Abstract
Introduction and Objectives
The Life-space assessment can be used to measure a patient's level of mobility. This study evaluated the relationship between Life-space mobility and frequency of hospitalization in the previous year and other clinical markers of health among adults with cystic fibrosis (CF).
Methods
The Life-space assessment was administered to ambulatory adults with CF in clinic or by telephone. Life-space mobility was correlated with the most recent FEV1 % predicted, body mass index (BMI) and number of hospitalizations in the previous year.
Results
Forty-eight subjects completed the Life-space assessment. Subjects had a Life-space score of 88 ± 26, FEV1 % predicted of 66% ± 26%, and BMI of 22.5 ± 3.3. There was a statistically significant negative linear correlation between the number of times a subject was hospitalized in the previous year and Life-space mobility (p = .001, R2 = .20). This association was independent of FEV1 % predicted and BMI.
Conclusion
The Life-space mobility score is associated with frequency of hospitalization in adults with CF. A prospective study should be undertaken to assess the ability of the Life-space assessment to predict hospitalization and other outcomes in adults with CF.
Keywords: cystic fibrosis, life space mobility, quality of life
Introduction
Cystic fibrosis (CF) is the most common fatal genetic disease among Caucasians in the United States.1 Advances in CF care have dramatically increased the life expectancy of patients with CF, but have not prevented the development over time of CF-associated conditions and diseases such as malnutrition, diabetes, and osteoporosis.2, 3 Many patients require hospitalization one or more times per year primarily for pulmonary exacerbations related to decline in lung function and/or infection.4, 5 In addition to the expense, inconvenience, and risk of acquiring infections during hospitalization, recent research has shown that damage sustained during an exacerbation causes a long-term decline in lung function.6 It is therefore crucial to be able to predict and mitigate pulmonary exacerbations and other causes of hospitalization.
Several physiological metrics including forced expiratory volume in one second as a percent of the predicted value (FEV1 % predicted) and body mass index (BMI) have been used to monitor disease progression and the need for hospitalization in CF patients.5 In addition to these objective measures, patient-reported outcomes, primarily in the form of disease-specific quality of life measures such as the Cystic Fibrosis Questionnaire – Revised (CFQ-R),7 CF Respiratory Diary,8 Cystic Fibrosis Quality of Life (CFQoL) questionnaire,9 and Questions on Life Satisfaction,10 have gained acceptance as supplemental outcome measures in CF. Data from these questionnaires have been shown to correlate with physiological measures, such as FEV1 % predicted, and also address functional and social status.11
An alternative way of gathering simple patient-reported information about health status and risk of adverse outcomes may be to use self-reports of physical activity or function related to specific tasks such as activities of daily living (ADLs). One study utilizing an assessment of physical activity demonstrated that female adolescents with CF who were more active had lower rates of decline in lung function compared with less active female adolescents with CF.12 Decreased physical activity and functional status may also be an early sign of decreasing lung function, as patients with a low FEV1 % predicted might not be capable of maintaining an active lifestyle. Improved physical activity and exercise have been demonstrated to slow the rate of decline in lung function in patients with CF.13 Other benefits of increased physical activity and exercise include improved airway clearance, muscle function, and quality of life.13
Assessing activity levels in CF patients may be difficult in clinical practice, and better tools need to be developed to assess physical activity in the CF population. The Life-space assessment is a simple five part mobility questionnaire that has been shown to be associated with various health characteristics in the geriatric population, including physical performance, cognitive abilities, mental health, and the rate of recovery after surgical and nonsurgical hospitalizations. Surveys of quality of life, depression, and activities of daily living have all been shown to correlate with the Life-space mobility score in this group. Additionally, the Life-space score has been shown to have excellent test-retest reliability within two weeks of face-to-face assessments.14-19 The objective of this study was to evaluate the use of the Life-Space score as a measure of health for adults with CF and to determine how well the Life-space score correlates with clinical outcomes including frequency of hospitalization. We hypothesized that those patients who are more mobile as reflected in higher Life-space scores would have better lung function and would have been hospitalized less frequently.
Methods
Participants
Participants were recruited from the Emory University Adult Cystic Fibrosis center. A list of patients was compiled from the clinic appointment schedule beginning with January 2009. Subjects were consented by telephone or in-person interview for participation in this study between May and October 2009. Subjects were included in this study if they had cystic fibrosis and were at least 18 years of age.
Study Protocol
The study was approved by the Emory human studies committee (IRB). Subjects who met study criteria completed the Life-space mobility assessment (detailed in next section) administered by one of two researchers (EG or ES). The following information was collected from each participant's medical record: subject demographics, medical history, most recent FEV1 % predicted, most recent BMI, and number of hospitalizations in the 12 months preceding the interview date.
Life-Space Mobility Assessment
The survey asked subjects how frequently they reached different zones extending outwards from the bedroom, such as going to other rooms in the home and leaving the home, and a score was calculated based on their responses. Each zone was assigned a value based on distance (leaving the bedroom was level 1, leaving the home was level 2, etc.). For each level the subject reported to have reached, the value for that level was multiplied by the frequency reported, from 1 (less than once a week) to 4 (daily), as well as by a factor for the level of assistance needed: subjects were assigned a score of 1 for that level if they needed personal assistance, 1.5 if they needed mobility-related equipment only, and 2 if they did not require any assistance. The scores for all the levels were summed to calculate a total score out of a possible 120 points. A higher Life-space score indicates greater mobility within and away from the home. An in-depth description of the Life-space assessment can be found in Peel, et. al., 2005.14
Statistical Methods
Single and multivariate linear regressions were performed comparing the number of hospitalizations in the year preceding the interview date with the Life-space mobility score. Covariates included FEV1 % predicted, BMI, and Life-space score. ANOVA tests were performed to test for the effects of potentially interfering factors, such as hospitalizations during the four week period measured in the survey, as well as population differences between patients interviewed by phone and those interviewed during clinic visits. All statistical analyses were performed using Microsoft Excel 2007 (Redmond, WA).
Results
Study Subjects
The subject demographics are presented in Table 1. Twenty-two subjects were interviewed by phone and 26 were interviewed during outpatient clinic visits. The 48 subjects enrolled were a mean age of 29 ± 8 years of age, with the majority self identified as white ethnicity (94%), and had a mean FEV1 % predicted of 66% ± 26%. The majority of subjects fell in the mild lung obstruction category (FEV1 % predicted > 69%). There were no statistical differences in age, FEV1 % predicted, or number of hospitalizations between patients who were interviewed over the phone and in clinic. Patients who were interviewed in clinic had a statistically significantly lower BMI (p = .003).
Table 1.
Subject Demographics
| Total | 48 |
| Age (years) | 29 ± 8 |
| Race | |
| White | 45 (94%) |
| Black | 3 (6%) |
| Sex | |
| Male | 25 (52%) |
| Female | 23 (48%) |
| FEV1 % Predicted | 66% ± 26% |
| Severity of Lung Disease by FEV1 % | |
| Predicted (number of patients) | |
| Very Severe (<35%) | 8 (17%) |
| Moderate to Severe (35%-69%) | 13 (27%) |
| Mild (>69%) | 27 (56%) |
| Life-Space Mobility Score | 88 ± 26 |
| BMI (kg/m2) | 22.5 ± 3.3 |
| Number of Hospitalizations in past year | 1.1 ± 1.3 |
All subjects had a mean Life-space score of 88 ± 26 out of 120 (Table 1). Life-space scores and numbers of hospitalizations by sub-groups of sex, severity of lung obstruction, age, and BMI are presented in Table 2. As expected, the mean Life-space score was statistically significantly lower in those subjects with lower FEV1 % predicted (p = 0.03). Similarly, the mean number of hospitalizations was statistically significantly higher in subjects with lower FEV1 % predicted (p = 0.0005). Subjects with a higher BMI had a statistically significantly lower number of hospitalizations (p = 0.01). In contrast, there was not a significant association between the Life-space score and BMI (p = 1.0).
Table 2.
Life-space mobility score and number of hospitalizations in the past year by sub-category
| Life- Space Score |
P-value (ANOVA) |
Number of Hospitalizations |
P-Value (ANOVA) |
|
|---|---|---|---|---|
| Total | 88 ± 26 | 1.1 ± 1.3 | ||
| Sex | ||||
| Male | 88 ± 22 | 0.8 | 1.1 ± 1.2 | 0.8 |
| Female | 87 ± 31 | 1.2 ± 1.4 | ||
| Severity of Lung Obstruction by | ||||
| FEV1 % Predicted | ||||
| Very Severe (<35%) | 67 ± 41 | 0.03* | 2.4 ± 1.7 | 0.0005* |
| Moderate to Severe (35%-69%) | 86 ± 22 | 1.5 ± 1.0 | ||
| Mild (>69%) | 95 ± 20 | 0.6 ± 0.9 | ||
| Age | ||||
| First tertile (18-24) | 93 ± 27 | 0.6 | 1.2 ± 1.6 | 0.3 |
| Second tertile (25-31) | 83 ± 26 | 1.4 ± .9 | ||
| Third Tertile (32-46) | 86 ± 26 | 0.8 ± 1.1 | ||
| BMI | ||||
| First tertile (16.1-20.6) | 88 ± 31 | 1.0 | 1.7 ± 1.4 | 0.01* |
| Second Tertile (20.7-23.9) | 87 ± 25 | 1.3 ± 1.1 | ||
| Third Tertile (24.0-28.6) | 88 ± 24 | 0.4 ± 0.9 |
Relationship between Life-Space and Hospitalizations
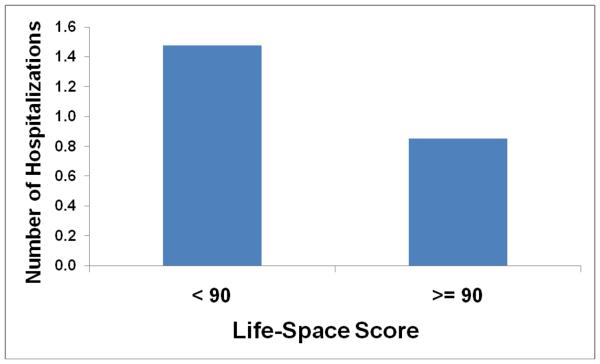
There was a statistically significant negative linear correlation between the number of times a subject was hospitalized in the previous year and his or her Life-space score (p = .001, R2 = .20) (Table 3). There was also a significant correlation comparing the number of hospitalizations to the Life-space score after controlling for FEV1 % predicted and BMI (p = 0.021, Table 3). The mean numbers of hospitalizations for subjects with high and low Life-space scores respectively are shown in Figure 1.
Table 3.
Regressions comparing number of hospitalizations to life-space score, FEV1 % Predicted, and BMI
| Variable | Regression Coefficient |
SE | P |
|---|---|---|---|
| Life-Space | −0.022 | 0.006 | 0.001 |
| R2 = 0.20 | |||
| Covariates |
Regression Coefficient |
SE | P |
|
| |||
| Life-Space | −0.014 | 0.006 | 0.021 |
| FEV1 % Predicted | −1.694 | 0.691 | 0.018 |
| BMI | −0.104 | 0.051 | 0.050 |
| R2 = 0.47 | |||
Figure 1.
Mean number of hospitalizations within the past year by life-space mobility score
Discussion
In this study, we sought to evaluate the Life-space mobility score as a new tool to objectively measure the health status of adults with CF. We found a significant negative correlation between the frequency of hospitalization of adults with CF and the Life-space score. The Life-space score was significantly associated with the number of hospitalizations after adjusting for BMI and FEV1 % predicted as covariates. This suggests that the Life-space score provides an additional measure of health status in CF patients independent of FEV1 % predicted and BMI to assess the risk of adverse outcomes requiring hospitalization.
Earlier detection of respiratory decline in CF patients is critical for preventing further declines in respiratory function and mortality. FEV1 % predicted has been a useful surrogate marker of health, and is strongly associated with pulmonary exacerbations5 and mortality.20 Body mass index (BMI) is another useful tool in predicting decline in pulmonary function in CF patients. BMI was significant in our multivariate regression (p = .05), but less so than FEV1 % predicted. This is consistent with previous studies demonstrating an association between lower BMI and FEV1 % predicted.5, 21 Other measures that have been shown to predict the frequency of exacerbations include age, courses of IV antibiotics, and certain medications taken.5
The Life-Space Mobility Score in Clinical Practice
Other questionnaires have been used to assess the health of CF patients.22 These include the Sickness Impact Profile (SIP),23 the Nottingham Health Profile (NHP),24 and the Short-Form Health Survey (SF-36).25 More recently, the Cystic Fibrosis Quality of Life (CFQoL) questionnaire and several other similar surveys have been developed to address issues specific to CF care.9 The CFQoL, for example, asks patients to respond to 52 statements regarding psychosocial implications of the disease. Examples of these statements are, “For the last two weeks, CF had made it difficult to move from my bed to my chair” and “I worry about CF shortening my life.”9 Like the Life-space score, such QOL surveys provide a unique patient perspective. Data from these surveys have also been shown to correlate with FEV1 % predicted, just as the Life-space score does.11 However, their short-term predictive value is diluted by more subjective questions about emotional wellbeing and broader impact of the disease and associated therapy. These surveys are also relatively long and time-consuming for the patient and clinician to complete. The Life-space mobility assessment, by contrast, takes just a few minutes to complete, and because the questions are based on a subject's mobility (as measured by the zones extending outwards from the bedroom that he or she has reached), the information it provides is more objective and more clearly linked to the risk of a pulmonary exacerbation.
The Life-space score might also be used by the clinician to initiate a discussion with the patient about his or her health. Treatment of CF is a complex, lifelong process that requires the active participation of the patient on a daily basis in order to reduce the frequency of exacerbations.26 Non-adherence is common for reasons including expense, discomfort, lack of understanding, and interference with daily life. Patients also fail to complete their treatments as a mechanism of denial of the severity of the illness.27 Nonetheless, adherence is better when patients understand and can perceive the benefits of the treatment.28 To this end, the Life-space score will simply and clearly demonstrate disease severity by showing how it is affecting the patient's mobility, and do so only with information that he or she has provided and can relate to. This might be a more relevant indicator to the patient than abstract physiological measures, thereby encouraging him or her to adhere to the prescribed treatment regimen.
Limitations and Next Steps
One limitation of this study is that it was not longitudinal and we did not test the Life-space score's capacity for predicting future hospitalizations. We also used a limited number of covariates, and it is possible that there are other explanations for a patient's frequency of hospitalization, such as socioeconomic status, which has been shown to affect outcomes in CF.7 Other possible confounding variables are 25-hydroxyvitamin D levels, which had not been tested in many of the subjects in this study, pancreatic enzyme insufficiency, and other measures of nutrition.21
A potential source of bias in our study is that we were not able to interview four patients because they were in the hospital when we attempted to contact them by phone. Additionally, the hospitalization period itself might reduce a subject's Life-space score. Ten out of 48 subjects in our sample had been hospitalized in the month prior to the interview, and although they were asked about activity in the past month, there was not a statistically significant impact on their Life-space scores. Further research should be undertaken to confirm the Life-space score's relevance to CF and refine the survey questions for this population. Its effectiveness should also be tested in children, who were not included in this study, but comprise more than 50% of the CF patient population.29 It would likely be less relevant for them, however, because children have less control than adults over their mobility away from the home, as this is more related to their parents' lifestyle and decisions about where they spend their time. Finally, while the test-retest reliability of the Life-space mobility assessment has been validated, future studies in the CF population should incorporate the mobility of family members as well.
Conclusion
The Life-space assessment is a simple survey used to measure mobility. It has been validated in the geriatric population, and is associated with standard indicators of health in patients with CF as well. It is objective to the extent that the patient objectively reports his or her mobility, and it may provide additional insight into the relationship between a patient's lifestyle and his or her health. A future prospective study should assess the capability of the life-space mobility score to predict health outcomes of patients with CF.
Acknowledgements
The authors would like to acknowledge Stephen Gottlieb MD and Joshua Gottlieb for their helpful feedback, Welela Berhanu RN, BSN for coordinating in-clinic interviews, and the staff of the Emory University Adult Cystic Fibrosis center for their cooperation. The Life-Space mobility assessment was developed by the University of Alabama at Birmingham Center for Aging with funding from the National Institute on Aging (Grant # NIA AG15062). Dr. Tangpricha is supported by the CF Foundation and NIH # K23 AR054334.
Footnotes
All of the authors do not have a conflict of interest to declare
References
- 1.Accurso FJ. Update in cystic fibrosis 2006. Am J Respir Crit Care Med. 2007;175:754–7. doi: 10.1164/rccm.200701-160UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belkin RA, Henig NR, Singer LG, et al. Risk factors for death of patients with cystic fibrosis awaiting lung transplantation. Am J Respir Crit Care Med. 2006;173:659–66. doi: 10.1164/rccm.200410-1369OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liou T, Adler F, FitzSimmons S, Cahill B, Hibbs J, Marshall B. Predictive 5-year survivorship model of cystic fibrosis. American journal of epidemiology. 2001;153:345. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zemanick ET, Harris JK, Conway S, et al. Measuring and improving respiratory outcomes in cystic fibrosis lung disease: Opportunities and challenges to therapy. J Cyst Fibros. 2009 doi: 10.1016/j.jcf.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block JK, Vandemheen KL, Tullis E, et al. Predictors of pulmonary exacerbations in patients with cystic fibrosis infected with multi-resistant bacteria. Thorax. 2006;61:969–74. doi: 10.1136/thx.2006.061366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amadori A, Antonelli A, Balteri I, Schreiber A, Bugiani M, De Rose V. Recurrent exacerbations affect FEV(1) decline in adult patients with cystic fibrosis. Respir Med. 2009;103:407–13. doi: 10.1016/j.rmed.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Quittner AL, Butt A, Messer MA, Modi AC, Watrous M. Development and validation of the cystic fibrosis questionnaire in the United States - A health-related quality-of-life measure for cystic fibrosis. Chest. 2005;128:2347–54. doi: 10.1378/chest.128.4.2347. [DOI] [PubMed] [Google Scholar]
- 8.Goss CH, Edwards TC, Ramsey BW, Aitken ML, Patrick DL. Patient-reported respiratory symptoms in cystic fibrosis. Journal of Cystic Fibrosis. 2009;8:245–52. doi: 10.1016/j.jcf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Gee L, Abbott J, Conway SP, Etherington C, Webb AK. Development of a disease specific health related quality of life measure for adults and adolescents with cystic fibrosis. Thorax. 2000;55:946–54. doi: 10.1136/thorax.55.11.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldbeck L, Schmitz TG, Heinrich G, Herschbach P. Questions on life satisfaction for adolescents and adults with cystic fibrosis - Development of a disease-specific questionnaire. Chest. 2003;123:42–8. doi: 10.1378/chest.123.1.42. [DOI] [PubMed] [Google Scholar]
- 11.Orenstein DM, Nixon PA, Ross EA, Kaplan RM. The Quality of Well-Being in Cystic-Fibrosis. Chest. 1989;95:344–7. doi: 10.1378/chest.95.2.344. [DOI] [PubMed] [Google Scholar]
- 12.Schneiderman-Walker J, Wilkes DL, Strug L, et al. Sex Differences in Habitual Physical Activity and Lung Function Decline in Children with Cystic Fibrosis. The Journal of Pediatrics. 2005;147:321–6. doi: 10.1016/j.jpeds.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 13.Wilkes DL, Schneiderman JE, Nguyen T, et al. Exercise and physical activity in children with cystic fibrosis. Paediatric Respiratory Reviews. 2009;10:105–9. doi: 10.1016/j.prrv.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Peel C, Baker P, Roth D, Brown C, Bodner E, Allman R. Assessing mobility in older adults: the UAB Study of Aging Life-Space Assessment. Physical Therapy. 2005;85:1008. [PubMed] [Google Scholar]
- 15.Brown CJ, Roth DL, Allman RM, Sawyer P, Ritchie CS, Roseman JM. Trajectories of Life-Space Mobility After Hospitalization. Ann Intern Med. 2009;150:372–W67. doi: 10.7326/0003-4819-150-6-200903170-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowe M, Andel R, Wadley VG, Okonkwo OC, Sawyer P, Allman RM. Life-space and cognitive decline in a community-based sample of African American and Caucasian older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1241–5. doi: 10.1093/gerona/63.11.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker P, Bodner E, Allman R. Measuring life-space mobility in community-dwelling older adults. Journal of the American Geriatrics Society. 2003;51:1610–4. doi: 10.1046/j.1532-5415.2003.51512.x. [DOI] [PubMed] [Google Scholar]
- 18.Allman R, Baker P, Maisiak R, Sims R, Roseman J. Racial similarities and differences in predictors of mobility change over eighteen months. Journal of general internal medicine. 2004;19:1118–26. doi: 10.1111/j.1525-1497.2004.30239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allman R, Sawyer P, Roseman J. The UAB Study of Aging: background and insights into life-space mobility among older Americans in rural and urban settings. Aging Health. 2006;2:417–29. [Google Scholar]
- 20.Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. N Engl J Med. 1992;326:1187–91. doi: 10.1056/NEJM199204303261804. [DOI] [PubMed] [Google Scholar]
- 21.Steinkamp G, Wiedemann B. Relationship between nutritional status and lung function in cystic fibrosis: cross sectional and longitudinal analyses from the German CF quality assurance (CFQA) project. Brit Med J. 2002;57:596. doi: 10.1136/thorax.57.7.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbott J, Webb K, Dodd M. Quality of life in cystic fibrosis. J R Soc Med. 1997;90(Suppl 31):37–42. doi: 10.1177/014107689709031s08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilson BS, Gilson JS, Bergner M, et al. Sickness Impact Profile - Development of an Outcome Measure of Health-Care. Am J Public Health. 1975;65:1304–10. doi: 10.2105/ajph.65.12.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt SM, Mckenna SP, Mcewen J, Williams J, Papp E. The Nottingham Health Profile - Subjective Health-Status and Medical Consultations. Soc Sci Med-Med Soc. 1981;15:221–9. doi: 10.1016/0271-7123(81)90005-5. [DOI] [PubMed] [Google Scholar]
- 25.Brazier JE, Harper R, Jones NMB, et al. Validating the Sf-36 Health Survey Questionnaire - New Outcome Measure for Primary Care. Brit Med J. 1992;305:160–4. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell SC, Robinson PJ. Exacerbations in cystic fibrosis: 2 . prevention. Thorax. 2007;62:723–32. doi: 10.1136/thx.2006.060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeffer PE, Pfeffer JM, Hodson ME. The psychosocial and psychiatric side of cystic fibrosis in adolescents and adults. J Cyst Fibros. 2003;2:61–8. doi: 10.1016/S1569-1993(03)00020-1. [DOI] [PubMed] [Google Scholar]
- 28.Abbott J, Dodd M, Bilton D, Webb AK. Treatment compliance in adults with cystic fibrosis. Thorax. 1994;49:115–20. doi: 10.1136/thx.49.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cystic Fibrosis Foundation . Patient Registry 2008 Annual Data Report. CF Foundation; 2009. [Google Scholar]