Abstract
Type 1 diabetes (T1D) is a chronic autoimmune disease that results in the specific immune destruction of insulin producing beta cells. Currently there is no cure for T1D and treatment for the disease consists of lifelong administration of insulin. Immunotherapies aimed at preventing beta cell destruction in T1D patients with residual c-peptide or in individuals developing T1D are being evaluated. Networks of researchers such as TrialNet and the Immune Tolerance Network in the U.S. and similar networks in Europe have been established to evaluate such immunotherapies. This review focuses on immune intervention for the prevention and amelioration of human T1D with a focus on potential immune suppressive, antigen specific and environmental therapies.
Keywords: Type 1 diabetes, autoantibodies, immunotherapy, T cells, tolerance
1. Introduction
Type 1A diabetes, the immune mediated form of diabetes, is a chronic autoimmune disease in which there is specific immune destruction of the insulin producing pancreatic β-cells [1]. Autoreactive T cells as well as other mononuclear cells infiltrate the islets (insulitis) and ultimately cause β-cell death, decreased insulin production, and a lifelong requirement for insulin therapy [2]. Despite treatment with insulin therapy long-term complications, including nephropathy, retinopathy, neuropathy, and cardiovascular disease, can result [3,4]. Type 1 diabetes (T1D) is believed to develop as a result of genetic predisposition (in particular with human leukocyte antigen (HLA) alleles contributing to disease risk) and unknown environmental factors. In terms of environmental factors, the incidence of T1D is increasing dramatically, doubling every 20 years especially in young children less than 5 years of age [5,6].
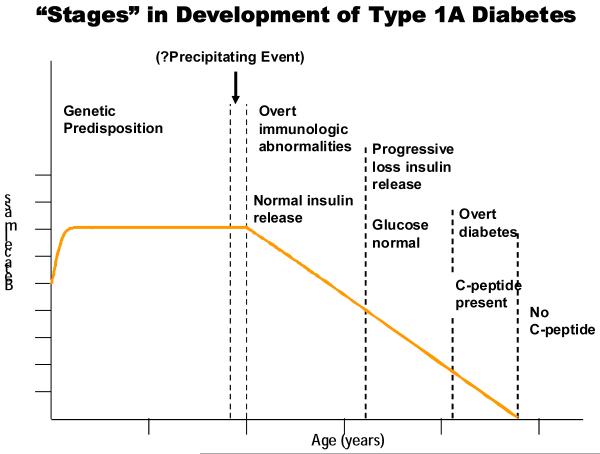
In a genetically susceptible individual the development of T1D can be divided into stages as depicted in figure 1 [7]. The presence of autoantibodies against islet cell antigens is the first indication for the development of diabetes and at the time autoantibodies appear individuals retain sufficient β-cell mass to maintain euglycemia. Following autoantibody development there is progressive loss of insulin release as the autoimmune response progresses [8]. During later stages patients progressively develop impaired glucose tolerance[9,10]. In the final stages of development, decreased c-peptide levels are present in patients with overt hyperglycemia. T1D is a predictable disease given multiple islet autoantibody positivity. There are currently four standard autoantibodies whose presence is used to predict the development of T1D: antibodies against insulin, glutamic acid decarboxylase (GAD65), a tyrosine phosphatase-like protein (ICA512 also termed IA-2), and the zinc T8 transporter (ZnT8) [11]. Relatives of patients with T1D who are positive for two or more autoantibodies have a greater than 70% risk of developing diabetes over a 7 year observation period [12], and this holds true for the general population as well [13].
Figure 1.
Hypothetical stages and loss of beta cells in an individual progressing to type 1A diabetes. Reproduced with permission from Eisenbarth, GS. From www.barbardaviscenter.org
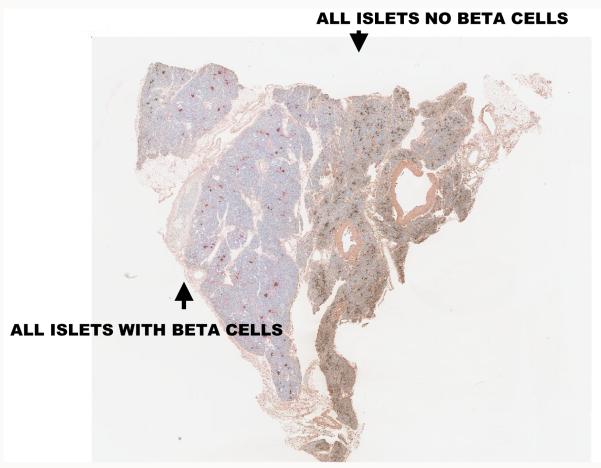
While the progress to complete insulin dependence in most patients (but not all) occurs quickly after clinical onset, initially after diagnosis the pancreas is able to produce a significant amount of insulin [14-17]. The Diabetes Control and Complications Trial (DCCT) found that 20% of patients studied, who were within 5 years of diagnosis, had remaining insulin production [18]; at this time immunologic intervention can potentially save beta cell function and reduce reliance on insulin administration. Even partial beta cell function is beneficial as patients that maintain endogenous insulin production have better metabolic control than those who rely solely on exogenous insulin [15], and improved metabolic control reduces the long-term complications from diabetes [19] and prevents hypoglycemia. Evaluation of the pancreas of patients with long-term T1D indicates that most patients retain some islet beta cells (approximately 1-2%) while some patients even with Type 1A (immune mediated) diabetes have significant beta cell mass[20]. Those patients (and presumably prediabetic autoantibody positive individuals) have lobular destruction of islet beta cells with areas with “normal appearing” islets interspersed with areas where all islets contain only non-beta cells (termed pseudoatrophic islets) [21,22]. Figure 2 illustrates a section of pancreas from the juvenile diabetes research foundation’s network for pancreatic organ donors with diabetes (nPOD) cadaveric program where one side of the slide has normal appearing islets and right next to those islets is a lobule in which no islet has insulin positive cells. There is minimal insulitis/inflammation (in contrast to the NOD mouse model) in the great majority of pancreases from patients after or at the onset of T1D [23]. A number of investigators are attempting to develop imaging modalities to define either beta cell mass or insulitis [24-26]. Though in animal models there has been some success, it is not yet clear if current technologies have the resolution necessary to follow the disease process in man. A recent report utilizing iron nanoparticles and magnetic resonance imaging, highlights loss of overall pancreatic mass (presumably due to atrophy of the acinar cells secondary to loss of trophic factors from beta cells that make up approximately 1% of normal pancreatic mass) as well as increased retention of the nanoparticles [26].
Figure 2.
Pancreatic section from a type 1 diabetic pancreatic organ donor showing lobular destruction of insulin producing beta cells in islets. On the left, there are islets with beta cells stained for insulin while the islets on the right lack beta cells and insulin staining. The histology section comes from the JDRF nPOD program, www.jdrfnpod.org.
2. Immune suppressive therapies for the treatment of type 1 diabetes
The first large-scale immunosuppressive trial in recent onset T1D studied cyclosporine A in the mid-1980s[27]. Continuous treatment reduced the need for exogenous insulin; however renal toxicity necessitated cessation of therapy [28,29]. After stopping treatment, endogenous insulin production diminished indicating immunologic tolerance to islet antigens was not induced. During the same time period, a pilot study administered horse antithymocyte globulin (ATG) and prednisone to new onset T1D individuals. Again, there was a reduction in insulin requirements; however, toxicity in the form of serum sickness and thrombocytopenia led to a discontinuation of ATG [30]. A phase II trial using rabbit ATG in new onset T1D is currently ongoing through the Immune Tolerance Network to evaluate the ability of this therapy to slow beta cell loss.
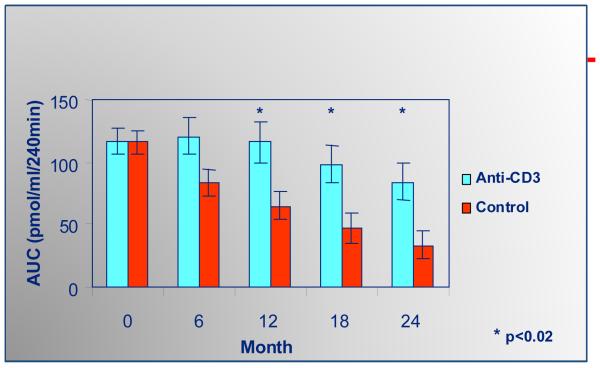
Following extensive studies in animal models [31,32] in 2002, Herold and colleagues reported the use of a humanized anti-CD3 monoclonal antibody (mutated in the IgG1 Fc chain to eliminate Fc receptor binding) in new onset T1D patients leading to sustained preservation of c-peptide production lasting up to five years following a single two week treatment (figure 3) [33-35]. Nevertheless after the first year of preservation of C-peptide, further loss of C-peptide appears to proceed in parallel to the slope of placebo treated patients. These results were confirmed by Keymeulen and coworkers with a different mutated anti-CD3 antibody (mutated to remove elimination of glycosylation sites in the Fc chain) reduced the loss of c-peptide in newly diagnosed T1D individuals [36,37]. Clinically, A1c and insulin usage improved and there were no long lasting adverse effects. Cytokine release syndrome developed in a number of treated subjects and particularly in the Keymeulen trial there was evidence of EBV reactivation that resolved spontaneously [38]. Circulating T cell levels returned to pretreatment levels one month following therapy. It is hypothesized that the preserved C-peptide following therapy is not only related to elimination of effector T cells but also the preservation of regulatory T cells. Studies in the NOD mouse, spontaneous animal model of autoimmune diabetes, reveal Tregs in pancreatic lymph nodes of these mice (which were lacking naturally occurring Tregs, CD28−/− mice) able to inhibit immune responses through a TGF-β-dependent mechanism following anti-CD3 treatment [39]. Tregs including IL-10 producing CD4+ cells have been isolated from humans treated with the monoclonal antibody [40-42] and it is possible that CD8+ Tregs are induced as well [43]. With the initial success of anti-CD3 monoclonal antibody treatment there are now clinical trials evaluating repeat dosing (AbATE) and use in T1D patients further removed from diagnosis, 4 months to 1 year, still producing c-peptide (DELAY). An anti-CD3 prevention trial is being considered for individuals with multiple islet autoantibodies but without hyperglycemia sponsored through the TrialNet organization [44].
Figure 3.
Anti-CD3 monoclonal antibody treatment delays loss of C-peptide in new onset patients with type 1 diabetes. From Herold et al Diabetes 54:1763-9, 2005.
T1D is a T cell mediated disease, however, B lymphocytes are implicated in disease pathogenesis as they have been identified in the pancreata of T1D individuals [45] and likely act as antigen presenting cells. A new onset study with rituximab, an anti-CD20 monoclonal antibody, showed modest improvement in c-peptide production 3 months after therapy but after 6 months the rate of c-peptide loss was similar between the treated and placebo groups [46]. Clinical responders could be differentiated from nonresponders by the amount of CD19+CD27+IgD+ cell depletion. Concerning was the fact that CD19+ levels did not return to pretreatment levels by one year post treatment and IgM levels remained depressed in the treated cohort. The applicability of this therapy must be weighed against the potential for chronic immune suppression.
There are a number of therapies currently in ongoing clinical trials or just beginning. CTLA4-Ig [47] is being used to block T cell activation. Anti-CD2 monoclonal antibodies which deplete memory and NK T cells will be used in a new onset T1D trial [48]. The anti-inflammatory pathway, specifically targeted to IL-1, will also be evaluated in T1D. Many chronic diseases have persistent inflammation such as neurodegenerative diseases, vascular disease, and metabolic diseases (type 2 diabetes) [49]. Type 2 diabetic patients treated with anakinra, an IL-1 receptor antagonist, improved glycemic control, reduced inflammation, and increased insulin sensitivity [50]. There appeared to be beneficial β-cells effects, in addition to reduced insulin resistance, as the treated patients had increased insulin:proinsulin ratios compared to controls [51]. β-cells may produce IL-1 in response to hyperglycemia, thereby perpetuating the cycle of inflammation and lymphocyte recruitment to the pancreas and pancreatic lymph nodes. Trials evaluating IL-1 blockade with anakinra and a monoclonal antibody to IL-1, canikinumab, are underway. Other therapies being used in T1D include alpha-1 antitrypsin (Aralast NP), a serine protease inhibitor, with anti-inflammatory properties [52] and Gleevec, a tyrosine kinase inhibitor used as a cancer drug to treat leukemia [53] as well as a peptide of a heat shock protein DiaPep277 (This peptide initially was considered an autoantigen but further studies suggest that it may influence innate immunity)[54]. Gleevec targets innate immune cells such as inflammatory macrophages involved in diabetes pathogenesis.
A trial of cyclophosphamide and ATG with autologous bone marrow transplantation is probably the most aggressive therapeutic regimen studied in new onset patients [55]. Probably associated with the severity of immunosuppression there was dramatic improvement n C-peptide secretion lasting several years and a subset of patients were able to discontinue insulin(see section 5 below).
3. Antigen specific therapies for the treatment and prevention of type 1 diabetes
It is ultimately hoped that forms of antigen specific therapy will be developed given their likely greater safety compared to immunosuppressive therapies. The antigens most studied to date are insulin and GAD65. The underlying rationale for antigen specific therapy is that the target autoantigens of the immune response can be utilized to delete specific pathogenic T cells or more likely to expand and activate T regulatory cells reacting with autoantigens at the target organ [56].
In a placebo controlled trial GAD-Alum injection delayed loss of C-peptide secretion in new onset patients following a single course of therapy[57]. Similar to anti-CD3 and rituximab between 6 months and one year loss of C-peptide secretion resumed. There was evidence of induction of both T and B lymphocyte (increased GAD autoantibodies) responses to GAD following the GAD-Alum injection documenting an immunologic effect of the “vaccine”[58].
There are multiple studies utilizing insulin, insulin peptides and proinsulin peptides to block anti-islet autoimmunity[59-63]. To date only one of those trials (Trialnet oral insulin) has provided any evidence of efficacy in a post-hoc analysis[12,64]. There is a large body of evidence in the NOD mouse model and man that insulin is likely a key target of the autoimmunity leading to T1D, beginning with the simple observation that islet beta cells are specifically destroyed, while autoantigens such as GAD65, IA-2, and ZnT8 are not specific to the islet beta cell. For instance to date in the NOD mouse model removing a specific insulin epitope recognized by CD4 T cells by mutating a single amino acid of the insulin peptide B:9-23 prevents all diabetes[65,66]. In man the insulin gene is the second most important genetic determinant of T1D with the protective genetic variant increasing insulin message within the thymus, and thus presumably deleting autoreactive T cells [67-69].
Trialnet/Diabetes Prevention Trial (DPT) has completed two studies of the potential of insulin to prevent the development of diabetes in islet autoantibody positive relatives of patients with T1D. The first gave insulin parenterally and the second oral insulin. Parenteral insulin did not decrease progression to diabetes[60]. Oral insulin overall also did not change progression to overt diabetes, but in the subset of patients with high levels of insulin autoantibodies there was a significant delay in progression, which for those with the highest levels the delay was estimated to be as much as 7 years[70]. In that the analysis was a post-hoc subgroup analysis, a repeat Trialnet study of oral insulin in relatives with multiple islet autoantibodies (including insulin autoantibodies) is underway[59]. In addition to insulin, peptides of the prohormone proinsulin are recognized by autoimmune T cells and Peakman and coworkers are studying peptides of proinsulin.
4. Environmental therapy for the prevention of islet autoimmunity
Early exposure to complex dietary proteins is a potential risk factor for developing islet autoimmunity [71]. The Trial to Reduce IDDM in the Genetically at Risk (TRIGR) conducted in Finland documents the rates of islet autoantibody development and progression to clinical T1D in children up to 10 years of age [72]. This double blind, randomized control trial assigned a highly hydrolyzed formula or conventional cow’s milk formula to infants having a first degree relative with T1D and high risk HLA alleles. The formula was to be given during the first 6 to 8 months of life whenever breast milk was not available. These children receiving the extensively hydrolyzed casein-based formula had less autoantibody positivity compared to the control group, 17% to 30%. In the study single autoantibodies (≥1) were significantly decreased (p=0.03) while multiple autoantibodies were not (p=0.12). Reasons why this maybe a beneficial approach to preventing islet autoimmunity comes from studies in animal models demonstrating the importance of diet and the intestinal microbiome in the development of T1D [73]. There was no difference in the development of clinical T1D, although there are relatively few events at this point in time. Fortunately a much larger TRIGR study, sponsored by the National Institute of Health, addressing the same issue is well underway with results expected for autoantibody expression in the next several years.
5. Future directions for treatment of type 1 diabetes
Monotherapy for T1D has not resulted in sustained tolerance and preservation of C-peptide production; individuals still progress to a complete loss of endogenous insulin producing ability. Combination therapy is likely to be necessary as different pathways and arms of the immune system can be targeted[74]. A very aggressive treatment approach from a group in Brazil performed autologous nonmyeloablative hematopoietic stem cell transplantation on selected new onset T1D patients[55]. Individuals were pretreated with cyclophosphamide and granulocyte-colony stimulating factor (G-CSF) to expand CD34+ cells which were harvested. Following treatment with ATG and a second course of cyclophosphamide, patients received their CD34+ cells. Fourteen of the 15 treated subjects were non-insulin requiring 16 months after therapy [55]. There were toxicities with the treatment but the radical approach suggests that T1D can be reversed and elements of the treatment protocol may be potentially useful in combinations. ATG or G-CSF could be used alone or in combination with other therapies. Other alternatives include using an immune suppressive agent (anti-CD3 or an anti-inflammatory therapy) with an antigen specific agent (GAD-Alum or BHT DNA proinsulin vaccine). The potential mechanism of tolerance induction would be allowing Tregs to develop from an antigen specific therapy while there is immune suppression or diminished inflammation.
Along with combination therapy, we believe targeted and specific immune therapies are needed to prevent and ultimately cure T1D. The immunologic mediators of T1D are becoming more defined. The trimolecular complexes consisting of class II major histocompatability (MHC II) molecules, peptides of autoantigens, and specific T cell receptors (TCR) that recognize peptide bound to MHC are essential for tissue specific targeting in autoimmune diseases. The trimolecular complex for insulin is now well characterized for the NOD mouse [75]. The major genetic determinant for many autoimmune disorders, including type 1 diabetes is MHC class II molecules [76,77]. Approximately 90% of T1D individuals have HLA-DQ8 or DQ2 alleles. Insulin has been focused on as a T1D autoantigen for decades since autoantibodies to this molecule were discovered in patients having T1D [78], and often precede the development of other islet autoantibodies [79]. Biostructural data has increased greatly over the last decade for the components of the anti-insulin trimolecular complex. A crystal structure exists for DQ8 with an insulin peptide, amino acids 9 to 23 of the B chain [80]. Recent studies indicate that insulin peptide B:9-23 is recognized in an unexpected low affinity register [81]. With understanding the structural determinants for autoantigen recognition, therapies can be designed to specifically target insulin-MHC complexes and the TCRs that recognize them[75]. Small molecule approaches can block autoantigen presentation or TCR recognition of autoantigen-MHC complexes. Another strategy includes designing monoclonal antibodies to recognize autoantigen peptide-MHC complexes with the peptide in a defined register recognized by the autoreactive T cell receptors. As the structural basis of autoantigen presentation and T cell recognition advances, these novel approaches become realistic.
6. Concluding remarks
With multiple trials underway, including phase III trials, it is possible that one or a combination of the currently studied agents will delay or hopefully prevent beta cell destruction. It is however very likely that we lack enough fundamental knowledge to rationally design trials/therapies that have a high probability of being both safe and effective. There are multiple unanswered questions, including what locks in the autoimmune process such that despite ability to delay beta cell destruction with multiple drugs, it recurs. In addition biomarkers are not available to rapidly assess efficacy and thus current trials are relatively long-term (one to two year outcomes for trials in new onset patients). Discovery of biomarkers that reflect numbers/activities of antigen specific pathogenic T cells or regulatory T cells we believe would be a major advance. Further structural knowledge of the T cells targeting islet autoantigens and the specific peptides recognized are likely to be needed. At present we can readily prevent autoimmune diabetes in animal models, predict the development of diabetes in man, but cannot yet safely prevent in man. As this review has highlighted considerable resources and thought is now devoted to achieving safe prevention in man.
Acknowledgements
This work was supported by grants from the National Institutes of Diabetes & Digestive & Kidney Diseases (R01 DK055969), the National Institutes of Health Diabetes & Endocrinology Research Core grant (P30 DK057516), the National Institute of Allergy & Infectious Diseases (U519 AI050864-08), the Juvenile Diabetes Foundation (4-2007-1056 and 17-2010-744), the Brehm Coalition, and the Children’s Diabetes Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Eisenbarth GS. Banting Lecture 2009: An Unfinished Journey: Molecular Pathogenesis to Prevention of Type 1A Diabetes. Diabetes. 2010;59(4):759–774. doi: 10.2337/db09-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Maahs DM, Rewers M. Editorial: Mortality and renal disease in type 1 diabetes mellitus--progress made, more to be done. J Clin Endocrinol Metab. 2006;91(10):3757–3759. doi: 10.1210/jc.2006-1730. [DOI] [PubMed] [Google Scholar]
- [4].Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26(3):832–836. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- [5].Harjutsalo V, Sjoberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371(9626):1777–1782. doi: 10.1016/S0140-6736(08)60765-5. [DOI] [PubMed] [Google Scholar]
- [6].Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- [7].Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- [8].Srikanta S, Ganda OP, Gleason RE, Jackson RA, Soeldner JS, Eisenbarth GS. Pre-type I diabetes. Linear loss of beta cell response to intravenous glucose. Diabetes. 1984;33(8):717–720. doi: 10.2337/diab.33.8.717. [DOI] [PubMed] [Google Scholar]
- [9].Sosenko JM, Krischer JP, Palmer JP, Mahon J, Cowie C, Greenbaum CJ, et al. A risk score for type 1 diabetes derived from autoantibody-positive participants in the diabetes prevention trial-type 1. Diabetes Care. 2008;31(3):528–533. doi: 10.2337/dc07-1459. [DOI] [PubMed] [Google Scholar]
- [10].Sosenko JM, Palmer JP, Rafkin LE, Krischer JP, Cuthbertson D, Greenbaum CJ, et al. Trends of earlier and later responses of C-peptide to oral glucose challenges with progression to type 1 diabetes in diabetes prevention trial-type 1 participants. Diabetes Care. 2010;33(3):620–625. doi: 10.2337/dc09-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A. 2007;104(43):17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sherr J, Sosenko J, Skyler JS, Herold KC. Prevention of type 1 diabetes: the time has come. Nat Clin Pract Endocrinol Metab. 2008 doi: 10.1038/ncpendmet0832. [DOI] [PubMed] [Google Scholar]
- [13].Achenbach P, Bonifacio E, Ziegler AG. Predicting type 1 diabetes. Curr Diab Rep. 2005;5(2):98–103. doi: 10.1007/s11892-005-0035-y. [DOI] [PubMed] [Google Scholar]
- [14].Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual beta-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT) The DCCT Research Group J Clin Endocrinol Metab. 1987;65(1):30–36. doi: 10.1210/jcem-65-1-30. [DOI] [PubMed] [Google Scholar]
- [15].Grajwer LA, Pildes RS, Horwitz DL, Rubenstein AH. Control of juvenile diabetes mellitus and its relationship to endogenous insulin secretion as measured by C-peptide immunoreactivity. J Pediatr. 1977;90(1):42–48. doi: 10.1016/s0022-3476(77)80762-2. [DOI] [PubMed] [Google Scholar]
- [16].Madsbad S, Faber OK, Binder C, McNair P, Christiansen C, Transbol I. Prevalence of residual beta-cell function in insulin-dependent diabetics in relation to age at onset and duration of diabetes. Diabetes. 1978;27(Suppl. 1):262–264. doi: 10.2337/diab.27.1.s262. [DOI] [PubMed] [Google Scholar]
- [17].O’Meara NM, Sturis J, Herold KC, Ostrega DM, Polonsky KS. Alterations in the patterns of insulin secretion before and after diagnosis of IDDM. Diabetes Care. 1995;18(4):568–571. doi: 10.2337/diacare.18.4.568. [DOI] [PubMed] [Google Scholar]
- [18].Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group Ann Intern Med. 1998;128(7):517–523. doi: 10.7326/0003-4819-128-7-199804010-00001. [DOI] [PubMed] [Google Scholar]
- [19].The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- [20].Keenan HA, Sun JK, Levine J, Doria A, Aiello LP, Eisenbarth G, et al. Residual insulin production and pancreatic ss-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59(11):2846–2853. doi: 10.2337/db10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Foulis AK, Stewart JA. The pancreas in recent-onset type 1 (insulin-dependent) diabetes mellitus: insulin content of islets, insulitis and associated changes in the exocrine acinar tissue. Diabetologia. 1984;26(6):456–461. doi: 10.1007/BF00262221. [DOI] [PubMed] [Google Scholar]
- [22].Gianani R, Campbell-Thompson M, Sarkar SA, Wasserfall C, Pugliese A, Solis JM, et al. Dimorphic histopathology of long-standing childhood-onset diabetes. Diabetologia. 2010 doi: 10.1007/s00125-009-1642-y. [DOI] [PubMed] [Google Scholar]
- [23].Gepts W. The pathology of the pancreas in human diabetes. In: Andreani D, DiMario U, Federlin KF, Heding LG, editors. Immunology in Diabetes. Kimpton Medical Publications; London: 1984. pp. 21–35. [Google Scholar]
- [24].Harris PE, Ferrara C, Barba P, Polito T, Freeby M, Maffei A. VMAT2 gene expression and function as it applies to imaging beta-cell mass. J Mol Med. 2008;86(1):5–16. doi: 10.1007/s00109-007-0242-x. [DOI] [PubMed] [Google Scholar]
- [25].Medarova Z, Tsai S, Evgenov N, Santamaria P, Moore A. In vivo imaging of a diabetogenic CD8+ T cell response during type 1 diabetes progression. Magn Reson Med. 2008;59(4):712–720. doi: 10.1002/mrm.21494. [DOI] [PubMed] [Google Scholar]
- [26].Gaglia JL, Guimaraes AR, Harisinghani M, Turvey SE, Jackson R, Benoist C, et al. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest. 2011;121(1):442–445. doi: 10.1172/JCI44339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Feutren G, Assan G, Karsenty G, DuRostu H, Sirmai J, Papoz L, et al. Cyclosporin increases the rate and length of remissions in insulin dependent diabetes of recent onset. Results of a multicentre double-blind trial. Lancet. 1986;2(8499):119–124. doi: 10.1016/s0140-6736(86)91943-4. [DOI] [PubMed] [Google Scholar]
- [28].Stiller CR, Dupre J, Gent M, Jenner MR, Keown PA, Laupacis A, et al. Effects of cyclosporine immunosuppression in insulin-dependent diabetes mellitus of recent onset. Science. 1984;223(4643):1362–1367. doi: 10.1126/science.6367043. [DOI] [PubMed] [Google Scholar]
- [29].Bougneres PF, Carel JC, Castano L, Boitard C, Gardin JP, Landais P, et al. Factors associated with early remission of type I diabetes in children treated with cyclosporine. N Engl J Med. 1988;318(11):663–670. doi: 10.1056/NEJM198803173181103. [DOI] [PubMed] [Google Scholar]
- [30].Eisenbarth GS, Srikanta S, Jackson R, Rabinowe S, Dolinar R, Aoki T, et al. Anti-thymocyte globulin and prednisone immunotherapy of recent onset type I diabetes mellitus. Diabetes Res. 1985;2(6):271–276. [PubMed] [Google Scholar]
- [31].Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci USA. 1994;91(1):123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chatenoud L, Bach JF. Regulatory T cells in the control of autoimmune diabetes: the case of the NOD mouse. Int Rev Immunol. 2005;24(3-4):247–267. doi: 10.1080/08830180590934994. [DOI] [PubMed] [Google Scholar]
- [33].Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- [34].Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, et al. A Single Course of Anti-CD3 Monoclonal Antibody hOKT3{gamma}1(Ala-Ala) Results in Improvement in C-Peptide Responses and Clinical Parameters for at Least 2 Years after Onset of Type 1 Diabetes. Diabetes. 2005;54(6):1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Herold KC, Gitelman S, Greenbaum C, Puck J, Hagopian W, Gottlieb P, et al. Treatment of patients with new onset Type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin Immunol. 2009;132(2):166–173. doi: 10.1016/j.clim.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352(25):2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- [37].Keymeulen B, Walter M, Mathieu C, Kaufman L, Gorus F, Hilbrands R, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010 doi: 10.1007/s00125-009-1644-9. [DOI] [PubMed] [Google Scholar]
- [38].Keymeulen B, Candon S, Fafi-Kremer S, Ziegler A, Leruez-Ville M, Mathieu C, et al. Transient Epstein-Barr virus reactivation in CD3 monoclonal antibody-treated patients. Blood. 2010;115(6):1145–1155. doi: 10.1182/blood-2009-02-204875. [DOI] [PubMed] [Google Scholar]
- [39].Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9(9):1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- [40].Chatenoud L. CD3 antibody treatment stimulates the functional capability of regulatory T cells. Novartis Found Symp. 2003;252:279–286. [PubMed] [Google Scholar]
- [41].Herold KC, Burton JB, Francois F, Poumian-Ruiz E, Glandt M, Bluestone JA. Activation of human T cells by FcR nonbinding anti-CD3 mAb, hOKT3gamma1(Ala-Ala) J Clin Invest. 2003;111(3):409–418. doi: 10.1172/JCI16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7(8):622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- [43].Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8 T cell population and induces CD8CD25 Tregs. J Clin Invest. 2005;115(10):2904–2913. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Skyler JS, Greenbaum CJ, Lachin JM, Leschek E, Rafkin-Mervis L, Savage P, et al. Type 1 Diabetes TrialNet--an international collaborative clinical trials network. Ann N Y Acad Sci. 2008;1150:14–24. doi: 10.1196/annals.1447.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155(2):173–181. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lee RS, Rusche JR, Maloney ME, Sachs DH, Sayegh MH, Madsen JC. CTLA4Ig-induced linked regulation of allogeneic T cell responses. J Immunol. 2001;166(3):1572–1582. doi: 10.4049/jimmunol.166.3.1572. [DOI] [PubMed] [Google Scholar]
- [48].Barlow AK, Like AA. Anti-CD2 monoclonal antibodies prevent spontaneous and adoptive transfer of diabetes in the BB/Wor rat. Am J Pathol. 1992;141(5):1043–1051. [PMC free article] [PubMed] [Google Scholar]
- [49].Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6(4):232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- [50].Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356(15):1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- [51].Larsen CM, Faulenbach M, Vaag A, Ehses JA, Donath MY, Mandrup-Poulsen T. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes Care. 2009;32(9):1663–1668. doi: 10.2337/dc09-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Koulmanda M, Bhasin M, Hoffman L, Fan Z, Qipo A, Shi H, et al. Curative and beta cell regenerative effects of alpha1-antitrypsin treatment in autoimmune diabetic NOD mice. Proc Natl Acad Sci U S A. 2008;105(42):16242–16247. doi: 10.1073/pnas.0808031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Louvet C, Szot GL, Lang J, Lee MR, Martinier N, Bollag G, et al. Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0810246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nussbaum G, Zanin-Zhorov A, Quintana F, Lider O, Cohen IR. Peptide p277 of HSP60 signals T cells: inhibition of inflammatory chemotaxis. Int Immunol. 2006 doi: 10.1093/intimm/dxl074. [DOI] [PubMed] [Google Scholar]
- [55].Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301(15):1573–1579. doi: 10.1001/jama.2009.470. [DOI] [PubMed] [Google Scholar]
- [56].Weiner HL. Oral tolerance, an active immunologic process mediated by multiple mechanisms. J Clin Invest. 2000;106(8):935–937. doi: 10.1172/JCI11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ludvigsson J, Faresjo M, Hjorth M, Axelsson S, Cheramy M, Pihl M, et al. GAD Treatment and Insulin Secretion in Recent-Onset Type 1 Diabetes. N Engl J Med. 2008;359:1909–1920. doi: 10.1056/NEJMoa0804328. [DOI] [PubMed] [Google Scholar]
- [58].Hjorth M, Axelsson S, Ryden A, Faresjo M, Ludvigsson J, Casas R. GAD-alum treatment induces GAD(65)-specific CD4(+)CD25(high)FOXP3(+) cells in type 1 diabetic patients. Clin Immunol. 2010 doi: 10.1016/j.clim.2010.10.004. [DOI] [PubMed] [Google Scholar]
- [59].Mahon JL, Sosenko JM, Rafkin-Mervis L, Krause-Steinrauf H, Lachin JM, Thompson C, et al. The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes. 2009;10(2):97–104. doi: 10.1111/j.1399-5448.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- [60].Skyler JS, Brown D, Chase HP, Collier E, Cowie C, Eisenbarth GS, et al. Effects of insulin in relatives of patients with type 1 diabetes mellitus. New Engl J Med. 2002;346(22):1685–1691B. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- [61].Nanto-Salonen K, Kupila A, Simell S, Siljander H, Salonsaari T, Hekkala A, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet. 2008;372(9651):1746–1755. doi: 10.1016/S0140-6736(08)61309-4. [DOI] [PubMed] [Google Scholar]
- [62].Walter M, Philotheou A, Bonnici F, Ziegler AG, Jimenez R. No effect of the altered peptide ligand NBI-6024 on beta-cell residual function and insulin needs in new-onset type 1 diabetes. Diabetes Care. 2009;32(11):2036–2040. doi: 10.2337/dc09-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Thrower SL, James L, Hall W, Green KM, Arif S, Allen JS, et al. Proinsulin peptide immunotherapy in type 1 diabetes: report of a first-in-man Phase I safety study. Clin Exp Immunol. 2009;155(2):156–165. doi: 10.1111/j.1365-2249.2008.03814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Skyler JS. Update on worldwide efforts to prevent type 1 diabetes. Ann N Y Acad Sci. 2008;1150:190–196. doi: 10.1196/annals.1447.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435(7039):220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med. 2009;360(16):1646–1654. doi: 10.1056/NEJMra0808284. [DOI] [PubMed] [Google Scholar]
- [67].Pugliese A, Fernandez A, Zeller M, Zalcberg LJ, Ricordi C, Pietropaolo M, et al. Ectopic transcription of IDDM-associated islet cell autoantigen genes and monoallelic expression of the insulin gene in human thymus. 1996 submitted. [Google Scholar]
- [68].Wicklow BA, Polychronakos C. Insulin auto-immunity: implications for the prevention of Type 1 diabetes mellitus. Expert Rev Clin Immunol. 2009;5(1):55–62. doi: 10.1586/1744666X.5.1.55. [DOI] [PubMed] [Google Scholar]
- [69].Barratt BJ, Payne F, Lowe CE, Hermann R, Healy BC, Harold D, et al. Remapping the Insulin Gene/IDDM2 Locus in Type 1 Diabetes. Diabetes. 2004;53(7):1884–1889. doi: 10.2337/diabetes.53.7.1884. [DOI] [PubMed] [Google Scholar]
- [70].Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial--Type 1. Diabetes Care. 2005;28(5):1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- [71].Virtanen SM, Knip M. Nutritional risk predictors of beta cell autoimmunity and type 1 diabetes at a young age. Am J Clin Nutr. 2003;78(6):1053–1067. doi: 10.1093/ajcn/78.6.1053. [DOI] [PubMed] [Google Scholar]
- [72].Knip M, Virtanen SM, Seppa K, Ilonen J, Savilahti E, Vaarala O, et al. Dietary intervention in infancy and later signs of beta-cell autoimmunity. N Engl J Med. 2010;363(20):1900–1908. doi: 10.1056/NEJMoa1004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Visser JT, Lammers K, Hoogendijk A, Boer MW, Brugman S, Beijer-Liefers S, et al. Restoration of impaired intestinal barrier function by the hydrolysed casein diet contributes to the prevention of type 1 diabetes in the diabetes-prone BioBreeding rat. Diabetologia. 2010;53(12):2621–2628. doi: 10.1007/s00125-010-1903-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Matthews JB, Staeva TP, Bernstein PL, Peakman M, von HM. Developing combination immunotherapies for type 1 diabetes: recommendations from the ITN-JDRF Type 1 Diabetes Combination Therapy Assessment Group. Clin Exp Immunol. 2010;160(2):176–184. doi: 10.1111/j.1365-2249.2010.04153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Michels AW, Nakayama M. 2The anti-insulin trimolecular complex in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010 doi: 10.1097/MED.0b013e32833aba41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, et al. HLA DR-DQ Haplotypes and Genotypes and Type 1 Diabetes Risk: Analysis of the Type 1 Diabetes Genetics Consortium Families. Diabetes. 2008;57:1084–1092. doi: 10.2337/db07-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Steenkiste A, Valdes AM, Feolo M, Hoffman D, Concannon P, Noble J, et al. 14th International HLA and Immunogenetics Workshop: report on the HLA component of type 1 diabetes. Tissue Antigens. 2007;69(Suppl 1):214–225. doi: 10.1111/j.1399-0039.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- [78].Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati O, Raghu PK, et al. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983;222(4630):1337–1339. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- [79].Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler AG, Bonifacio E. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest. 2004;114(4):589–597. doi: 10.1172/JCI21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lee KH, Wucherpfennig KW, Wiley DC. Structure of a human insulin peptide/HLA-DQ8 complex and susceptibility to type 1 diabetes. Nature Immunology. 2001;2:501–507. doi: 10.1038/88694. [DOI] [PubMed] [Google Scholar]
- [81].Stadinski BD, Zhang L, Crawford F, Marrack P, Eisenbarth GS, Kappler JW. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc Natl Acad Sci U S A. 2010;107(24):10978–10983. doi: 10.1073/pnas.1006545107. [DOI] [PMC free article] [PubMed] [Google Scholar]