
Discussion
Our data show that receptors for GLP-1 are present in sciatic nerve of rats and mice and that protein levels are not affected by STZ-induced type 1 diabetes. The functional status of the GLP-1R was confirmed in nerve from diabetic rats by detection of GLP-1 induced phosphorylation of ERK1/2. Induction of ERK-signaling was attributed to GLP-1 activation of the GLP-1R by co-incubation with exendin 9–39, an antagonist of GLP-1R. We extended our studies to assess the effects of the GLP-1 analog, exenatide, on peripheral nerve of normal and STZ-diabetic mice. Exenatide treatment increased GLP-1R protein levels in nerve of both control and diabetic rats, although we cannot yet offer evidence for the mechanism by which this occurs. Consistent with our ex vivo studies, phopshorylation of ERK1/2 was significantly increased in the sciatic nerve from diabetic mice treated with exenatide for 8 weeks. This was accompanied by prevention of NCV slowing and paw IENF loss in STZ-diabetic mice. No effects of exenatide treatment were seen on ERK-signaling or MNCV in control mice or on systemic indices of type 1 diabetes. Our data suggest that GLP-1 agonists selectively activate ERK-signaling in the sciatic nerve during diabetes and that this is associated with neuroprotective effects that are independent of modulation of plasma glucose or insulin levels.
The pancreas is a primary site of GLP-1 binding to the GLP-1R. Depletion of GLP-1R protein in the pancreas of STZ-diabetic rats is consistent with localization of the GLP-1R to β cells in the pancreas [24], as these cells are selectively destroyed by STZ. The GLP-1R is also expressed in a number of other tissues, including kidney, heart, brain and skin [25, 26], a distribution that is indicative of the increasingly diverse physiological functions attributed to the hormone [27]. Our finding of GLP-1R protein in the peripheral nerve of rats and mice, and its localization to neurons agree with the demonstration of GLP1-R in nerve terminals of the portal vein [28] and by previous reports suggesting that GLP-1R agonists have neurotrophic and neuroprotective effects on cultures of PC12 cells and CNS-derived neurons [15, 16]. It is also consistent with the protective effect of GLP-1R agonists against pyridoxine-induced peripheral sensory neuropathy [21] and in models of stroke, Parkinson's disease and Alzheimer's disease [29, 30]. GLP1-Rs were also expressed in the Schwann cell of the mouse sciatic nerve, and this localization has also been noted in a report published during preparation of this manuscript [26]. GLP-1R protein levels in peripheral nerve were not altered by insulin-deficient diabetes. The identification of GLP-1R in the PNS offers the possibility that endogenous circulating GLP-1 may have direct effects on nerve phenotype or function and that systemic delivery of GLP-1R agonists or antagonists can have direct effects on the nervous system independent of effects on other organs that express the GLP-1R.
Phosphorylation of ERK1/2 proteins was measured as an indicator of the functional activity of the GLP-1R. These proteins were chosen because the ERK-signaling pathway is activated when GLP-1R agonists are applied to cultures of pancreatic cells [31], mouse skin cells [32] and PC12 cells [16]. The fidelity of the GLP-1:GLP1-R:ERK1/2 pathway in our assay systems was confirmed using isolated pancreas tissue in which the STZ-induced ablation of pancreatic β cells resulted in the expected absence of both GLP-1R protein and GLP-1-induced ERK1/2 phosphorylation. A particularly striking subsequent finding was that while GLP-1 induced a rapid and transient ERK1/2 phosphorylation when applied to endoneurial preparations of nerve from diabetic rats, no such activation was noted in similar preparations from normal rats. This divergence occurred despite the presence of equal amounts of GLP-1R protein in nerve from both control and diabetic rats. It is possible that activation of the GLP-1R may promote cell signaling via alternate unknown pathways in normal peripheral nerve and that diabetes initiates ERK1/2 signaling. Indeed, GLP-1Rs are known to signal using other pathways such as those mediated by protein kinase A [33] or PI3K [34]. At present, we have measured activity of the PI3K/GSK3 pathway in normal nerve and find no activation by GLP-1R agonists (Jolivalt, unpublished data). Even if alternate GLP-1R signaling pathways operate in normal nerve, exenatide treatment did not alter any parameters of nerve function and structure measured in control mice, suggesting that activation of alternate pathways downstream of GLP-1R in peripheral nerve has no overt functional or structural consequences. An alternative possibility is that the GLP-1R may be inactive in normal peripheral nerve and become activated during hyperglycemia. This hypothesis is consistent with the ability of GLP-1 and GLP-1R agonists to stimulate insulin secretion while suppressing glucagon secretion in the hyperglycaemic state, but not in the hypoglycaemic state [35, 36]. How glucose levels may regulate GLP-1R activation in peripheral nerves remains to be determined.
Previous studies have reported increased ERK phosphorylation in the DRG of STZ-diabetic rats, but not in sciatic nerve [37, 38]. Our data agree with these findings, as we found increased basal ERK phosphorylation in the DRG, but not sciatic nerve, of STZ-diabetic rats and did not find any increase in ERK phosphorylation in the sciatic nerve of STZ-diabetic mice. ERK is a serine/threonine protein kinase that transduces extracellular stimuli into a variety of intracellular posttranslational and transcriptional responses and promotes outcomes that are often positive for the cell, such as survival, growth, differentiation, and maintenance of phenotype [39]. Diabetes-induced activation of ERK-signaling in DRG may therefore be a protective response to metabolic stresses induced by diabetes. The activation of ERK-signaling by GLP-1R agonists in peripheral neurons and/or Schwann cells from diabetic rats and mice may also have beneficial ramifications. Treating diabetic mice with a GLP-1 agonist resulted in both ERK activation in nerve and protection against MNCV slowing, although at present we cannot conclude that the two are causally related. In short-term diabetic rodents, MNCV slowing is frequently viewed as a consequence of hyperglycemia-induced metabolic stresses, along with loss of neurotrophic support that would normally allowed tolerance of metabolic stress [40]. In time, axonal atrophy may contribute to NCV slowing, while physical damage to myelin is not commonly reported in the sciatic nerve of short-term STZ-diabetic rodents [41]. The presence of the GLP-1R in both axons and Schwann cells leaves open the possibility that exenatide is acting on either or both cell types. The limited amount of mouse nerve available from our study precluded us from exploring potential mechanistic links between ERK-signaling and MNCV in the nerve of exenatide-treated diabetic mice. Further studies using rat models of diabetic neuropathy may be warranted to address these speculations.
The attenuation of MNCV slowing in STZ-diabetic mice by exenatide indicates efficacy against large fibre dysfunction. Exenatide also showed some efficacy against the IENF loss that had developed after 8 weeks of diabetes and which represents a structural index of small fibre neuropathy. This effect is consistent with a recent study in long-term STZ-diabetic mice that showed prevention of paw IENF loss when plasma levels of endogenous GLP-1 were increased by inhibiting its proteolytic degradation [42]. It is interesting to note that the maintenance of normal paw IENF profile number by exenatide was not accompanied by prevention of paw thermal hypoalgesia, a behavioral disorder that has been associated with loss of C fibres in the skin [43]. However, it takes loss of 50% or more of total IENF to promote detectable thermal hypoalgesia in human subjects [44] and factors other than discernable IENF loss may be contributing to acute thermal hypoalgesia in diabetic mice [23]. Conversely, IENF loss in diabetic animals need not reflect loss of the sub-population of heat-sensitive fibres. As GLP-1R agonists promote neurite outgrowth in vitro via an ERK-dependent mechanism [16], it is also possible that the increase in IENF profile number seen in exenatide-treated diabetic mice represents regenerative or collateral sprouts from IENF that are not yet functional. Our data may add to the expanding list of studies that are revealing a less than simple association between epidermal fibre number and thermal nociceptive capacity. However, they also indicate that exenatide can preserve nerve numbers in the epidermis during diabetes, a measurement that is becoming increasingly viewed as a means of quantifying early distal sensory neuropathy in diabetic patients [45].
In contrast to the beneficial consequences of ERK-signaling discussed above, there have been reports that increased ERK-signaling is associated with neuropathic pain states [46] and neuronal cell death [47]. It was therefore feasible that ERK signaling induced by GLP-1R agonists in DRG or nerve trunks of diabetic rodents could have some detrimental consequences. However, neither control nor diabetic mice treated with exenatide showed any overt behavioral indices of discomfort during observation or handling and exenatide did not induce thermal hyperalgesia. Control mice treated with exenatide also did not show indications of neurotoxicity as illustrated by normal MNCV and paw epidermal fibre density. Our data suggest that GLP-1R agonist treatment and subsequent activation of ERK-signaling in the nerve of diabetic mice does not promote any overt neurotoxicity.
In summary, we have presented evidence that the GLP-1R is found in peripheral nerve and that GLP-1R agonists induce ERK activation in peripheral nerve of diabetic rats and mice. GLP-1 agonism also protected large motor fibre function and small sensory fibre structure of STZ-diabetic mice by a mechanism that is independent of glycaemic control. Use of GLP-1 agonists by diabetic patients to augment glycaemic control may confer additional protection against diabetic neuropathy that is independent of effects on blood glucose regulation.
Acknowledgments
This work was supported by Novo Nordisk (Denmark), Amylin Pharmaceuticals (San Diego, USA), NIH grant DK57629 (NAC) and a Career Development Award from the Juvenile Diabetes Research Foundation (CJG). The authors would like to thank Lucie Guernsey and Victor Arballo for excellent technical assistance.
Abbreviations
- DRG
dorsal root ganglia
- ECL
enhanced Chemiluminescence
- ERK
extracellular signal-regulated kinase
- DPP IV
dipeptidyl peptidase IV
- GLP-1
glucagon-like peptide-1
- GLP-1R
glucagon-like peptide-1 receptor
- IENF
intraepidermal nerve fibre
- MAPK
mitogen-activated protein kinase
- MNCV
motor nerve conduction velocity
- PI3K
phosphoinositol-3 kinase
- STZ
streptozotocin
- VIP
vasoactive intestinal peptide
Footnotes
Conflict of interest details: CG Jolivalt: design, conduct, data collection, analysis, writing M. Fineman: design, conduct/data collection, analysis CF Deacon: design, analysis RD Carr: design NA Calcutt: design, analysis, writing
There are no other conflicts of interest to declare
References
- [1].Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- [2].Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- [3].Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372:1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- [4].Behme MT, Dupre J, McDonald TJ. Glucagon-like peptide 1 improved glycemic control in type 1 diabetes. BMC Endocr Disord. 2003;3:3. doi: 10.1186/1472-6823-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kielgast U, Holst JJ, Madsbad S. Treatment of type 1 diabetic patients with glucagon-like peptide-1 (GLP-1) and GLP-1R agonists. Curr Diabetes Rev. 2009;5:266–275. doi: 10.2174/157339909789804413. [DOI] [PubMed] [Google Scholar]
- [6].Creutzfeldt WO, Kleine N, Willms B, Orskov C, Holst JJ, Nauck MA. Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7–36) amide in type I diabetic patients. Diabetes Care. 1996;19:580–586. doi: 10.2337/diacare.19.6.580. [DOI] [PubMed] [Google Scholar]
- [7].Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7–36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992;326:1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- [8].Valverde I, Merida E, Delgado E, Trapote MA, Villanueva-Penacarrillo ML. Presence and characterization of glucagon-like peptide-1(7–36) amide receptors in solubilized membranes of rat adipose tissue. Endocrinology. 1993;132:75–79. doi: 10.1210/endo.132.1.8380388. [DOI] [PubMed] [Google Scholar]
- [9].Villanueva-Penacarrillo ML, Alcantara AI, Clemente F, Delgado E, Valverde I. Potent glycogenic effect of GLP-1(7–36)amide in rat skeletal muscle. Diabetologia. 1994;37:1163–1166. doi: 10.1007/BF00418382. [DOI] [PubMed] [Google Scholar]
- [10].Villanueva-Penacarrillo ML, Delgado E, Trapote MA, et al. Glucagon-like peptide-1 binding to rat hepatic membranes. J Endocrinol. 1995;146:183–189. doi: 10.1677/joe.0.1460183. [DOI] [PubMed] [Google Scholar]
- [11].Peak M, Rochford JJ, Borthwick AC, Yeaman SJ, Agius L. Signalling pathways involved in the stimulation of glycogen synthesis by insulin in rat hepatocytes. Diabetologia. 1998;41:16–25. doi: 10.1007/s001250050861. [DOI] [PubMed] [Google Scholar]
- [12].Redondo A, Trigo MV, Acitores A, Valverde I, Villanueva-Penacarrillo ML. Cell signalling of the GLP-1 action in rat liver. Mol Cell Endocrinol. 2003;204:43–50. doi: 10.1016/s0303-7207(03)00146-1. [DOI] [PubMed] [Google Scholar]
- [13].Brubaker PL. Minireview: update on incretin biology: focus on glucagon-like peptide-1. Endocrinology. 2010;151:1984–1989. doi: 10.1210/en.2010-0115. [DOI] [PubMed] [Google Scholar]
- [14].Mudaliar S, Henry RR. Effects of incretin hormones on beta-cell mass and function, body weight, and hepatic and myocardial function. Am J Med. 2010;123:S19–27. doi: 10.1016/j.amjmed.2009.12.006. [DOI] [PubMed] [Google Scholar]
- [15].Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther. 2002;302:881–888. doi: 10.1124/jpet.102.037481. [DOI] [PubMed] [Google Scholar]
- [16].Perry T, Lahiri DK, Chen D, et al. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther. 2002;300:958–966. doi: 10.1124/jpet.300.3.958. [DOI] [PubMed] [Google Scholar]
- [17].Perry T, Lahiri DK, Sambamurti K, et al. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci Res. 2003;72:603–612. doi: 10.1002/jnr.10611. [DOI] [PubMed] [Google Scholar]
- [18].Bertilsson G, Patrone C, Zachrisson O, et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson's disease. J Neurosci Res. 2008;86:326–338. doi: 10.1002/jnr.21483. [DOI] [PubMed] [Google Scholar]
- [19].Holscher C. Incretin analogues that have been developed to treat type 2 diabetes hold promise as a novel treatment strategy for Alzheimer's disease. Recent Pat CNS Drug Discov. 2010;5:109–117. doi: 10.2174/157488910791213130. [DOI] [PubMed] [Google Scholar]
- [20].Martin B, Golden E, Carlson OD, et al. Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington's disease. Diabetes. 2009;58:318–328. doi: 10.2337/db08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Perry T, Holloway HW, Weerasuriya A, et al. Evidence of GLP-1-mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Exp Neurol. 2007;203:293–301. doi: 10.1016/j.expneurol.2006.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Calcutt NA. Modeling diabetic sensory neuropathy in rats. Methods Mol Med. 2004;99:55–65. doi: 10.1385/1-59259-770-x:225. [DOI] [PubMed] [Google Scholar]
- [23].Beiswenger KK, Calcutt NA, Mizisin AP. Dissociation of thermal hypoalgesia and epidermal denervation in streptozotocin-diabetic mice. Neurosci Lett. 2008;442:267–272. doi: 10.1016/j.neulet.2008.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tornehave D, Kristensen P, Romer J, Knudsen LB, Heller RS. Expression of the GLP-1 receptor in mouse, rat, and human pancreas. J Histochem Cytochem. 2008;56:841–851. doi: 10.1369/jhc.2008.951319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wei Y, Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995;358:219–224. doi: 10.1016/0014-5793(94)01430-9. [DOI] [PubMed] [Google Scholar]
- [26].Liu WJ, Jin HY, Lee KA, Xie SH, Baek HS, Park TS. Neuroprotective effect of the glucagon-like peptide-1 receptor agonist, synthetic exendin-4, in streptozotocin-induced diabetic rats. Br J Pharmacol. doi: 10.1111/j.1476-5381.2011.01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Burcelin R, Dejager S. GLP-1: What is known, new and controversial in 2010? Diabetes Metab. 2010;36:503–509. doi: 10.1016/j.diabet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- [28].Vahl TP, Tauchi M, Durler TS, et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148:4965–4973. doi: 10.1210/en.2006-0153. [DOI] [PubMed] [Google Scholar]
- [29].Li Y, Duffy KB, Ottinger MA, et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. J Alzheimers Dis. 2010;19:1205–1219. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li Y, Perry T, Kindy MS, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Blandino-Rosano M, Perez-Arana G, Mellado-Gil JM, Segundo C, Aguilar-Diosdado M. Anti-proliferative effect of pro-inflammatory cytokines in cultured beta cells is associated with extracellular signal-regulated kinase 1/2 pathway inhibition: protective role of glucagon-like peptide -1. J Mol Endocrinol. 2008;41:35–44. doi: 10.1677/JME-07-0154. [DOI] [PubMed] [Google Scholar]
- [32].List JF, He H, Habener JF. Glucagon-like peptide-1 receptor and proglucagon expression in mouse skin. Regul Pept. 2006;134:149–157. doi: 10.1016/j.regpep.2006.02.007. [DOI] [PubMed] [Google Scholar]
- [33].Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, Sillje HH. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol. 2010;30:1407–1414. doi: 10.1161/ATVBAHA.110.206425. [DOI] [PubMed] [Google Scholar]
- [34].Erdogdu O, Nathanson D, Sjoholm A, Nystrom T, Zhang Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol. 2010;325:26–35. doi: 10.1016/j.mce.2010.04.022. [DOI] [PubMed] [Google Scholar]
- [35].Degn KB, Brock B, Juhl CB, et al. Effect of intravenous infusion of exenatide (synthetic exendin-4) on glucose-dependent insulin secretion and counterregulation during hypoglycemia. Diabetes. 2004;53:2397–2403. doi: 10.2337/diabetes.53.9.2397. [DOI] [PubMed] [Google Scholar]
- [36].Fernandez J, Valdeolmillos M. Glucose-dependent stimulatory effect of glucagon-like peptide 1(7–36) amide on the electrical activity of pancreatic beta-cells recorded in vivo. Diabetes. 1999;48:754–757. doi: 10.2337/diabetes.48.4.754. [DOI] [PubMed] [Google Scholar]
- [37].Fernyhough P, Gallagher A, Averill SA, et al. Aberrant neurofilament phosphorylation in sensory neurons of rats with diabetic neuropathy. Diabetes. 1999;48:881–889. doi: 10.2337/diabetes.48.4.881. [DOI] [PubMed] [Google Scholar]
- [38].Purves T, Middlemas A, Agthong S, et al. A role for mitogen-activated protein kinases in the etiology of diabetic neuropathy. FASEB J. 2001;15:2508–2514. doi: 10.1096/fj.01-0253hyp. [DOI] [PubMed] [Google Scholar]
- [39].Rubinfeld H, Seger R. The ERK cascade: a prototype of MAPK signaling. Mol Biotechnol. 2005;31:151–174. doi: 10.1385/MB:31:2:151. [DOI] [PubMed] [Google Scholar]
- [40].Calcutt NA, Cooper ME, Kern TS, Schmidt AM. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov. 2009;8:417–429. doi: 10.1038/nrd2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Calcutt NA, Allendoerfer KL, Mizisin AP, et al. Therapeutic efficacy of sonic hedgehog protein in experimental diabetic neuropathy. J Clin Invest. 2003;111:507–514. doi: 10.1172/JCI15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jin HY, Liu WJ, Park JH, Baek HS, Park TS. Effect of dipeptidyl peptidase-IV (DPP-IV) inhibitor (Vildagliptin) on peripheral nerves in streptozotocin-induced diabetic rats. Arch Med Res. 2009;40:536–544. doi: 10.1016/j.arcmed.2009.09.005. [DOI] [PubMed] [Google Scholar]
- [43].Johnson MS, Ryals JM, Wright DE. Early loss of peptidergic intraepidermal nerve fibers in an STZ-induced mouse model of insensate diabetic neuropathy. Pain. 2008;140:35–47. doi: 10.1016/j.pain.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Malmberg AB, Mizisin AP, Calcutt NA, von Stein T, Robbins WR, Bley KR. Reduced heat sensitivity and epidermal nerve fiber immunostaining following single applications of a high-concentration capsaicin patch. Pain. 2004;111:360–367. doi: 10.1016/j.pain.2004.07.017. [DOI] [PubMed] [Google Scholar]
- [45].Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ji RR, Gereau RWt, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Subramaniam S, Unsicker K. ERK and cell death: ERK1/2 in neuronal death. FEBS J. 2010;277:22–29. doi: 10.1111/j.1742-4658.2009.07367.x. [DOI] [PubMed] [Google Scholar]