Abstract
Objective
To characterize infants ≤90 days old enrolled in an international, multi-center, prospective registry of children < 18 years old with acute liver failure (ALF). Study design The Pediatric Acute Liver Failure (PALF) Study Group collects prospective data on children from birth to 18 years. We analyzed data from infants ≤ 90 days enrolled in PALF Study before May 18, 2009.
Results
148 infants were identified in the PALF registry (median age, 18 days). Common ALF etiologies were indeterminate (38%), neonatal hemochromatosis (13.6%) and HSV (12.8%). Spontaneous survival occurred in 60%, 16% underwent liver transplantation and 24% died without liver trsansplantation. Subjects with indeterminate ALF were more likely to undergo liver trsansplantation than those with viral induced ALF (p=0.0002). The cumulative incidence of death without liver trsansplantation was higher in those with viral ALF (64%) compared withneonatal hemochromatosis (16%) or indeterminate ALF (14%), p=0.0007.
Conclusion
ALF in young infants presentsunique diagnostic considerations. Spontaneous survival is better than previously expected. Liver trsansplantation provides an additional option for care.
Keywords: Liver failure, Neonate, Young Infant
Acute liver failure (ALF) is a rare, though frequently fatal, event in pediatrics. For an adult to be classified as having ALF, hepatic encephalopathy within 8 weeks of the development of clinical jaundice, in the absence of a pre-existing liver condition is required. However, hepatic encephalopathy is particularly difficult to assess in children and neonates. Therefore, the Pediatric Acute Liver Failure (PALF) Study Group established entry criteria for enrollment into the registry to include patients with no known underlying liver disease who had evidence of a severe liver-based coagulopathy without encephalopathy(1). The outcome of ALF in childrenhas been suggested to be poor, especially in children younger than one year old. Use of urgent liver transplantation in the setting of ALF has improved the outcome of some of these children(2). Infants less than 3 months old represent a particularly challenging cohort of patients within the spectrum of ALF because of their small size, severity of illness at presentation and primary diagnoses. Previous studies on this unique population have been single center, retrospective studies with small numbers of patients(2-8).
The purpose of this study was to characterize ALF in infants≤ 90 days old using data from an international, multi-center, prospectively collected registry of children less than 18 years of age.
Methods
Data were obtained from the PALF Study Group, a multi-center, prospective study, initiated in 1999, that collects data on children < 18 years old with ALF from 24 participating centers in the United States, Canada, and the United Kingdom. Entry criteria requiredevidence of acute liver injury combined with either severe coagulopathy (International Normalized Ratio (INR) >2.0 or prothrombin time (PT) >20 seconds) or encephalopathy with moderate coagulopathy (INR ≥ 1.5 or PT≥ 15 seconds). Encephalopathy was graded as mild, moderate or severeusing a modified criteria for infants (9). Patients with known chronic liver disease were excluded. The protocol was approved by individualcenter Institutional Review Boards. Data collected by each center weretransmitted to a central data coordinating center where they were subject to data editing and quality control procedures. This study population was limited to infants ≤90 days, enrolled in the PALF Study before May 18, 2009. The study population was compared withsubjects>90 days of age enrolled in the PALF Study with non-acetaminophen (non-APAP) induced ALF. Follow up of patients was available from the time of enrollment into the study until 21 days post-enrollment, transplant, death or hospital discharge, whichever came first.
Statistical Analysis
Data are reported as percentages if categorical, means ± standard error if normally distributed, or median (25 % (Q1) and 75 % (Q3) quartiles) if not normally distributed. Pearson’s chi-square test for association (without a continuity correction) was used to compare the probability of receiving the diagnosis of indeterminate etiology of ALF in this infant cohort, compared with the cohort of subjects > 90 days of age. These analyses were conducted using SAS Statistical Software (SAS Institute, Cary, NC). The cumulative incidence of transplantation and death without transplantation in a competing risk setting was estimated, with p-values used to compare the weighted average of the hazards of cumulative incidence function reported (R program, CRAN website). Multivariate analysis using a generalized estimating equation (GEE) model was applied to determine the relationship between a combination of biochemical parameters (ALT, AST, direct bilirubin, and INR) and etiology of ALF. A p value of p<0.05 was considered to be statistically significant.
Results
Of the 841 patients enrolled in the PALF registry, 148 were ≤ 90 days old at enrollment (57.4 % male and 73.0 % Caucasian). The median age at enrollment was 18 days (1st quartile (Q1)=11 days, 3rd quartile (Q3)= 37 days). The median weight at enrollment was 3.5 (Q1=2.8, Q3= 4.2) kilograms and the median weight for age z score was -1.3 (Q1= -2, Q3= -0.2). The smallest infant weighed 1.7 kilograms (weight for age z score: -3.10) and the largest infant weighed 6.4 kilograms (weight for age z score: 3.32). The most common presenting symptoms were lethargy (49%), fever (20%), and nausea/vomiting (20%). Common physical signs included hepatomegaly (71%), splenomegaly (41%), ascites (39%) and peripheral edema (38%).
A standard diagnostic evaluation revealed elevated aminotransferases, cholestasis and synthetic dysfunction consistent with the diagnosis of ALF. The median ALT of 156 IU/L (Q1= 47, Q3=526) and AST of 215 IU/L (Q1= 84, Q3=1186)were lower in young infants compared with older children with non-APAP induced ALF (median ALT 1616, Q1=615, Q3=3078 and median AST 1778, Q1=629, Q3=3476), p < 0.0001. Sub-group analysis of infants with neonatal hemochromatosis (NH) showed a median ALT of 35 (Q1= 29, Q3=44) and AST of 84 IU/L (Q1= 57, Q3=143), compared with infants with other causes of liver failure, in whom the median ALT was 211 (Q1= 71, Q3=2858) and median AST 289 IU/L (Q1= 94, Q3=1842), p <0.0001. Direct hyperbilirubinemia in young infants with non-NH induced liver failure was less pronounced (median 5.1 mg/dL, Q1= 2.3, Q3= 9.8) than in older children with non-APAP induced liver failure (median 7.6 mg/dL, Q1=2.2, Q3=13.7), p= 0.02. White blood cell count, hemoglobin, arterial and venous ammonia level, and plasma lactate and pyruvate concentration were similar between groups.
Based on the available clinical data, the etiology of ALF was assigned by the local investigator(Table I). Fifty six (38%) patients were classified as having an indeterminate etiology, lower than the indeterminate rate (47%) in patients > 90 days at enrollment, p=0.05.
Table 1.
Etiology of ALF in infants 0-90 days of age.
| Diagnosis | N (total=148) | % |
|---|---|---|
| Metabolic Diseases | 28 | 18.9 |
| Galactosemia | 12 | 8.1 |
| Respiratory Chain Defect | 5 | 3.4 |
| Tyrosinemia | 3 | 2.0 |
| Neiman Pick Type C | 3 | 2.0 |
| Mitochondrial Disorder | 3 | 2.0 |
| Urea Cycle Defect | 1 | 0.7 |
| OTC Deficiency | 1 | 0.7 |
|
| ||
| Neonatal Hemochromatosis | 20 | 13.5 |
|
| ||
| Viral Infections | 24 | 16.2 |
| HSV | 19 | 12.8 |
| Enterovirus | 4 | 2.7 |
| CMV | 1 | 0.7 |
|
| ||
| Other Etiologies | 19 | 12.8 |
| Shock | 6 | 4.1 |
| Hemophagocytic Syndrome | 4 | 2.7 |
| E- Coli Sepsis | 2 | 1.4 |
| Hemangioendothelioma | 2 | 1.4 |
| Acetaminophen | 1 | 0.7 |
| Down’s Syndrome | 1 | 0.7 |
| Leukemia | 1 | 0.7 |
| Intra-ventricular Hemorrhage | 1 | 0.7 |
| Multiple diagnoses | 2 | 1.4 |
|
| ||
| Indeterminate | 56 | 37.8 |
OTC: Ornithine Transcarbamylase Deficiency, HSV: Herpes Simplex Virus, CMV: Cytomegalic Virus
Significant differences in ALT, AST, total and direct bilirubin, GGT, INR, BUN and creatinine were found between different groups, based on ALF etiology(metabolic, viral, NH, indeterminate and other), p<0.05 (Table II). Therefore, the relationship between the aggregate of four biochemical parameters (ALT, AST, direct bilirubin and INR) and ALF etiology was assessed using multivariate analysis ona subset of 69 patients with complete data, using neonatal hemochromatosisas the reference group. There was a strong relationship between the cumulative biochemical profile and the presence of viral induced ALF, with extremely high aminotransferase levels, severe cholestasis and coagulopathy, as compared withNH, (effect size 1.66, 95% CI: 1.05-2.28; p <0.0001). There was a moderate relationship between the cumulative biochemical profile and the presence of metabolic induced ALF, with moderately elevated aminotransferase levels, moderate cholestasis and minimal coagulopathy compared withNH, (effect size 0.24, 95% CI: 0.02-0.46; p= 0.03). There was a moderate relationship between the cumulative biochemical profile and an indeterminate etiology of ALF, with moderately elevated aminotransferase levels, moderate cholestasis and moderate coagulopathy as compared withNH, (effect size 0.29, 95% CI: 0.07-0.52; p= 0.01). White blood cell count, hemoglobin, platelet, arterial and venous ammonia level, plasma lactate and pyruvate concentrationsand albumin were similar between groups.
Table 2.
Laboratory values of infants 0-90 days of age at enrollment in the PALF Study by etiology of acute liver failure.
| Metabolic N=20 | NH=20 | Viral Infection N=24 | Indeterminate N= 26 | Other N=20 | p-value (Wilcoxon) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median (25%, 75%) | N | Median (25%, 75%) | N | Median (25%, 75%) | N | Median (25%, 75%) | N | Median (25%, 75%) | ||
| ALT (IU/L) | 20 | 117 (68, 213) | 16 | 35 (29,44) | 18 | 618 (377,993) | 42 | 109 (41,339) | 15 | 483 (321,1896) | <0.0001 |
| AST (IU/L) | 24 | 159 (72, 290) | 16 | 84 (57,143) | 23 | 2863 (847, 6150) | 52 | 153 (63,677) | 18 | 1066 (244, 2749) | <0.0001 |
| GGT (IU/L) | 24 | 55.5 (37.5, 102) | 15 | 23.0 (15.0, 33.0) | 19 | 110.0 (97.0,143) | 51 | 43.0 (26.0, 80.0) | 12 | 106.0 (82.0, 180.5) | <0.0001 |
| Total Bilirubin (mg/dL) | 27 | 13.9 (7.2, 18.6) | 17 | 12.2 (8.6, 162) | 20 | 7.9 (3.5, 10.0) | 54 | 15.2 (7.6, 19.6) | 19 | 10.6 (3.4, 15.9) | 0.03 |
| Direct Bilirubin (mg/dL) | 22 | 5.1 (2.6, 8.5) | 15 | 3.8 (2.3, 6.3) | 15 | 2.0 (1.1, 4.6) | 39 | 7.5 (2.6, 8.5) | 14 | 5.8 (2.2, 10.0) | 0.004 |
| PT (sec) | 21 | 22.7 (21.7, 24.8) | 16 | 29.1 (23.3,40.5) | 19 | 30.6 (26.0, 42.9) | 43 | 28.0 (21.8, 46.2) | 14 | 25.0 (21.0, 32.5) | 0.03 |
| INR | 20 | 2.5 (2.6,2.8) | 16 | 2.8 (2.0, 3.8) | 24 | 3.9 (2.8, 5.2) | 45 | 2.8 (2.0, 4.0) | 17 | 2.6 (1.8, 3.5) | 0.03 |
| BUN (mg/dL) | 18 | 4.0 (2.0,5.0) | 15 | 4.0 (2.0, 5.0) | 20 | 11.0 (8.5,17.0) | 38 | 7.9 (4.0, 12.0) | 17 | 23.0 (11.0, 30.0) | <0.0001 |
| Creatinine (mg/dL) | 26 | 0.4 (0.3,0.6) | 15 | 0.3 (0.3, 0.5) | 24 | 0.7 (0.4, 1.0) | 53 | 0.4 (0.3, 0.6) | 20 | 0.6 (0.4, 1.1) | 0.004 |
NH: Neonatal Hemochromatosis
Of note, the p values presented in this table represent multiple comparisons between the 5 potential etiologies for acute liver failure.
The outcomes of infants with encephalopathy at entry into the PALF Study were analyzed. Encephalopathy was assessed in 129 patients at enrollment(9). At entry, no encephalopathy was present in 83 (64%) subjects, of whom 67% spontaneously survived, 17% survived with transplant and 16% died without transplant. Mild encephalopathy (grades 1 and 2) was present in 34 subjects, of whom 53% spontaneously survived, 21% survived with transplant and 26% died without transplant. Moderate to severe encephalopathy (grades 3 and 4) was present in 12 subjects, of whom33% spontaneously survived, 9% survived with transplant and 58% died without transplant. Spontaneous survival was highest in those with no or mild encephalopathy, p=0.03. Poor outcomes (death or transplant), however, occurred frequently, even in subjects with no or mild encephalopathy.
The outcomes of infants with ALF based on etiology of liver failure were assessed. Of 148 subjects, 88 (60%) survived without transplant. Of these, 18 (20.5%) had a metabolic disease, 13 (19.8%) had NH, 9 (10.2%) had a viral infection, 34 (38.6%) were indeterminate and 14(15.9%) had other diagnoses. An additional 24 patients underwent liver transplant: 4 with metabolic diseases, 4 with NH, 15 with indeterminate disease and 1 with other disease. Only 41% of young infants with ALF were listed for transplant, compared with 58% of the older non-APAP cohort, p=0.008. Similar to the older cohort, 20 (71%) of 28 infants with metabolic diseases were not listed for transplant. Of 20 infants with NH, only 6 (30%) were not listed for transplant. Of the 24 infants with viral induced ALF, 20 (83.3%) were not listed for transplant. In contrast, in the older ALF cohort, only 41.2% of those with viral induced ALF were not listed for transplant, p=0.001. Similar to the older ALF cohort, 24 (43%) of the 56 infants with an indeterminate etiology of ALF were not listed for transplant. Only 40% of infants in liver failure listed for transplant received an organ by 21 days post-enrollment into the study, as compared with 66% of older patients, p <0.0001. Young infants experienced a mortality rate of 24% (n=36), higher than that of patients > 90 days(10.5%), p=0.001. The etiology of death in these young infants included multi-organ failure (64%), sepsis (8%), pulmonary hemorrhage or edema (8%) and intracranial edema(3%). A competing risk analysis demonstrated age as an important factor in the likelihood of subjects undergoing liver transplantation or dying without liver transplantation.
At up to 21 days post-enrollment, subjects with an indeterminate etiology were most likely to have been transplanted (32%), followed by NH induced ALF(20%), p=0.0002 (Figure 1). Figure 2 provides an analysis of the cumulative incidence of death without transplant at up to 21 days post-enrollment, taking into account the competing risk of transplant. The percentage of patients who died was higher for infants diagnosed with a viral etiology (64%) comparedwiththose with metabolic disease (29%), NH (16%) or indeterminate etiology (14%), p=0.0007. Data on the use of specific therapeutic regimens such as anti-viral medications or anti-oxidant cocktails were unavailable.
Figure 1.
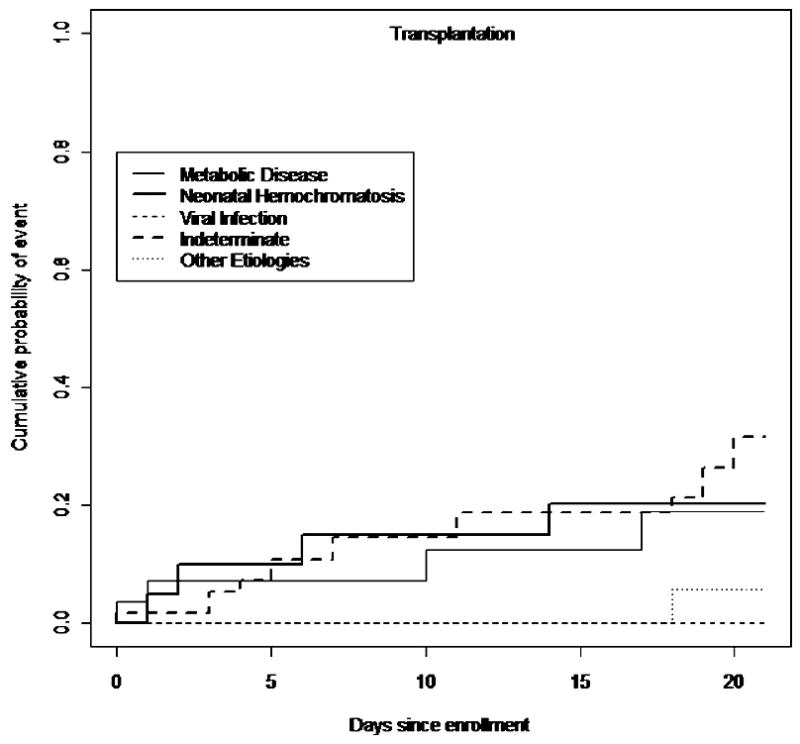
The cumulative incidence of transplantation by etiology in infants 0-90 days of age in the PALF Study.
Figure 2.
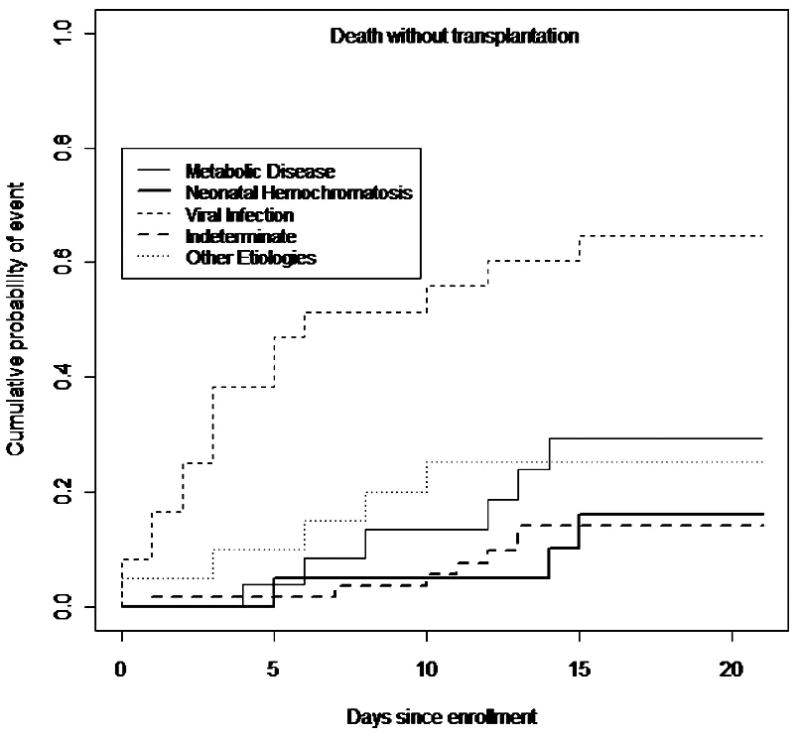
The cumulative incidence of death without transplantation by etiology in infants 0-90 days of age in the PALF Study.
Discussion
This large prospective multicenter study characterizedALF in young infants. As might be expected, the etiology of ALF in young infants was different from older pediatric patients. The most common cause of ALF in young infants was neonatal hemochromatosis, a disease limited to the newborn. Viral infections were also prominent (16%), mainlyherpes. A variety of metabolic diseases, dominated by galactosemia, also caused ALF. In this series, respiratory chain defects were more common than tyrosinemia, typically cited as a common cause of neonatal ALF. Therefore, we suggest the highest priority studies in young infants with ALF focus on diagnosing neonatal hemochromatosis and viral infections, both potentially treatable diseases, and a thorough evaluation for metabolic diseases (10).
The overall biochemical profile of infants presenting with ALF may help further guide this evaluation. Young infants with evidence of liver failure characterized by severe coagulopathy and relatively normal aminotransferase levels, typical for NH, should be screened with a serum alpha feto-protein and serum ferritin (11). Infants with suspected viral induced ALF(high aminotransferase levels and severe coagulopathy), should be tested for enteroviruses, HSV and CMV. Preemptive anti-viral therapy should be considered pending a definitivediagnosis. Infants with a moderate hepatitis and coagulopathy, as seen in metabolic diseases, should be screened for tyrosinemia (urine succinylacetone) and galactosemia (galactose-1-phosphate uridyl transferase (GalPUT) assay)(12). Infants with suspected galactosemia should be placed on a galactose free diet until GalPUT results are available. In addition, screening for respiratory chain defects and other mitochondropathiesis recommended using serum lactate and pyruvate levels.
An indeterminate etiology of ALF occurred in 38% of infants. Some infants classified as indeterminate may have had an incomplete or insufficient evaluation, as suggested by Narkewicz et al (13). Smaller blood volumes may limit diagnostic testing and require prioritization of diagnostic evaluation. The small size and severe coagulopathy present in these infants also limits the ability to perform a liver biopsy. Despite these potential issues, a trend towards fewer infants being labeled as indeterminate ALF compared with the older cohort was observed.
This study focuses on the unique aspects of young infants presenting with evidence of ALFwho should be considered separately in terms of medical decision making. These infants are small for age, which may represent intra-uterine growth retardation as occurs in neonatal hemochromatosis or other metabolic diseases and generally have loweraminotransferase levels than older infants and children(14). Encephalopathy, when present, was primarily mild, althoughit was not recorded in all subjects. Death or transplant, however, occurred in a third of subjects even without encephalopathy. This emphasizes that, particularly in young infants, the presence of encephalopathy is unnecessary for the diagnosis of ALF and does not adequately predict survival (1).
This multi-center experiencethat demonstrates age at presentation in ALFis a clear predictor of outcome. Spontaneous survival in these young infants was 59.5%, higher than the 24% previously reported in children less than 1 of age (2). Spontaneous survival was, however, notably lower than in older children with ALF (79% spontaneous survival rate)(1). The mortality rate of young infants without transplantation in this study was 24%, much lower than 48% cited by Durand et al, but significantly higher than the 10.5% seen in older patients in the ALF cohort(2). In addition, only 40% of young infants withALFwho were listed for transplant in this study received an organ, significantly lower than the 66% transplant rate seen in the older ALF cohort. These differences in outcomes may reflect the severity of illness in these young infants, together with a scarcity of organs appropriate for recipients of this size. There may also be hesitation by transplant teams to proceed with transplantation because of concerns regarding post-operative outcomes, particularly in centers relatively inexperienced in liver transplantation in young infants. Young infants undergoing liver transplant experience prolonged hospitalizations, longer intensive care unit stays and longer ventilation(15). In addition, young infants have high re-operation rates for bleeding, sepsis, wound and biliary complications(15). Nevertheless, young infants undergoing liver transplant have good over overall graft (76.1%) and patient survival (87.8%) at one year, statistically similar to older children(15). Moreover, long term outcome with normal liver function may be good, as suggested by an 80% 5-year survival in a small group of neonates who had undergone liver transplant(16).
Additional factors affecting outcomes in young infants with ALF were also analyzed. Infants with NH induced liver failure had a high probability of undergoing liver transplant. Those who did not undergo transplant, however, had a low probability of death. This likely reflects the broad spectrum of liver injury among infants with NH(17-19). Conversely, young infants with a viral etiology were the most likely to die without liver transplantation and the least likely to receive a liver transplant. Viral induced multi-organ failure often renders these patients poor transplant candidates. The presence of a disseminated viral infection would also be a relative contraindication to the significant immunosuppression required in the immediate post-operative period. Patients with an indeterminate etiology were much more likely than infants with other etiologies to undergo a liver transplant and paradoxically less likely to die without liver transplantation. The high transplant rate in the indeterminate group may reflect the belief that patients with indeterminate liver failure have low spontaneous recovery rates and poor transplant free survival. This high degree of uncertainty regarding outcome prompts a lower threshold for transplant. Yet, the high spontaneous recovery rate and low mortality without transplant suggests that at least some patients who received a liver transplant may have survived without transplantation. Better predictive models of survival or death in patients with an indeterminate etiology are needed, including young infants.
This study has several limitations. Based on the design of the PALF Study, in which liver transplant is an endpoint, follow up on young infants after transplant was unavailable. A previous study, however, has shown that young infants experience graft and patient survival similar to older cohorts of liver transplant recipients, despitehigher post-transplant complication rates(15). In addition, the PALF Study collectedonly 3 weeks of follow up data by design. Therefore, it is possible that some infants with ALF received a liver transplant or died without liver transplant more than 3 weeks after entry in the study. This limitation will be remedied in future studies, as the PALF Study is now collecting 6 and 12 month follow up data.
We conclude that ALF in young infants requires careful attention to unique characteristics of this particular age group. Systematic evaluation of the etiology of ALF, initially prioritized by the clinical and biochemical profile, may help guide treatment decisions. Spontaneous survival, though poor, may be better than previously expected. Liver transplantation provides additional options for care by experienced transplant teams.
Acknowledgments
The authors wish to thank the PALF Study Group for their access to its clinical registry data. The authors are also grateful for support from National Institutes of Health (Patricia R. Robuck PhD-MPH, Director Clinical Trials Program, DDDN-NIDDK) and for assistance from members of the Data Coordinating Center at the University of Pittsburgh (directed by Steven Belle, PhD MScHyg).
PALF Study Group member institutions are supported by the NIH (grants U01-DK 072146-04, ULI RR025014-01, #UL1 RR024131, #UL1 RR024153) and General Clinical Research Centersgrants(#M01 RR 08084 to University of Cincinnati and MO1 RR00069to University of Colorado).
Pediatric Acute Liver Failure Study Group principal and co-investigators include: Baylor College of Medicine (Saul Karpen, MD-PhD, Ruben E. Quiros, MD), Birmingham Children’s Hospital, UK (Dominic Dell Olio, MD, Deirdre Kelly, MD), Children’s Hospital of Pittsburgh (Robert Squires, MD, Ben Schneider, MD), Columbia Presbyterian (Steven Lobritto, MD), Drexel University (Humberto Soriano, MD), Emory University (Rene Romero, MD), Harvard Medical School (Scott Elisofon, MD, Maureen Jonas MD), Hospital for Sick Children, Toronto (Vick Ng, MD), Indiana University Riley Hospital (GirishSubbarao, MD), Johns Hopkins University (Kathleen Schwartz, MD), King’s College Hospital, UK (Anil Dhawan, MD), Mt. Sinai School of Medicine (Nanda Kerkar, MD, SukruEmre, MD), Northwestern University (Estella Alonso, MD), University of Alabama (Brendan M. McGuire, MD), University of California at Los Angeles (Martin G. Martin, MD), University of California, San Diego, (Joel E. Lavine, MD), University of California, San Francisco (Philip Rosenthal, MD), University of Cincinnati (John Bucuvalas, MD, Nada Yazigi MD), University of Colorado Denver (Michael Narkewicz, MD), University of Michigan (M. James Lopez, MD-PhD), University of Nebraska (Simon Horslen, MD), University of Pennsylvania (Liz Rand MD), University of Southern California (Daniel W. Thomas, MD), University of Texas Southwestern (Norberto Rodriguez-Baez, MD), University of Washington (Seattle, Karen Murray, MD), and Washington University (David Rudnick, MD-PhD, Ross Shepherd, MD).
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Squires RH, Jr, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148:652–8. doi: 10.1016/j.jpeds.2005.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durand P, Debray D, Mandel R, Baujard C, Branchereau S, Gauthier F, et al. Acute liver failure in infancy: a 14-year experience of a pediatric liver transplantation center. J Pediatr. 2001;139:871–6. doi: 10.1067/mpd.2001.119989. [DOI] [PubMed] [Google Scholar]
- 3.Woodle ES, Millis JM, So SK, McDiarmid SV, Busuttil RW, Esquivel CO, et al. Liver transplantation in the first three months of life. Transplantation. 1998;66:606–9. doi: 10.1097/00007890-199809150-00010. [DOI] [PubMed] [Google Scholar]
- 4.Bonatti H, Muiesan P, Connelly S, Baker A, Mieli-Vergani G, Gibbs P, et al. Hepatic transplantation in children under 3 months of age: a single centre’s experience. Journal of pediatric surgery. 1997;32:486–8. doi: 10.1016/s0022-3468(97)90612-6. [DOI] [PubMed] [Google Scholar]
- 5.Noujaim HM, Mayer DA, Buckles JA, Beath SV, Kelly DA, McKiernan PJ, et al. Techniques for and outcome of liver transplantation in neonates and infants weighing up to 5 kilograms. Journal of pediatric surgery. 2002;37:159–64. doi: 10.1053/jpsu.2002.30242. [DOI] [PubMed] [Google Scholar]
- 6.Lund DP, Lillehei CW, Kevy S, Perez-Atayde A, Maller E, Treacy S, et al. Liver transplantation in newborn liver failure: treatment for neonatal hemochromatosis. Transplant Proc. 1993;25:1068–71. [PubMed] [Google Scholar]
- 7.Srinivasan P, Vilca-Melendez H, Muiesan P, Prachalias A, Heaton ND, Rela M. Liver transplantation with monosegments. Surgery. 1999;126:10–2. doi: 10.1067/msy.1999.98686. [DOI] [PubMed] [Google Scholar]
- 8.Sundaram SS, Alonso EM, Whitington PF. Liver transplantation in neonates. Liver Transpl. 2003;9:783–8. doi: 10.1053/jlts.2003.50104. [DOI] [PubMed] [Google Scholar]
- 9.Alonso E, Squires RH, Whitington PF. Acute Liver Failure in Children. In: Suchy FJ, S R, Balistreri WF, editors. Liver Disease in Children. 3. Cambridge: Cambridge University Press; 2007. pp. 71–96. [Google Scholar]
- 10.Lee WS, Sokol RJ. Liver disease in mitochondrial disorders. Semin Liver Dis. 2007;27:259–73. doi: 10.1055/s-2007-985071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitington PF. Fetal and infantile hemochromatosis. Hepatology. 2006;43:654–60. doi: 10.1002/hep.21129. [DOI] [PubMed] [Google Scholar]
- 12.Xu YK, Kaufman FR, Donnell GN, Ng WG. Radiochemical assay of minute quantities of galactose-1-phosphate uridyltransferase activity in erythrocytes and leukocytes of galactosemia patients. Clin Chim Acta. 1995;235:125–36. doi: 10.1016/0009-8981(95)06013-x. [DOI] [PubMed] [Google Scholar]
- 13.Narkewicz MR, Dell Olio D, Karpen SJ, Murray KF, Schwarz K, Yazigi N, et al. Pattern of diagnostic evaluation for the causes of pediatric acute liver failure: an opportunity for quality improvement. J Pediatr. 2009;155:801–6 e1. doi: 10.1016/j.jpeds.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitington PF. Neonatal hemochromatosis: a congenital alloimmune hepatitis. Semin Liver Dis. 2007;27:243–50. doi: 10.1055/s-2007-985069. [DOI] [PubMed] [Google Scholar]
- 15.Sundaram SS, Alonso EM, Anand R. Outcomes after liver transplantation in young infants. J Pediatr Gastroenterol Nutr. 2008;47:486–92. doi: 10.1097/MPG.0b013e318175d7d2. [DOI] [PubMed] [Google Scholar]
- 16.Grabhorn E, Richter A, Fischer L, Ganschow R. Emergency liver transplantation in neonates with acute liver failure: long-term follow-up. Transplantation. 2008;86:932–6. doi: 10.1097/TP.0b013e318186d64a. [DOI] [PubMed] [Google Scholar]
- 17.Ekong UD, Kelly S, Whitington PF. Disparate clinical presentation of neonatal hemochromatosis in twins. Pediatrics. 2005;116:e880–4. doi: 10.1542/peds.2005-0784. [DOI] [PubMed] [Google Scholar]
- 18.Ekong UD, Melin-Aldana H, Whitington PF. Regression of severe fibrotic liver disease in 2 children with neonatal hemochromatosis. J Pediatr Gastroenterol Nutr. 2008;46:329–33. doi: 10.1097/MPG.0b013e318046772f. [DOI] [PubMed] [Google Scholar]
- 19.Whitington PF, Kelly S, Ekong UD. Neonatal hemochromatosis: fetal liver disease leading to liver failure in the fetus and newborn. Pediatric transplantation. 2005;9:640–5. doi: 10.1111/j.1399-3046.2005.00357.x. [DOI] [PubMed] [Google Scholar]


