1. Introduction
In the early 1950's, Billingham, Brent and Medawar reported the induction of transplantation tolerance by injection of donor hematopoietic cells intravenously into neonatal mice within 24 hours of birth [1]. Since that time, the induction of tolerance for organ transplants has been a major goal of both basic and clinical transplantation immunology. At the theoretical level, understanding transplantation tolerance is an essential part of understanding the mechanism of self/non-self discrimination in the immune system. At a practical level, understanding tolerance may provide the means for inducing it in patients receiving organ transplants, thus liberating them from the complications of life-long chronic immunosuppression. We hope to review here our own attempts to contribute to this understanding through a series of studies, first in small and then in large animal models, and most recently in clinical practice.
2. Studies in Mice
Initial studies of tolerance induction through mixed chimerism involved lethal irradiation of recipient mice and reconstitution of these mice with a mixture of T cell depleted host plus donor bone marrow [2]. These animals reconstituted as mixed lymphohematopoietic chimeras, in which all lymphoid and hematopoietic elements remained a mixture of host and donor type for the rest of the lives of the animals. These studies provided proof of principle that mixed chimerism was associated with donor-specific skin graft tolerance and that the tolerant state was systemic, as evidenced by in vitro measures of alloreactivity. Graft-versus-host disease (GvHD) was avoided by T cell depletion of the donor marrow component and depletion of the recipient marrow component was essential to avoid rejection of the donor marrow.
Subsequent skin grafts of donor tail skin onto the lateral thorax of these tolerant animals were accepted long term [2]. However, these recipients showed side effects of the lethal irradiation used as part of the preparative regimen. While lethal irradiation and chemotherapy is acceptable as part of the treatment for a malignant disease, it is both too toxic and not sufficiently reliable in permitting engraftment of T cell-depleted allogeneic bone marrow to be considered appropriate for the conditioning of patients requiring only an organ transplant. Nevertheless, this initial study proved the principal that mixed chimerism could lead to long term, donor-specific tolerance. Therefore, much effort was subsequently devoted to developing non-myeloablative methods for inducing mixed chimerism [3–5].
Similarly, while some degree of GvHD is considered acceptable in patients with malignancies because of the associated beneficial graft-vs-leukemia (GvL) effects [6], this complication would be unacceptable in patients without malignant disease, who receive hematopoietic cell transplantation (HCT) expressly for the purpose of organ allograft tolerance induction via mixed chimerism. Since organ transplantation is routinely performed across extensive HLA barriers, the formidable challenge of avoiding GvHD while crossing HLA barriers must be met if HCT is to be applied expressly for the purpose of tolerance induction.
Thus, to make the mixed chimerism approach clinically relevant, a protocol was needed that excluded lethal TBI, and was nevertheless sufficient to reliably overcome the immune barrier to MHC-mismatched HCT, even after the donor graft was T cell-depleted to prevent GvHD. T cells pose the major immune barrier to allogeneic hematopoietic cell engraftment, and this barrier can be overcome by recipient conditioning that either eliminates mature host immune cells or permits pre-existing T cells to be rendered tolerant. Conditioning therapy must overcome both peripheral and intrathymic T cell-mediated alloreactivity. Under such conditions, we have shown that both donor- and host-reactive T cells arising de novo in the thymus are specifically deleted [7–11]. Coexistence of donor and recipient hematopoietic stem cells (HSCs) in the recipient marrow environment is needed to assure the life-long presence of APCs of each type to maintain lifelong central, deletional T cell tolerance [7–11].
In an early regimen, in vivo depletion of host T-cells along with TBI (at least 6 Gy) permitted MHC-mismatched allogeneic marrow engraftment and induction of donor-specific tolerance [12]. After finding that lower doses of irradiation and T cell-depleting mAbs almost completely eliminated peripheral but not intrathymic T cells, we showed that adding thymic irradiation (7 Gy) to the regimen permitted this outcome in animals receiving only 3 Gy TBI [4]. Thymic irradiation eliminates mature alloreactive thymocytes [13]. Tolerance can be abrogated and chimerism eliminated in established mixed chimeras by depleting donor cells with anti-donor MHC-specific mAbs. Loss of tolerance is associated with the de novo appearance in the periphery of T-cells bearing donor-reactive TCR [10]. Removal of the recipient thymus before depletion of donor cells prevents loss of tolerance and appearance in the periphery of T cells with donor-reactive TCR [10], proving definitively that intrathymic chimerism is necessary and sufficient for the maintenance of tolerance. Since persistent antigen is required to maintain anergy and suppression, these results argue against any role for such mechanisms in maintaining this tolerance. This conclusion is supported by the ease with which new thymic emigrants break tolerance after donor cell depletion by mAb or with infusion of non-tolerant recipient lymphocytes [10]. Thus, lifelong central, deletional tolerance is the mechanism of tolerance in this model.
We have subsequently attempted to develop conditioning regimens that are less T cell depleting than that described above. Conditioning regimens that do not fully deplete recipient T cells involve more complex mechanisms of tolerance induction in order to achieve initial mixed chimerism. Both thymic irradiation and T cell depleting mAbs in the conditioning regimen discussed above can be replaced by costimulatory blockade [14]. These approaches are of interest clinically, since T cell recovery from the thymus might be dangerously slow if we had a means of achieving exhaustive T cell depletion in humans, especially in older patients with impaired thymic function (reviewed in [15]).
Following tolerization of the peripheral T cell repertoire to achieve mixed chimerism, long-term tolerance is maintained by intrathymic deletion in mixed chimeras prepared with costimulatory blockade [14;16;17]. Initial tolerance of preexisting peripheral T cells in recipients of anti-CD154 plus allogeneic BMT involves specific deletion of pre-existing peripheral donor-reactive CD4 [18] and CD8 [19] T cells. Specific donor-reactive CD8 deletion occurs within 1–2 weeks and requires CD4 cells that do not appear to be classical Tregs [19]. Also, arguing against a role for “adaptive” or “induced” Tregs, CD4 cells are not required for maintenance of tolerance after this initial 2-week period [19]. Thus, while CD4 cells are clearly required for CD8 tolerance in this model, the evidence does not implicate a specific subset of CD4 cells that is differentiated to mediate suppression. The expression of MHC class II on recipient APCs, as well as recipient dendritic cells and B cells, all are required to tolerize pre-existing CD8 cells, but not CD4 cells, in this model [20]. Moreover, PD-1/PD-L1 interactions are required to tolerize CD8 cells but not CD4 cells [21]. Deletion of peripheral donor-reactive CD4 and CD8 cells is preceded by a state of specific unresponsiveness to the donor [18;22]. Regulatory cells do not maintain long-term tolerance in this model [18;19]. Since donor HSC engraftment ensures central deletion of donor-reactive T cells developing after the achievement of chimerism [14;16;17;23], and specific peripheral deletion is quite complete, insufficient persistence of donor-reactive effector T cells may preclude the expansion and maintenance of specific regulatory cells. However, models using CTLA4Ig and anti-CD154 as conditioning for allogeneic BMT have been associated with less complete deletion of pre-existing donor-reactive T cells and appear to involve long-term regulatory mechanisms [24;25].
Mixed chimerism also been assessed in the treatment of hematologic malignancies in mouse models [26–29]. This approach, which involves using mixed chimerism to help achieve GvL without GVHD following donor leukocyte infusion, has been translated to clinical tolerance protocols for patients with leukemias and lymphomas. It was during the course of studies aimed at translating a strategy for separating GVHD from GVL in the mouse model to a clinical model [30;31], that we developed clinical regimens to be used for renal allograft tolerance induction in the HLA-identical setting in patients with multiple myeloma and later in HLA-mismatched patients without malignant disease (see Clinical studies, below [32–34]).
3. Studies in large animals
Over the course of the decade-long translational research effort, we have observed significant biological differences among rodents, pigs and nonhuman primates (NHP). This variability is attributed to their genetic and developmental differences, which can affect innate and adaptive immunologic functions as well as metabolic responses to various medications. Elucidating these biological differences between species has proved pivotal to the successful extension of the basic observations in mice to NHP and humans.
Studies in pigs
Extensive studies of organ and tissue tolerance induction via bone marrow transplantation were carried out early in the development of the miniature swine model [35–39]. Many of the studies of mixed chimerism in this model have been directed toward the potential use of this modality for the treatment of hematologic malignancies [40;41] and have therefore utilized mobilized peripheral stem cells rather than bone marrow as source of hematopoietic stem cells (HSC) [40;42].
Organ allograft tolerance has also been studied in mixed chimeras [43–45], and Horner et al. have recently summarized these studies in terms of the relationship between tolerance and a variety of parameters of HSC engraftment [46]. Twenty-two HSC transplant recipients that subsequently received donor renal allografts without immunosuppression, were examined for a possible correlation of tolerance induction with the presence of donor cells in the bone marrow, thymus and lineages of peripheral blood. PCR assays for presence of donor colony-forming units (CFU) in bone marrow, assessment of thymic and peripheral blood chimerism by flow cytometry and PCR and assays of in vitro responsiveness to donor MHC were performed.
Engraftment, as indicated by the presence of donor derived CFU in the bone marrow, was found to have the highest correlation with tolerance induction for a subsequent renal allograft (p<0.0001). Thymic chimerism and multilineage peripheral blood chimerism at the time of organ transplant were highly correlated with the presence of donor CFU in the bone marrow and with subsequent allograft tolerance (p<0.001 and p<0.005, respectively). In vitro assays of responsiveness were also predictive of tolerance (p < 0.002), but were not as highly correlated as the presence of donor derived CFU in the bone marrow.
Studies in nonhuman primates (NHP)
Before clinical application of nonmyeloablative mixed chimerism regimens for induction of renal allograft tolerance, it was considered essential to develop a preclinical model in NHP, based on the nonmyeloablative regimen established in mice. The essential components of the regimen were: 1) nonmyeloablative total body irradiation (TBI); 2) thymic irradiation; and 3) effective T cell depletion. However, reagents capable of completely depleting T cells were not available for primates, and severe depletion with available reagents resulted in unacceptable mortality due to infectious complications and/or lymphoma development. In addition probable homeostatic proliferation of T cells led to the selective recovery of memory-type cells, which could potentially inhibit chimerism induction and allograft tolerance [47].
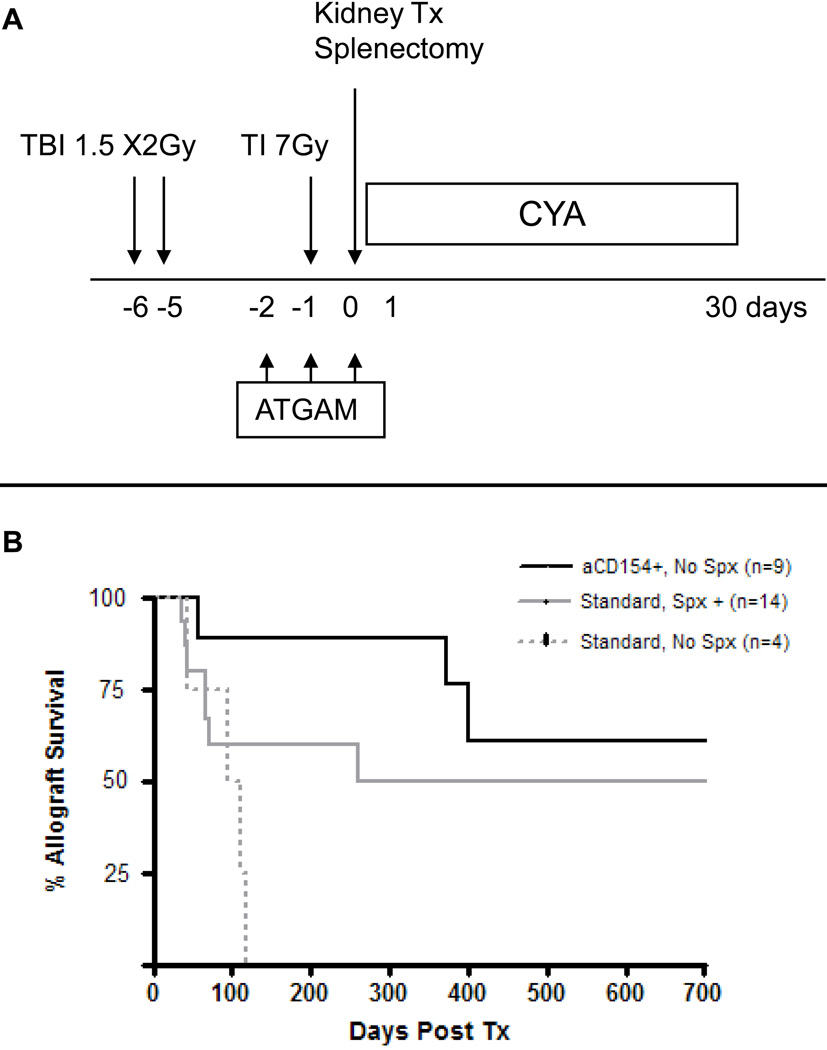
Thus, in the initial regimen, we included horse ATG (ATGAM). Since T cell depletion by ATGAM was incomplete, the initial regimen with TBI (3Gy on day -6), TI (7Gy on day -1) and ATGAM (50mg/kg on days -2,-1 and 0) failed to induce chimerism and the recipients rejected kidney allografts within 15 days. Therefore, we added splenectomy (day 0) and a one month course of CyA to the conditioning regimen, in order to reduce/suppress residual T cells. Multilineage hematopoietic chimerism was detected in the first recipient treated with this regimen on day 7, and this chimerism increased until day 20, with a peak of >60% donor myeloid cells [48]. However, unlike the permanent mixed chimerism of mice, the chimerism in this monkey subsequently declined and became undetectable by day 30. We anticipated that tolerance would be lost and that the renal allograft would be rejected after the discontinuation of CyA. To our surprise, however, the monkey did not reject its renal allograft. Kidney function remained stable for years, with no histopathologic evidence of rejection [48].
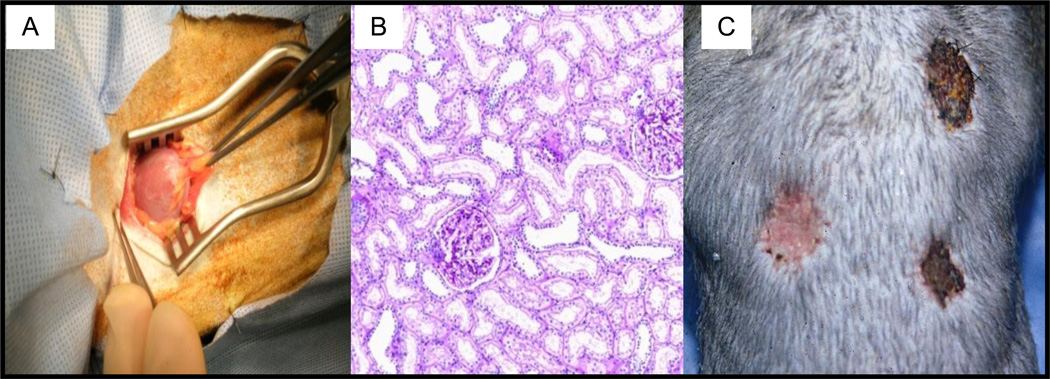
To reduce the morbidity and mortality of the regimen, the protocol was subsequently modified by fractionating the TBI to 1.5 Gy on days –6 and –5 (Fig. 1A, Standard Regimen). With this modification, 11/14 recipients developed transient chimerism and 8/14 survived long-term without immunosuppression (Fig. 1B, Standard Spx+). The longest survival exceeded 14 years, with a normal kidney (Figs. 2A and 2B). Stable tolerance was confirmed in some of these monkeys by skin transplantation, where the skin graft from the kidney donor was accepted while skin allografts from third party donors were rejected within 10 days (Fig. 2C).
Figure 1.
Tolerance induction regimen in cynomolgus monkeys: A) The initial preparative and treatment regimens; B) Renal allograft survivals with initial regimen or with modified regimen (anti-CD154 with or without splenectomy)
Figure 2.
Tolerance of kidneys in cynomolgus monkeys: A) Gross appearance of transplanted kidney at biopsy (normal); B) Microscopic appearance of same kidney, (normal histology); C) Frozen donor skin grafted onto thorax of recipient approximately one year after renal transplant is accepted (left), while third party skin, either fresh (top right) or frozen (bottom right) is rejected by day 15 after skin grafting.
Costimulatory blockade and mixed chimerism
As described above, splenectomy was a component of the initial regimen. Without splenectomy, all recipients developed alloantibodies and rejected their allografts (Fig. 1B, Standard No Spx). The importance of splenectomy at the time of DBMT was also demonstrated in another approach, the delayed kidney transplant model. In this protocol, conditioning and DBMT was given with or without splenectomy, followed by kidney transplantation 3–12 weeks later, without further immunosuppression [49]. If splenectomy was performed simultaneously with DBMT, the subsequent kidney allografts survived long-term. In contrast, if splenectomy was not performed at the time of DBMT, all recipients developed antibody mediated rejection of the kidney within 12 days. Significant residual T cells remained in lymph nodes or spleen after conditioning and these may have been sensitized by donor marrow. Replacement of splenectomy with CD154 blockade was associated with significantly greater and more durable chimerism and eight of nine kidney grafts survived long-term (Fig. 1B, +aCD154, No Spl). Since anti-CD154 mAb is not clinically applicable because of its thrombogenic complications [50;51], we are currently evaluating alternate methods for costimulatory blockade.
Mechanisms of tolerance by transient mixed chimerism
These results in monkeys emphasized that renal allograft tolerance can be induced in primates even with transient chimerism. In murine recipients with stable mixed chimerism, thymic deletion of donor reactive T cells has been demonstrated and the loss of chimerism leads to allograft rejection [10] However, in our monkeys (as well as in our patients, see below), persistence of tolerance after loss of chimerism suggested that peripheral tolerance mechanisms may be responsible for the maintenance of tolerance. We hypothesized that T regulatory cells might be implicated on the basis of the following observations: 1) donor specific MLR responsiveness can be restored by removing CD25+ cells from the MLR (Aoyama et al., manuscript in preparation); 2) Foxp 3+ cells specifically proliferate after MLR with donor cells but not third party cells (Aoyama et al., manuscript in preparation); and 3) significantly higher mRNA for Foxp3 has been detected in the renal allograft of the tolerant patients, comparing with regular transplant recipients with conventional immunosuppression [34]. As is detailed below, additional observations support a role for Tregs in the tolerant patients.
Modification of the protocol for deceased donor transplantation
Our current NHP and clinical regimens require treatment of subjects beginning 6 days prior to organ transplantation, which limits applicability to only living donor allograft recipients. Simple compression of all treatments to the 24-hour peri-operative transplant period not only failed to induce chimerism but also led to unacceptable toxicity (unpublished data). Therefore, we have more recently developed a “delayed tolerance” protocol, in which kidney transplantation is performed first with conventional immunosuppressive therapy, followed by non-myeloablative conditioning and donor bone marrow transplantation several months later. If this approach proves feasible, any stable recipient of either living donor or deceased donor kidney transplantation (KTx) could be a potential candidate, using either fresh (living donor) or cryopreserved (deceased donor) DBM.
However, institution of a tolerance inducing regimen following a period of conventional immunosuppression post-transplantation has the theoretical disadvantage that donor specific memory T cells might be elicited against the allograft even during administration of potent suppressive agents. Analyses of T cell subsets in our NHP recipients revealed that a substantial number of memory T cells remained after the conditioning regimen, in spite of effective depletion of naïve T cells. Using CD95 and CD28 to define memory T cells [52], we observed persistence of CD4 central memory cells following conditioning [53]. Both CD8 effector memory T cells (TEM) and CD8 central memory T cells (TCM) were initially depleted effectively but rapid post-conditioning expansion of CD8 TEM was observed after day 5, while CD8 TCM counts remained unchanged. We therefore added to the conditioning regimen a humanized anti-CD8 mAb, cM-T807, which effectively inhibited expansion of CD8 TEM. Preliminary results showed that the regimen with cM-T807 induced improved chimerism and allograft tolerance in approximately 40% of the NHP studied. Thus, the mixed chimerism approach can induce tolerance even several months after organ transplantation if additional treatments to eliminate memory T cells are included [54].
Attempts to extend this protocol to non-kidney allografts (heart, lung, and islet) are in progress. In a heterotopic cardiac allograft model, three of 5 recipients have developed multilineage chimerism, and allograft survival in these recipients has been prolonged to 138, 428, and 509 days, but without evidence for tolerance. Islet transplantation has been tested by inducing diabetes with Streptozocin in a kidney transplant recipient whose allograft kidney function had been stable for more than 2 years after immunosuppression was withdrawn [55]. Islets obtained by partial pancreatectomy of the original BM and kidney donor were transplanted without immunosuppression and exogenous insulin administration became unnecessary two weeks thereafter. At autopsy viable islets with strong insulin staining were observed both in the liver and under the kidney capsule, with no histological evidence of rejection. Attempts to induce tolerance in lung transplant recipients are also underway, with the understanding that organ specific modifications of the regimen may be necessary to achieve tolerance of different tissues and organs.
4. Clinical Studies
The initial observations indicating that human organ allografts transplanted in conjunction with donor bone marrow (DBM) can survive without administration of chronic immunosuppressive therapy were made in patients who had undergone successful bone marrow transplantation (BMT) for treatment of hematologic malignancies and subsequently developed end-stage renal disease (ESRD). Kidney transplantation from the original BM donor was accomplished in these long-term chimeric recipients without requiring immunosuppressive therapy [56–59]. However, as previously noted, the toxicity associated with conventional conditioning and the possible complication of graft-versus-host disease (GVHD) following myeloablative therapy and BMT precluded application of this strategy for routine organ transplantation. As detailed above, a major advance for this approach was the series of studies in mice showing that transplant tolerance could be induced in recipients treated with a non-myeloablative conditioning regimen that resulted in only “mixed chimerism”. Following the demonstration that this approach could be successfully extended to swine and nonhuman primates, and following demonstration of safety and efficacy of a protocol for achieving mixed chimerism in patients with hematologic malignancies [30;31;60], we decided in 1998 to first evaluate its clinical applicability in patients with ESRD secondary to multiple myeloma (MM). Optimal treatment for these individuals had, until then, been limited by the dilemma that conventional BMT was contraindicated as treatment for the MM because of the patient’s ESRD, while renal transplantation was contraindicated because of their malignancy. We concluded that combined renal transplantation with BMT might provide new hope to this patient group, considered by many to be untreatable.
HLA-Matched Renal Allografts
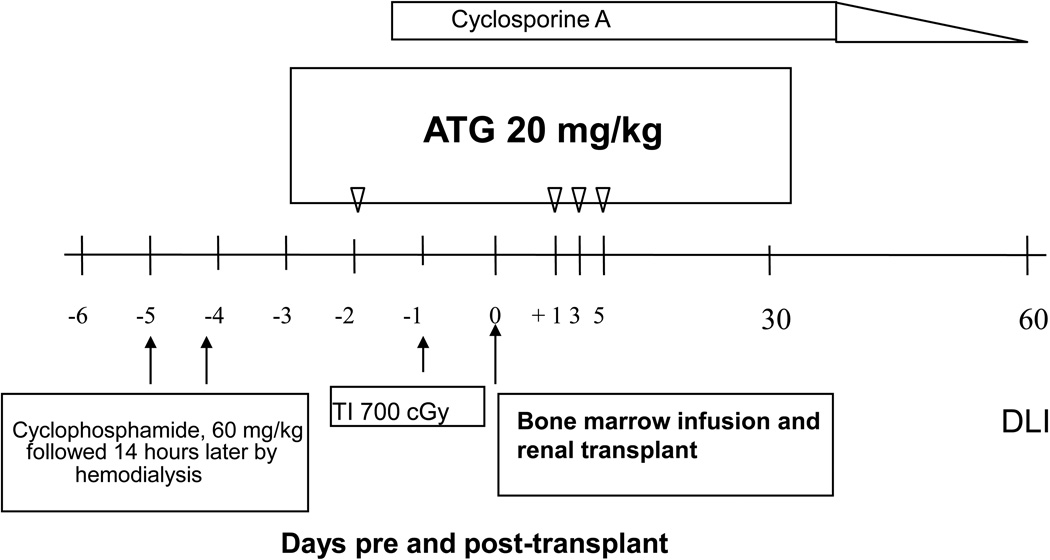
The first patient enrolled into this novel clinical study was a 55-year-old woman who presented with the classical findings of lytic, painful MM bone lesions, poor tolerance of dialysis, and rapidly deteriorating general health [61]. Her HLA-identical sister was in good health and willing to donate both BM and a kidney. The peri-transplant recipient conditioning for the planned kidney/BM transplant included cyclophosphamide, thymic irradiation, equine anti-thymocyte globulin (ATGAM, Pharmacia & Upjohn Co.), and cyclosporine as summarized in Figure 3. Post-operatively, the patient’s serum creatinine and BUN levels returned rapidly to normal and renal allograft function has remained normal thereafter. Peripheral blood donor-derived hematopoietic elements, was detectable by week 2 by analysis of microsatellite markers. Beginning 7 weeks post-transplantation, the percentage of donor cells declined and no evidence of hematopoietic chimerism has been detectable since 5 months after kidney/BM transplantation. Nevertheless, maintenance immunosuppressive therapy (cyclosporine) was discontinued at 10 weeks post-transplantation, as planned. Subsequently, the MM has remained in remission and kidney allograft function has remained normal, with no evidence of rejection, for more than 12 years of post-transplant, immunosuppression-free survival.
Figure 3.
Clinical tolerance induction regimen: Preparative and treatment regimen for HLA-matched sibling donors in first clinical trial for patients with ESRD and intractable multiple myeloma.
To date 9 patients with ESRD, MM and an HLA-identical donor, have been treated with this protocol [32;62]. Only one recipient developed evidence of renal allograft rejection, after stopping immunosuppression, and this responded to temporary reintroduction of immunosuppression, which was then successfully discontinued. The long-term, operational renal allograft tolerance observed in these patients, despite loss of detectable hematopoietic chimerism, suggests the presence of active immune regulation in addition to (or instead of) the deletional tolerance demonstrable in mice. The detection of anti-donor alloresponses in vitro after loss of chimerism and the enrichment for CD25+CD4 T cells after transplant is consistent with this hypothesis [32]. Control of the MM has been encouraging, as only 3 of these patients have developed recurrent disease, one of whom expired at 7 years post-transplantation due to progressive MM. In view of the essentially 100% 3–5 year mortality rate typically expected with alternative treatments for this challenging population, it has been suggested that combined kidney/BM transplantation following non-myeloablative conditioning will likely become the standard therapy for patients with ESRD secondary to MM [63].
HLA-Mismatched Renal Allografts
Encouraged by these observations in recipients of HLA-identical kidney/BM allografts, we next extended the mixed chimerism approach to HLA-mismatched transplants in ESRD patients without complicating neoplasia [34]. This was made possible by the development of a conditioning regimen in patients with hematologic malignancies that induced transient mixed chimerism across full HLA haplotype barriers without GVHD [33]. This conditioning regimen was similar to that use for the MM study (Fig. 3), except that the humanized anti-CD-2 monoclonal antibody (MEDI-507, Medimmune) was substituted for ATGAM. MEDI-507 had been shown to be more effective than ATGAM for T cell depletion in clinical trials of haploidentical BMT for treatment of lymphoma, thereby avoiding the complication of GVHD, which was seen when HLA barriers were transgressed with the ATGAM regimen [64;65].
This mixed chimerism clinical trial was initiated in 2002. The first recipient was a 22-year-old woman who had 10 years earlier received a renal transplant from her father for treatment of ESRD secondary to Alport’s syndrome. Her course, while receiving conventional immunosuppression after that transplant, had been complicated by disabling viral infections. This had prompted gradual discontinuation of the drugs, resulting in rejection of the kidney allograft. Faced with the choices of either chronic dialysis therapy or another attempted transplant with conventional therapy, she requested selection as the first study subject in our mixed chimerism approach to prolonging allograft survival in patients without HLA-identical donors and without malignancy. Following the conditioning described above, she underwent combined kidney/BM transplantation from her mother.
Transient mixed chimerism became undetectable in this recipient’s peripheral blood after only 3 weeks. Nevertheless, her post-operative course was essentially uneventful with no evidence of renal allograft rejection. Immunosuppression withdrawal in this protocol was planned to be slower than in the previous recipients of HLA-identical allografts, so that final discontinuation in this patient was at 8 months post-transplantation. She has never shown any evidence of kidney rejection and continues with stable function (serum creatinine, 1.2 mg/dl) after nearly 8 years of immunosuppressive-free follow-up.
This protocol was modified after the third patient treated in this manner developed irreversible humoral rejection on the tenth post transplant day [34]. This patient had been found pre-transplant to have high levels of pre-existing alloantibody activity, but without detectable donor-specific antibody (DSA). Reasoning that his rapid development of DSA post-transplantation likely indicated pre-sensitization at the B cell level, the chimeric monoclonal antibody to CD20 (Rituximab, Genentech) was added to the therapeutic regimen, to provide anti-B cell depletion in subsequent patients. Seven additional recipients of HLA-mismatched kidney/BM transplants have now been enrolled into this and a subsequent ongoing trial.
Withdrawal of immunosuppressive therapy was accomplished according to the protocol design in 2 of the first 3 and 6 of the last 7 subjects. As noted above, one of the first 3 patients developed early, irreversible humoral rejection. He subsequently underwent successful deceased donor transplantation with conventional immunosuppression. Stable renal allograft function has been maintained in 7 of the 8 subjects in whom immunosuppression was discontinued for follow-up periods to date of 10 to over 90 months. One subject (the most recently treated) developed an episode of acute cellular rejection seven weeks after immunosuppressive therapy had been discontinued. Following re-institution of immunosuppression, his renal allograft function has improved, though not yet to the previously established baseline level.
The clinical course of one of the 7 patients treated with the modified protocol was more complex. This individual, like most of the others, developed the typical “Engraftment Syndrome” (see below) around day 10. Unlike the other study participants, however, her renal allograft function then improved, as anticipated, but failed to return to baseline. Serial biopsies showed gradually progressive thrombotic microangiography (TMA) thought likely to be the consequence of calcineurin inhibitor (CNI) toxicity. Humoral rejection was ruled out as the cause of this condition by the absence of C4d staining in general kidney biopsies and failure to detect serum DSA. CNI immunosuppression was discontinued, but the allograft eventually failed and she was returned to dialysis, six months after transplantation.
Morbidity
Several morbidities have been observed using this mixed chimerism approach to induction of tolerance across HLA-mismatched barriers:
Myelodepression: All recipients have developed pancytopenia in conjunction with the cytoreductive conditioning regimen. Neutrophil recovery to >500/cmm was observed in all subjects between days 14 and 17 post transplantation. Platelet recovery to >20,000/cmm was observed in all subjects by day 10 post transplantation.
-
Engraftment Syndrome: Transient acute renal dysfunction, accompanied by a constellation of symptoms and signs including fever and fluid retention, was observed in 9 of the 10 recipients of HLA-mismatched kidney/BM allografts, but none of the recipients of HLA-identical transplants (Farris et al., manuscript submitted). This phenomenon has been reported to also occur in patients who receive autologous or allogeneic BM transplants during the period of hematopoietic recovery [66;67]. Accordingly, it has been termed “engraftment syndrome” or “capillary leak syndrome”.
In our subjects, the renal dysfunction consistently began between post-transplant days 10–12. Allograft dysfunction reached a peak in all subjects within 5–7 days and returned to baseline by day 60 in all subjects except the one described above, who subsequently developed TMA. Renal biopsies showed acute tubular injury, with interstitial edema, hemorrhage and capillary congestion, with little or no interstitial infiltrate (≤10%). Electron microscopy showed glomerular and peritubular (PTC) endothelial injury. While the molecular mediators of this unusual syndrome remain to be defined, we believe it is caused by an attack on the graft endothelium related to immune dysregulation or the innate immune system during recovery from the profound leukocyte depletion, which leads to transient auto- or allo-reactivity by CD8+ T cells (and in some cases, B cells) and accumulation of macrophages. This vascular injury appears to be completely reversible, even without treatment, in the absence of additional renal insult, such as CNI nephrotoxicity or humoral rejection.
Rejection following drug withdrawal: As summarized above, acute rejection was diagnosed in one of 9 recipients of HLA-identical allografts and one of 8 recipients of HLA-mismatched allografts after immunosuppressive agents were discontinued. The rejection episode was completely reversible and the re-introduced immunosuppression was then successfully withdrawn in the HLA-identical patient. The rejection episode to date has been only partially reversed (serum creatinine: 3.0–3.5 mg/dl) in the mismatched patient.
The only evidence of chronic rejection has appeared in one of the recipients of HLA-mismatched allografts. This patient developed low levels of class II antibodies two months after immunosuppression was withdrawn. Subsequent allograft biopsies have shown C4d deposits and spotty duplication of glomerular basement membranes, but renal function has remained normal, with no proteinuria, for over 60 months without resuming immunosuppressive treatment.
Mechanism of Tolerance Induction
In vitro analyses revealed the progressive development of complete donor-specific unresponsiveness in both mixed lymphocyte responses (MLR) and cell-mediated lympholysis (CML) assays in the tolerant recipients of combined kidney and BMT, with recovery of normal third party alloresponses [34]. Thus, the state of donor-specific tolerance was systemic, involving the entire immune system. In vitro results differed significantly between recipients of CKBMT and patients with hematologic malignancies who received the same haploidentical BMT regimen without a kidney transplant. In contrast to the combined transplant recipients, those receiving BMT alone, who showed generally weak alloresponses, tended to have stronger anti-donor than anti-third party responses following the loss of chimerism [68]. In combination, these results point to a role for the kidney graft itself in the long-term tolerance achieved in the recipients of CKBMT.
The development of long-term donor-specific unresponsiveness in recipients of HLA-mismatched CKBMT contrasts with in vitro results in the recipients of HLA-identical CKBMT mentioned above, who sometimes demonstrated sensitization to donor hematopoietic antigens in association with loss of chimerism [32]. While these results suggest that the mechanisms of tolerance might differ in the HLA-identical versus the mismatched transplants, a more cohesive explanation is that in both groups tolerance is restricted to antigens expressed by the kidney and does not extend to antigens that are expressed by hematopoietic cells and not the kidney. If this were the case, in patients receiving HLA-mismatched donor transplants, the strong anti-donor response existing prior to transplant would disappear following the transplant because the majority of donor MHC/peptide complexes inducing direct alloresponses are shared by both the kidney and the hematopoietic cells. Only a small minority of T cells are likely to recognize MHC alloantigen/peptide complexes expressed only on hematopoietic cells and not on other tissues. Thus, tolerance of donor antigens expressed on the kidney would lead to loss of the bulk MLR and CML response. This would not be the case in the HLA-identical setting, in which the pool of minor histocompatibility antigens expressed by the kidney is likely to overlap only partially with those expressed by hematopoietic cells. Thus, a sensitized anti-donor hematopoietic antigen-directed response (to antigens not shared by the kidney) could occur while tolerance to minor antigens expressed by the kidney (some, but not all, of which are shared by the hematopoietic cells) develops.
An alternative explanation for the early loss of chimerism in the patients receiving HLA-mismatched CKBMT, which occurred within a few weeks of transplant, when T cells were markedly depleted by the conditioning, is that the loss of chimerism may not reflect a T cell-mediated immune response but instead may reflect inadequate donor hematopoietic stem cell engraftment. Consistent with a non-immune basis for the loss of chimerism and the rapid development of donor-specific tolerance, we have detected donor-specific unresponsiveness of IL-2-producing T cells as early as 24 days post-HLA-mismatched CKBMT (Locascio et al., Transplantation, 2010, in Press}.
Evidence from our studies in monkeys suggests that the mechanism of tolerance may switch from central deletion to a peripheral mechanism that may include regulatory T cells. After this switch, the kidney allograft may be essential to the maintenance of the tolerant state. High levels of FOXP3+, a marker of regulatory T cells, without a concomitant inflammatory response (as indicated by decreased granzyme B expression) in the renal allografts of our patients [34], suggest such a regulatory mechanism, and may indicate active involvement of intragraft regulatory T cells for the maintenance of unresponsiveness, as has been shown in mice [69–72] and in swine [73–77]. Regulatory cells are enriched among the T cells initially present in recipients of this regimen ([68] and Andreola et al, manuscript submitted), and functional studies in vitro suggest that these cells may play an early role in tolerance induction, while peripheral deletion may be responsible for the long-term state of tolerance, which is systemic and complete (Andreola et al, manuscript submitted).
5. Conclusions and Future Plans
While the mouse remains the most versatile animal model for initial studies of immunologic phenomena, it is clear that there are many therapeutic modalities that have led to transplantation tolerance in mice that have not been able to be translated to large animals or humans [78;79]. Fortunately, mixed chimerism appears to be an exception, and, as reviewed here, there is considerable evidence that this modality can provide an effective means of inducing tolerance in mice, swine, monkeys and now in humans. Nevertheless, there are differences in both the methodology and the mechanisms by which tolerance is induced in the different animal models. In mice, where complete T cell depletion is possible, mixed chimerism may be long-lasting and tolerance is then based on central, clonal deletion. The maintenance of such tolerance does not depend on presence of an organ allograft and it persists even after such a graft is removed. In monkeys and in humans, T cell depletion is less complete and while central deletion may be involved early after HSC transplantation, maintenance of tolerance long-term, after peripheral chimerism has disappeared, depends on the presence of the donor organ allograft and appears to involve control of donor-specific responses by regulatory T cells. In swine, mixed chimerism can be either transient or long-lasting, depending on the preparative regimen, and both central deletion and regulatory mechanisms co-exist.
As described here, the protocol for tolerance induction through mixed chimerism that we have developed for clinical application evolved through a rational progression from mouse to large animals and then to humans. We considered this a necessary course to follow in the field of transplantation, where there are other alternative therapies that promise at least short-term success. The differences we have observed in the conditioning required and the mechanism of tolerance in these different species substantiates this consideration.
In both NHP and humans, the non-myeloablative preparative regimen that led to mixed chimerism and renal allograft tolerance has required treatment beginning several days prior to the planned organ transplantation procedure. Therefore, the clinical applicability of this regimen is currently limited to living donor recipients. Ongoing preclinical studies are therefore now focused upon defining a comparably effective regimen that can be introduced following the organ transplant procedure -- i.e. the “Delayed Tolerance” conditioning protocol. As described above, significant progress has already been achieved and we are hopeful that this much more widely applicable approach may be introduced into clinical trials in the near future.
Transplantation tolerance avoids the complications caused by immunosuppressive drugs
Mixed hematopoietic chimerism induces transplantation tolerance
Tolerance through mixed chimerism has been achieved in animal models and in humans
Both central and peripheral mechanisms can achieve long-term tolerance
Acknowledgments
The authors thank the Immune Tolerance Network and the National Institute of Allergy and infectious Diseases, NIH, for support of the clinical studies reported in this communication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance to foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 2.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307(5947):168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 3.Sachs DH, Sharabi Y, Sykes M. Mixed chimerism and transplantation tolerance. In: Melchers F, et al., editors. Progress in Immunology. Vol. VII. Berlin Heidelberg: Springer-Verlag; 1989. pp. 1171–1176. [Google Scholar]
- 4.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sykes M, Sharabi Y, Sachs DH. Achieving alloengraftment without graft-versus-host disease: approaches using mixed allogeneic bone marrow transplantation. Bone Marrow Transplant. 1988;3:379–386. [PubMed] [Google Scholar]
- 6.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease. New Engl J Med. 1981;304:1529–1533. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 7.Tomita Y, Khan A, Sykes M. Role of intrathymic clonal deletion and peripheral anergy in transplantation tolerance induced by bone marrow transplantation in mice conditioned with a non-myeloablative regimen. J Immunol. 1994;153:1087–1098. [PubMed] [Google Scholar]
- 8.Tomita Y, Sachs DH, Khan A, Sykes M. Additional mAb injections can replace thymic irradiation to allow induction of mixed chimerism and tolerance in mice receiving bone marrow transplantation after conditioning with anti-T cell mAbs and 3 Gy whole body irradiation. Transplantation. 1996;61:469–477. doi: 10.1097/00007890-199602150-00027. [DOI] [PubMed] [Google Scholar]
- 9.Tomita Y, Khan A, Sykes M. Mechanism by which additional monoclonal antibody injections overcome the requirement for thymic irradiation to achieve mixed chimerism in mice receiving bone marrow transplantation after conditioning with anti-T cell mAbs and 3 Gy whole body irradiation. Transplantation. 1996;61:477–485. doi: 10.1097/00007890-199602150-00028. [DOI] [PubMed] [Google Scholar]
- 10.Khan A, Tomita Y, Sykes M. Thymic dependence of loss of tolerance in mixed allogeneic bone marrow chimeras after depletion of donor antigen. Peripheral mechanisms do not contribute to maintenance of tolerance. Transplantation. 1996;62:380–387. doi: 10.1097/00007890-199608150-00014. [DOI] [PubMed] [Google Scholar]
- 11.Manilay JO, Pearson DA, Sergio JJ, Swenson KG, Sykes M. Intrathymic deletion of alloreactive T cells in mixed bone marrow chimeras prepared with a nonmyeloablative conditioning regimen. Transplantation. 1998;66:96–102. doi: 10.1097/00007890-199807150-00015. [DOI] [PubMed] [Google Scholar]
- 12.Cobbold SP, Qin S, Waldmann H. Reprogramming the immune system for tolerance with monoclonal antibodies. Sem Immunol. 1990;2:377–387. [PubMed] [Google Scholar]
- 13.Nikolic B, Khan A, Sykes M. Induction of tolerance by mixed chimerism with nonmyeloblative host conditioning: the importance of overcoming intrathymic alloresistance. Biol Blood Marrow Transplant. 2001;7:144–153. doi: 10.1053/bbmt.2001.v7.pm11302548. [DOI] [PubMed] [Google Scholar]
- 14.Wekerle T, Sayegh MH, Hill J, Zhao Y, Chandraker A, Swenson KG, et al. Extrathymic T cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T cell tolerance. J Exp Med. 1998;187:2037–2044. doi: 10.1084/jem.187.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Ann Rev Immunol. 2000;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 16.Wekerle T, Kurtz J, Ito H, Ronquillo JV, Dong V, Zhao G, et al. Allogeneic bone marrow transplantation with costimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nature Med. 2000;6:464–469. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 17.Ito H, Kurtz J, Shaffer J, Sykes M. CD4 T cell-mediated alloresistance to fully MHC-mismatched allogeneic bone marrow engraftment is dependent on CD40-CD40L interactions, and lasting T cell tolerance is induced by bone marrow transplantation with initial blockade of this pathway. J Immunol. 2001;166:2970–2981. doi: 10.4049/jimmunol.166.5.2970. [DOI] [PubMed] [Google Scholar]
- 18.Kurtz J, Shaffer J, Anosova N, Benichou G, Sykes M. Mechanisms of early peripheral CD4 T cell tolerance induction by anti-CD154 monoclonal antibody and allogeneic bone marrow transplantation: Evidence for anergy and deletion, but not regulatory cells. Blood. 2004;103:4336–4343. doi: 10.1182/blood-2003-08-2642. [DOI] [PubMed] [Google Scholar]
- 19.Fehr T, Takeuchi Y, Kurtz J, Sykes M. Early regulation of CD8 T cell alloreactivity by CD4+CD25- T cells in recipients of anti-CD154 antibody and allogeneic BMT is followed by rapid peripheral deletion of donor-reactive CD8+ T cells, precluding a role for sustained regulation. Eur J Immunol. 2005;35:2679–2690. doi: 10.1002/eji.200526190. [DOI] [PubMed] [Google Scholar]
- 20.Fehr T, Haspot F, Mollov J, Chittenden M, Hogan T, Sykes M. Alloreactive CD8 T cell tolerance requires recipient B cells, dendritic cells and MHC class II. J Immunol. 2008;181:165–173. doi: 10.4049/jimmunol.181.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haspot F, Fehr T, Gibbons C, Zhao G, Hogan T, Honjo T, et al. Peripheral deletional tolerance of alloreactive CD8 but not CD4 cells is dependent on the PD-1/PD-L1 pathway. Blood. 2008;112:2149–2155. doi: 10.1182/blood-2007-12-127449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtz J, Ito H, Wekerle T, Shaffer J, Sykes M. Mechanisms involved in the establishment of tolerance through costimulatory blockade and BMT: Lack of requirement for CD40L-mediated signaling for tolerance or deletion of donor-reactive CD4+ cells. Am J Transplant. 2001;1:339–349. doi: 10.1034/j.1600-6143.2001.10409.x. [DOI] [PubMed] [Google Scholar]
- 23.Wekerle T, Sayegh MH, Chandraker A, Swenson KG, Zhao Y, Sykes M. Role of peripheral clonal deletion in tolerance induction with bone marrow transplantation and costimulatory blockade. Transplant Proc. 1999;31:680. doi: 10.1016/s0041-1345(98)01605-4. [DOI] [PubMed] [Google Scholar]
- 24.Bigenzahn S, Blaha P, Koporc Z, Pree I, Selzer E, Bergmeister H, et al. The role of non-deletional tolerance mechanisms in a murine model of mixed chimerism with costimulation blockade. Am J Transplant. 2005;5:1237–1247. doi: 10.1111/j.1600-6143.2005.00862.x. [DOI] [PubMed] [Google Scholar]
- 25.Domenig C, Sanchez-Fueyo A, Kurtz J, Alexopoulos SP, Mariat C, Sykes M, et al. Roles of deletion and regulation in creating mixed chimerism and allograft tolerance using a nonlymphoablative irradiation-free protocol. J Immunol. 2005;175:51–60. doi: 10.4049/jimmunol.175.1.51. [DOI] [PubMed] [Google Scholar]
- 26.Mapara MY, Kim Y-M, Marx J, Sykes M. DLI-mediated GVL effects in mixed chimeras established with a non-myeloablative conditioning regimen: extinction of GVL effects coincides with loss of alloreactive cells following conversion to full donor chimerism. Transplantation. 2003;76:297–305. doi: 10.1097/01.TP.0000072014.83469.2D. [DOI] [PubMed] [Google Scholar]
- 27.Mapara MY, Kim Y-M, Wang S-P, Bronson R, Sachs DH, Sykes M. Donor lymphocyte infusions mediate superior graft-versus-leukemia effects in mixed compared to fully allogeneic chimeras: a critical role for host antigen-presenting cells. Blood. 2002;100:1903–1909. doi: 10.1182/blood-2002-01-0023. [DOI] [PubMed] [Google Scholar]
- 28.Boyd RL, Hugo P. Towards an integrated view of thymopoiesis. Immunol Today. 1991;12:71–79. doi: 10.1016/0167-5699(91)90161-L. [DOI] [PubMed] [Google Scholar]
- 29.Chakraverty R, Eom HS, Sachs J, Buchli J, Cotter P, Hsu R, et al. Host MHC Class II+ antigen-presenting cells and CD4 cells are required for CD8-mediated graft-versus-leukemia responses following delayed donor leukocyte infusions. Blood. 2006;108:2106–2113. doi: 10.1182/blood-2006-03-007427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spitzer TR, MCafee S, Sackstein R, Colby C, Toh HC, Multani P, et al. The intentional induction of mixed chimerism and achievement of anti-tumor responses following non-myeloablative conditioning therapy and HLA-matched and mismatched donor bone marrow transplantation for refractory hematologic malignancies. Biol Blood Marrow Transplant. 2000;6:309–320. doi: 10.1016/s1083-8791(00)70056-5. [DOI] [PubMed] [Google Scholar]
- 31.Dey BR, MCafee S, Colby C, Sackstein R, Saidman S, Tarbell N, et al. Impact of prophylactic donor leukocyte infusions on mixed chimerism, graft-vs-host disease and anti-tumor response in patients with advanced hematologic malignancies treated with nonmyeloablative conditioning and allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2003;9:320–329. doi: 10.1016/s1083-8791(03)00077-6. [DOI] [PubMed] [Google Scholar]
- 32.Fudaba Y, Spitzer TR, Shaffer J, Kawai T, Fehr T, Delmonico F, et al. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: in vivo and in vitro analyses. Am J Transplant. 2006;6:2121–2133. doi: 10.1111/j.1600-6143.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- 33.Spitzer TR, McAfee SL, Dey BR, Colby C, Hope J, Grossberg H, et al. Nonmyeloablative haploidentical stem-cell transplantation using anti-CD2 monoclonal antibody (MEDI-507)-based conditioning for refractory hematologic malignancies. Transplantation. 2003;75:1748–1751. doi: 10.1097/01.TP.0000064211.23536.AD. [DOI] [PubMed] [Google Scholar]
- 34.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gress R, Moses R, Suzuki T, Lowman M, Pennington L, Sakamoto K, et al. Biparental bone marrow transplantation as a means of tolerance induction. Transplant Proc. 1987;19:95–97. [PubMed] [Google Scholar]
- 36.Pennington LR, Sakamoto K, Popitz-Bergez FA, Pescovitz MD, McDonough MA, MacVittie TJ, et al. Bone marrow transplantation in miniature swine. I. Development of the model. Transplantation. 1988;45:21–26. doi: 10.1097/00007890-198801000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Pennington LR, Popitz F, Sakamoto K, Pescovitz MD, Sachs DH. Bone marrow transplantation in miniature swine: I. Autologous and SLA matched allografts. In: Tumbleson ME, editor. Swine in biomedical research. New York: Plenum Press; 1986. pp. 377–383. [Google Scholar]
- 38.Popitz-Bergez FA, Sakamoto K, Pennington LR, Pescovitz MD, McDonough MA, MacVittie TJ, et al. Bone marrow transplantation in miniature swine. II. Effect of selective genetic differences on marrow engraftment and recipient survival. Transplantation. 1988;45:27–31. [PubMed] [Google Scholar]
- 39.Sakamoto K, Sachs DH, Shimada S, Popitz-Bergez FA, Pennington LR, Pescovitz MD, et al. Bone marrow transplantation in miniature swine. III Graft-versus-host disease and the effect of T cell depletion of marrow. Transplantation. 1988;45:869–875. [PubMed] [Google Scholar]
- 40.Huang CA, Fuchimoto Y, Scheier-Dolberg R, Murphy MC, Neville DMJ, Sachs DH. Stable mixed chimerism and tolerance using a nonmyeloablative preparative regimen in a large-animal model. J Clin Invest. 2000;105:173–181. doi: 10.1172/JCI7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lima B, Gleit ZL, Cameron AM, Germana S, Murphy MC, Consorti R, et al. Engraftment of quiescent progenitors and conversion to full chimerism after nonmyelosuppressive conditioning and hematopoietic cell transplantation in miniature swine. Biol Blood Marrow Transplant. 2003;9:571–582. doi: 10.1016/s1083-8791(03)00227-1. [DOI] [PubMed] [Google Scholar]
- 42.Colby C, Chang Q, Fuchimoto Y, Ferrara V, Murphy M, Sackstein R, et al. Cytokine-mobilized peripheral blood progenitor cells for allogeneic reconstitution of miniature swine. Transplantation. 2000;69:135–140. doi: 10.1097/00007890-200001150-00023. [DOI] [PubMed] [Google Scholar]
- 43.Fuchimoto Y, Huang CA, Yamada K, Shimizu A, Kitamura H, Colvin RB, et al. Mixed chimerism and tolerance without whole body irradiation in a large animal model. J Clin Invest. 2000;105:1779–1789. doi: 10.1172/JCI8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gleit ZL, Fuchimoto Y, Yamada K, Melendy E, Scheier-Dolberg R, Monajati L, et al. Variable relationship between chimerism and tolerance after hematopoietic cell transplantation without myelosuppressive conditioning. Transplantation. 2002;74:1535–1544. doi: 10.1097/00007890-200212150-00010. [DOI] [PubMed] [Google Scholar]
- 45.Schwarze ML, Menard MT, Fuchimoto Y, Huang CA, Houser S, Mawulawde K, et al. Mixed hematopoietic chimerism induces long term tolerance to cardiac allografts in miniature swine. Ann Thorac Surg. 2000;70:131–138. doi: 10.1016/s0003-4975(00)01564-2. [DOI] [PubMed] [Google Scholar]
- 46.Horner BM, Cina RA, Wikiel KJ, Lima B, Ghazi A, Lo DP, et al. Predictors of organ allograft tolerance following hematopoietic cell transplantation. Am J Transplant. 2006;6:2894–2902. doi: 10.1111/j.1600-6143.2006.01563.x. [DOI] [PubMed] [Google Scholar]
- 47.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256–262. [PubMed] [Google Scholar]
- 49.Kawai T, Poncelet A, Sachs DH, Mauiyyedi S, Boskovic S, Wee SL, et al. Long-term outcome and alloantibody production in a non-myeloablative regimen for induction of renal allograft tolerance. Transplantation. 1999;68:1767–1775. doi: 10.1097/00007890-199912150-00022. [DOI] [PubMed] [Google Scholar]
- 50.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand [letter] Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 51.Koyama I, Kawai T, Andrews D, Boskovic S, Nadazdin O, Wee SL, et al. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation. 2004;77:460–462. doi: 10.1097/01.TP.0000110291.29370.C0. [DOI] [PubMed] [Google Scholar]
- 52.Pitcher CJ, Hagen SI, Walker JM, Lum R, Maino VC, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 53.Nadazdin O, Boskovic S, Murakami T, O'Connor DH, Wiseman RW, Karl JA, et al. Phenotype, distribution and alloreactive properties of memory T cells from cynomolgus monkeys. Am J Transplant. 2010;10:1375–1384. doi: 10.1111/j.1600-6143.2010.03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koyama I, Nadazdin O, Boskovic S, Ochiai T, Smith RN, Sykes M, et al. Depletion of CD8 memory T cells for induction of tolerance of a previously transplanted kidney allograft. Am J Transplant. 2007;7:1055–1061. doi: 10.1111/j.1600-6143.2006.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawai T, Sogawa H, Koulmanda M, Smith RN, O'Neil JJ, Wee SL, et al. Long-term islet allograft function in the absence of chronic immunosuppression: a case report of a nonhuman primate previously made tolerant to a renal allograft from the same donor. Transplantation. 2001;72:351–354. doi: 10.1097/00007890-200107270-00036. [DOI] [PubMed] [Google Scholar]
- 56.Sayegh MH, Fine NA, Smith JL, Rennke HG, Milford EL, Tilney NL. Immunologic tolerance to renal allografts after bone marrow transplants from the same donors. Ann Intern Med. 1991;114:954–955. doi: 10.7326/0003-4819-114-11-954. [DOI] [PubMed] [Google Scholar]
- 57.Helg C, Chapuis B, Bolle J-F, Morel P, Salomon D, Roux E, et al. Renal transplantation without immunosuppression in a host with tolerance induced by allogeneic bone marrow transplantation. Transplantation. 1994;58:1420–1422. [PubMed] [Google Scholar]
- 58.Jacobsen N, Taaning E, Ladefoged J, Kristensen JK, Pedersen FK. Tolerance to an HLA-B,DR disparate kidney allograft after bone- marrow transplantation from same donor. Lancet. 1994;343:800. doi: 10.1016/s0140-6736(94)91881-3. [DOI] [PubMed] [Google Scholar]
- 59.Dey B, Sykes M, Spitzer TR. Outcomes of recipients of both bone marrow and solid organ transplants. A review. Medicine (Baltimore) 1998;77:355–369. doi: 10.1097/00005792-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Dey BR, MCafee S, Sackstein R, Colby C, Saidman S, Weymouth D, et al. Successful allogeneic stem cell transplantation with nonmyeloablative conditioning in patients with relapsed hematologic malignancy following autologous stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:604–612. doi: 10.1053/bbmt.2001.v7.pm11760148. [DOI] [PubMed] [Google Scholar]
- 61.Spitzer TR, Delmonico F, Tolkoff-Rubin N, McAfee S, Sackstein R, Saidman S, et al. Combined histocompatibility leukocyte antigen-matched donor bone marrow and renal transplantation for multiple myeloma with end stage renal disease: the induction of allograft tolerance through mixed lymphohematopoietic chimerism. Transplantation. 1999;68:480–484. doi: 10.1097/00007890-199908270-00006. [DOI] [PubMed] [Google Scholar]
- 62.Buhler LH, Spitzer TR, Sykes M, Sachs DH, Delmonico FL, Tolkoff-Rubin N, et al. Induction of kidney allograft tolerance after transient lymphohematopoietic chimerism in patients with multiple myeloma and end-stage renal disease. Transplantation. 2002;74:1405–1409. doi: 10.1097/00007890-200211270-00011. [DOI] [PubMed] [Google Scholar]
- 63.Newell KA, Larsen CP. Toward transplantation tolerance: a large step on a long road. Am J Transplant. 2006;6:1989–1990. doi: 10.1111/j.1600-6143.2006.01481.x. [DOI] [PubMed] [Google Scholar]
- 64.Sykes M, Preffer F, McAffee S, Saidman SL, Colby C, Sackstein R, et al. Mixed lymphohematopoietic chimerism and graft-vs-lymphoma effects are achievable in adult humans following non-myeloablative therapy and HLA-mismatched donor bone marrow transplantation. Lancet. 1999;353:1755–1759. doi: 10.1016/S0140-6736(98)11135-2. [DOI] [PubMed] [Google Scholar]
- 65.Spitzer TR, MCafee S, Sackstein R, Colby C, Dey B, Saidman S, et al. Mixed lymphohematopoietic chimerism and delayed donor leukocyte infusions following non-myeloablative conditioning and HLA-matched and mismatched donor bone marrow transplantation. In: Dicke KA, Keating A, editors. Autologous Bone Marrow Transplantation: Proceedings of the 10th International Symposium; Carden Jennings, Charlottesville: 2001. pp. 321–330. [Google Scholar]
- 66.Cahill RA, Spitzer TR, Mazumder A. Marrow engraftment and clinical manifestations of capillary leak syndrome. Bone Marrow Transplant. 1996;18:177–184. [PubMed] [Google Scholar]
- 67.Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:893–898. doi: 10.1038/sj.bmt.1703015. [DOI] [PubMed] [Google Scholar]
- 68.Shaffer J, Villard J, Means TK, Alexander S, Dombkowski D, Dey BR, et al. Regulatory T-cell recovery in recipients of haploidentical nonmyeloablative hematopoietic cell transplantation with a humanized anti-CD2 mAb, MEDI-507, with or without fludarabine. Exp Hematol. 2007;35:1140–1152. doi: 10.1016/j.exphem.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, et al. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 70.Fehervari Z, Sakaguchi S. Development and function of CD25+CD4+ regulatory T cells. Curr Opin Immunol. 2004;16:203–208. doi: 10.1016/j.coi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 71.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med. 2002;195:1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kurtz J, Shaffer J, Lie A, Anosova N, Benichou G, Sykes M. Mechanisms of early peripheral CD4 T-cell tolerance induction by anti-CD154 monoclonal antibody and allogeneic bone marrow transplantation: evidence for anergy and deletion but not regulatory cells. Blood. 2004;103:4336–4343. doi: 10.1182/blood-2003-08-2642. [DOI] [PubMed] [Google Scholar]
- 73.Baron C, McMorrow I, Sachs DH, LeGuern C. Persistence of dominant T cell clones in accepted solid organ transplants. J Immunol. 2001;167:4154–4160. doi: 10.4049/jimmunol.167.8.4154. [DOI] [PubMed] [Google Scholar]
- 74.Griesemer AD, LaMattina JC, Okumi M, Etter JD, Shimizu A, Sachs DH, et al. Linked suppression across an MHC-mismatched barrier in a miniature swine kidney transplantation model. J Immunol. 2008;181:4027–4036. doi: 10.4049/jimmunol.181.6.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ierino FL, Yamada K, Hatch T, Sachs DH. Preliminary in vitro evidence for regulatory cells in a miniature swine renal allograft model. Transplant Proc. 1997;29:1165. doi: 10.1016/s0041-1345(96)00515-5. [DOI] [PubMed] [Google Scholar]
- 76.Mezrich JD, Kesselheim JA, Johnston DR, Yamada K, Sachs DH, Madsen JC. The role of regulatory cells in miniature swine rendered tolerant to cardiac allografts by donor kidney cotransplantation. Am J Transplant. 2003;3:1107–1115. doi: 10.1046/j.1600-6143.2003.00202.x. [DOI] [PubMed] [Google Scholar]
- 77.Wu A, Yamada K, Ierino FL, Vagefi PA, Sachs DH. Regulatory mechanism of peripheral tolerance: in vitro evidence for dominant suppression of host responses during the maintenance phase of tolerance to renal allografts in miniature swine. Transpl Immunol. 2003;11:367–374. doi: 10.1016/S0966-3274(03)00006-6. [DOI] [PubMed] [Google Scholar]
- 78.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sachs DH. Tolerance: of mice and men. J Clin Invest. 2003;111:1819–1821. doi: 10.1172/JCI18926. [DOI] [PMC free article] [PubMed] [Google Scholar]