Abstract
Recent evidence suggests that yawning is a thermoregulatory behavior. To explore this possibility further, the frequency of contagious yawning in humans was measured while outdoors in a desert climate in the United States during two distinct temperature ranges and seasons (winter: 22°C; early summer: 37°C). As predicted, the proportion of pedestrians who yawned in response to seeing pictures of people yawning differed significantly between the two conditions (winter: 45%; summer: 24%). Across conditions yawning occurred at lower ambient temperatures, and the tendency to yawn during each season was associated with the length of time spent outside prior to being tested. Participants were more likely to yawn in the milder climate after spending long periods of time outside, while prolonged exposure to ambient temperatures at or above body temperature was associated with reduced yawning. This is the first report to show that the incidence of yawning in humans is associated with seasonal climate variation, further demonstrating that yawn-induced contagion effects can be mediated by factors unrelated to individual social characteristics or cognitive development.
Keywords: yawning, contagious yawning, brain cooling, thermoregulation, thermal window
Introduction
Numerous hypotheses have been proposed to explain why vertebrates yawn, and the two prevailing hypotheses favor either physiological or social function (Guggisberg et al., 2010). Physiological hypotheses predict that yawning acts to aid in the regulation of a given body state (e.g., brain temperature or arousal), while the social hypothesis predicts that yawning functions to synchronize group behavior by communicating unpleasant mental and physical states. While experimental and observational evidence of contagious yawning in humans (e.g., Provine, 1986; Platek et al., 2003), a few select non-human primate species (chimpanzees, Pan troglodytes; stumptail macaques, Macaca arctoides; gelada baboons, Theropithecus gelada; Anderson et al., 2004; Paukner and Anderson, 2006; Campbell et al., 2009; Palagi et al., 2009), and dogs (Joly-Mascheroni et al., 2008; but see Harr et al., 2009 and O’Hara and Reeve, 2011) provides support that yawning may have a social role, tests of the specific predictions of the social model are lacking and it is unknown how yawning could reliably transmit specific information during varying contexts (Gallup, 2011). Furthermore, the ubiquity of non-social yawning across vertebrate classes (e.g., Baenninger, 1987) suggests that it has an underlying physiological significance, and thus may have multiple functional outcomes across species.
Although it is still commonly believed that yawning functions to equilibrate O2 and CO2 levels in the blood, there is no support for this hypothesis and it has been concluded that yawning and breathing operate by different mechanisms (Provine et al., 1987). On the other hand, one of the physiological hypotheses receiving growing support proposes a role of yawning in brain thermoregulation (Gallup and Gallup, 2007, 2008). Recent evidence for this model comes from prelimbic brain temperature recordings in rats (Rattus norvegicus), showing that yawning is preceded by rapid increases in brain temperature and followed by corresponding decreases in brain temperature (Shoup-Knox et al., 2010). According to this model, cooling is primarily the result of enhanced cerebral blood flow and countercurrent heat exchange with ambient air. Accordingly, the temperature of the ambient air is what gives a yawn its utility. Thus yawning should be counterproductive – and therefore suppressed – in ambient temperatures at or exceeding body temperature, as taking a deep inhalation of air would no longer promote cooling. In other words, there should be a “thermal window” or a relatively narrow range of ambient temperatures in which to expect highest rates of yawning (Gallup and Gallup, 2007). Experimental studies on birds (Melopsittacus undulatus) and rats (Rattus norvegicus) support this view, showing that changes solely in ambient temperature are sufficient to influence yawning frequency (Gallup et al., 2009, 2010, 2011). In particular, yawns become more frequent during initial increases in ambient temperature, but then decrease as temperatures approach body temperature.
The current study explored this relationship in humans by measuring the incidence of contagious yawning while outdoors during two distinct periods of temperature and season (winter and early summer) in Arizona, USA. The summer condition provided temperatures that matched or slightly exceeded body temperature with relatively low humidity, while the corresponding winter condition included milder temperatures and slightly higher humidity. It was predicted that yawning would be less frequent in summer trials, but that it may be common just after entering the outside conditions since adaptive thermoregulatory responses take time to occur after encountering sudden climate changes. However, the rate of yawning should diminish sharply as the amount of time spent outside increases, since it would no longer result in a cooling response. Contagious yawning was used as a proxy for yawning in both conditions because it is indistinguishable from spontaneous yawning (aside from the fact that the triggers differ), and it can be manipulated using visual stimuli (e.g., Platek et al., 2003). In other words, a stretching of the jaw and a deep inhalation of air accompany both responses, and thus the physiological consequences should be similar. Furthermore, the study of contagious yawning provides the opportunity to determine whether underlying features related to thermal homeostasis mediate socially derived aspects of this behavior.
Materials and Methods
Participants
These experiments were conducted in the city of Tucson, AR, USA (Lat: 32.12 Lon: 110.93) separately during the months of June 2010 (early summer) and February 2011 (winter). All testing occurred on days that were mostly sunny and did not rain (average temperature range 22–37°C). In total, 80 participants were recruited during each season (winter: 38 males, 42 females; summer: 32 males, 48 females). All participants were over 18 years of age, gave verbal consent to participate in this study, and the Human Subjects Review Board at Binghamton University approved this research.
Procedure
Pedestrians were approached by trained researchers while walking outside in public (sidewalks and courtyards) and asked to participate in a survey about contagious yawning. The survey itself served as the contagious yawning stimulus, which included 20 images of people yawning followed by questions focused on the individuals’ own yawning and sleeping behavior. After viewing the images, participants self-reported on (1) whether they yawned during the experiment (for validity of this approach see Greco and Baenninger, 1989), (2) how many hours they slept the night before, and (3) how long they had been continuously outside before being approached to partake in this study. One person in the winter condition and two individuals in the summer condition did not respond to the last question.
While participants were taking the survey, the experimenters recorded the relative humidity and temperature of the ambient air at the time of the experiment using a digital thermometer/hygrometer that was positioned in the shade. Because research suggests that participants are less likely to yawn when they are being observed (Baenninger and Greco, 1991), the experimenters immediately walked away from the participants after they agreed to take the survey and intentionally avoided directing their attention toward them until it was completed. While post-survey yawns were not used in the analysis, it is notable that four participants in the winter condition did not report yawning during the experiment but yawned while handing in the survey to the experimenter.
Based on recent research showing no difference in contagious yawning rates between the hours of 1230 and 1630 (Giganti and Zilli, 2011), all participant recruitment occurred from 1300 to 1500 h. While it is possible that differences in the day/light cycles (February: sunrise 7:05 AM, sunset 6:10 PM; June: sunrise 5:17 AM, sunset 7:32 PM) may influence circadian yawning rates, comparable shifts in our testing range would encompass periods of the day where contagious yawning frequencies are similar. Therefore any difference in the incidence of contagious yawning between seasons should not be result of this distinction.
Statistical analyses were performed across and between conditions. Chi-square tests were used to test for differences in yawning frequency between seasonal climate conditions. Independent samples t-tests were performed to investigate differences between the two conditions in relative humidity, ambient temperature, time spent outside prior to testing, and sleep reported the night before. Lastly, a binary logistic regression was used to explore which variables were associated with the tendency to yawn across and between conditions, with Nagelkerke R2 being reported for significant results.
Results
Across conditions
In total, 55 of the 160 (34.4%) participants reported yawning while being exposed to the contagious yawning stimulus. There were no sex differences in the response of social contagion, with 24 of 70 (34.3%) males and 31 of 90 (34.4%) females reporting yawning. When taking into account the amount of sleep the night before, the time spent outside prior to testing, and the relative humidity and temperature of the ambient air, temperature was the only significant predictor of contagious yawning (binary logistic regression: R2 = 0.101, β = −0.074, p = 0.005). In other words, yawning occurred more frequently at lower ambient temperatures after controlling for other variables.
Between conditions
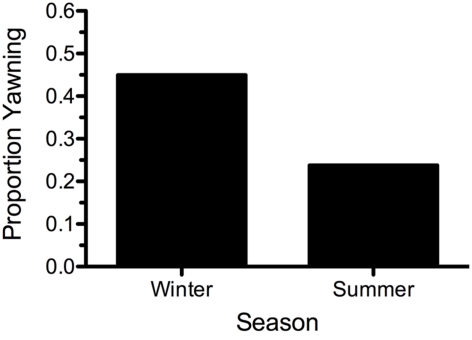
Table 1 shows the descriptive statistics for all variables both within and across conditions. During the summer when the climate was significantly warmer and dryer, pedestrians spent less time outdoors prior to testing. The proportion of participants who reported yawning differed significantly between the two conditions (χ2 (1) = 10.391, p = 0.001, Cohen’s w = 0.255; see Figure 1), with 36 of 80 (45.0%) individuals yawning in the winter, and only 19 of 80 (23.8%) yawning in the summer.
Table 1.
Descriptive statistics.
| Total | Winter | Summer | t-Value | p-Value | Cohen’s d | |
|---|---|---|---|---|---|---|
| Temp (°C) | 29.46 ± 8.01 | 21.92 ± 3.60 | 37.00 ± 0.94 | −36.225 | <0.001 | −5.732 |
| Humidity | 12.12 ± 13.75 | 19.00 ± 16.83 | 5.21 ± 1.40 | 7.276 | <0.001 | 1.155 |
| Time (min) | 23.13 ± 29.24 | 30.05 ± 33.22 | 16.13 ± 22.70 | 3.062 | 0.003 | 0.499 |
| Sleep (h) | 6.86 ± 1.64 | 6.93 ± 1.78 | 6.78 ± 1.49 | 0.578 | 0.564 | 0.091 |
Mean and SD are provided for each variable across and between conditions (time represents the time spent outside prior to participating). Independent t-test comparisons are between the winter and summer conditions.
Figure 1.
The proportion of participants yawning per seasonal climate condition.
Similar binary logistic regression analyses were conducted separately for each seasonal condition. In the winter, the amount of time spent outside was positively associated with the incidence of yawning (R2 = 0.242, β = 0.024, p = 0.047), with no other variables showing significant effects. However, the summer condition showed the exact inverse relationship, indicating that the less time an individual was exposed to the climate conditions (i.e., ambient temperatures at or exceeding body temperature with low relative humidity) the more likely they were to yawn (R2 = 0.297, β = −0.190, p = 0.029).
Discussion
As predicted from the thermal window hypothesis of thermoregulatory model, the incidence of contagious yawning in humans was influenced by seasonal variation in climate conditions. According to this hypothesis, it is the temperature of the ambient air that gives a yawn its cooling utility and thus it was expected that yawning should be diminished when outdoor temperatures reach or exceed body temperature. Across seasonal trials yawning was associated with lower ambient temperatures, and in particular, yawning was less frequent in the summer condition when temperatures were higher and humidity was lower. Furthermore, the proportion of individuals yawning in the summer dropped greatly as the length of time spent outside increased, suggesting that the expression of social yawning may reflect a compromise with thermal effects. On the other hand, there was a positive relationship between time spent outdoors prior to testing and yawning in the winter condition. Since all trials were conducted in direct sunlight (where temperature exceeded that recorded in the shade), it seems likely that thermoregulatory responses will increase over time under these conditions, thus explaining this relationship.
The findings of this report are consistent with previous research suggesting that the triggers for spontaneous yawning are sensitive to ambient air temperature and mechanisms of brain thermoregulation. Recent experimental and observational research has shown a connection between yawning and ambient temperature manipulation/variation in other animals (Deputte, 1994; Campos and Fedigan, 2009; Gallup et al., 2009, 2010, 2011). Due to inherent limitations of field research, future studies should experimentally investigate the association between human yawning and more refined ambient temperature ranges in the laboratory. In addition, research should investigate the frequency of yawning in extreme cold climates since yawning in severe cold temperatures would be expected to have adverse effects on brain thermoregulation, and thus should be inhibited. Moreover, considering yawning or yawn-like behaviors are ubiquitous across vertebrate classes, research should explore the relationship between yawning and seasonal climate variation in poikilotherms.
This study is the first to show that the expression of yawning in response to contagious stimuli can be altered by variation in seasonal climatic conditions. Furthermore, while previous research has shown that specific individual social characteristics and cognitive developmental factors are involved with the susceptibility to yawning contagiously in humans (e.g., Anderson and Meno, 2003; Platek et al., 2003; Senju et al., 2007; Millen and Anderson, 2010), this study further indicates that yawn-induced contagion effects can also be mediated by non-social factors (e.g., ambient temperature). These findings extend the logic of the thermal window hypothesis to humans, demonstrating a highly conserved, comparative aspect to the expression of this behavior. Consistent with the thermoregulatory model, these results dovetail with recent research showing that methods of behavioral brain cooling (nasal breathing and forehead cooling) inhibit contagious yawning in humans (Gallup and Gallup, 2007). Taken together, future studies of contagious yawning should be assessed under conditions where thermal effects are essentially neutral, or at least consistent, across conditions.
In summary, while the utility of yawning as a contagious behavior is still under debate, the current findings suggest the underlying mechanism for this response may be involved with thermoregulatory physiology, further challenging the view that the origin of yawning is social (Guggisberg et al., 2010).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the three reviewers for their helpful comments during revisions. We would also like to thank Michael Miller and Gordon Gallup for providing comments on earlier drafts of this paper, and Kyle Rizzo and Hassibullah Kushkaki for helping with data collection. Funding was provided by the Center for Insect Science, University of Arizona, NIH grant 5 K12 GM000708.
References
- Anderson J. R., Meno P. (2003). Psychological influences on yawning in children. Curr. Psychol. Lett. Behav. Brain Cog. 11 [Google Scholar]
- Anderson J. R., Myowa-Yamakoshi M., Matsuzawa T. (2004). Contagious yawning in chimpanzees. Proc. R. Soc. Lond. B Biol. Sci. 271, S468–S470 10.1098/rsbl.2004.0224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenninger R. (1987). Some comparative aspects of yawning in Betta splendens, Homo sapiens, Panthera leo, and Papio sphinx. J. Comp. Psychol. 101, 349–354 10.1037/0735-7036.101.4.349 [DOI] [Google Scholar]
- Baenninger R., Greco M. (1991). Some antecedents and consequences of yawning. Psychol. Rec. 41, 453–460 [Google Scholar]
- Campbell M. W., Carter J. D., Proctor D., Eisenberg M. L., de Waal F. B. M. (2009). Computer animations stimulate contagious yawning in chimpanzees. Proc. R. Soc. Lond. B Biol. Sci. 276, 4255–4259 10.1098/rspb.2009.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos F. A., Fedigan L. M. (2009). Behavioural adaptations to heat stress and water scarcity in white-faced capuchins (Cebus capucinus) in Santa Rosa National Park, Costa Rica. Am. J. Phys. Anthropol. 138, 101–111 10.1002/ajpa.20908 [DOI] [PubMed] [Google Scholar]
- Deputte B. L. (1994). Ethological study of yawning in primates. I. Quantitative analysis and study of causation in two species of old world monkeys (Cercocebus albigena and Macaca fascicularis). Ethology 98, 221–245 10.1111/j.1439-0310.1994.tb01073.x [DOI] [Google Scholar]
- Gallup A. C. (2011). Why do we yawn? Primitive versus derived features. Neurosci. Biobehav. Rev. 35, 765–769 10.1016/j.neubiorev.2010.09.009 [DOI] [PubMed] [Google Scholar]
- Gallup A. C., Gallup G. G., Jr. (2007). Yawning as a brain cooling mechanism: nasal breathing and forehead cooling diminish the incidence of contagious yawning. Evol. Psychol. 5, 92–101 [Google Scholar]
- Gallup A. C., Gallup G. G., Jr. (2008). Yawning and thermoregulation. Physiol. Behav. 95, 10–16 10.1016/j.physbeh.2008.05.003 [DOI] [PubMed] [Google Scholar]
- Gallup A. C., Miller M. L., Clark A. B. (2009). Yawning and thermoregulation in budgerigars (Melopsittacus undulatus). Anim. Behav. 77, 109–113 10.1016/j.anbehav.2008.09.014 [DOI] [Google Scholar]
- Gallup A. C., Miller M. L., Clark A. B. (2010). The direction and range of ambient temperature influences yawning in budgerigars (Melopsittacus undulatus). J. Comp. Psychol. 124, 133–138 10.1037/a0018006 [DOI] [PubMed] [Google Scholar]
- Gallup A. C., Miller R. R., Clark A. B. (2011). Changes in ambient temperature trigger yawning but not stretching in rats. Ethology 117, 145–153 10.1111/j.1439-0310.2010.01854.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giganti F., Zilli I. (2011). The daily time course of contagious and spontaneous yawning among humans. J. Ethol. 29, 215–219 10.1007/s10164-010-0242-0 [DOI] [Google Scholar]
- Greco M., Baenninger R. (1989). Self-report as a valid measure of yawning in the laboratory. Bull. Psychon. Soc. 27, 75–76 [Google Scholar]
- Guggisberg A. G., Mathis J., Schnider A., Hess C. W. (2010). Why do we yawn? Neurosci. Biobehav. Rev. 34, 1267–1276 10.1016/j.neubiorev.2010.03.008 [DOI] [PubMed] [Google Scholar]
- Harr A., Gilbert V., Phillips K. (2009). Do dogs (Canis familiaris) show contagious yawning? Anim. Cogn. 12, 833–837 10.1007/s10071-009-0233-0 [DOI] [PubMed] [Google Scholar]
- Joly-Mascheroni R. M., Senju A., Shepherd A. J. (2008). Dogs catch human yawns. Biol. Lett. 4, 446–448 10.1098/rsbl.2008.0333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen A., Anderson J. R. (2010). Neither infants nor toddlers catch yawns from their mothers. Biol. Lett. 7, 440–442 10.1098/rsbl.2010.0966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara S. J., Reeve A. V. (2011). A test of the yawning contagion and emotional connectedness hypothesis in dogs, Canis familiaris. Anim. Behav. 81, 335–340 10.1016/j.anbehav.2010.11.005 [DOI] [Google Scholar]
- Palagi E., Leone A., Mancini G., Ferrari P. F. (2009). Contagious yawning in gelada baboons as a possible expression of empathy. Proc. Natl. Acad. Sci. U.S.A. 106, 19262–19267 10.1073/pnas.0910891106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukner A., Anderson J. R. (2006). Video-induced yawning in stumptail macaques (Macaca arctoides). Biol. Lett. 2, 36–38 10.1098/rsbl.2005.0411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek S. M., Critton S. R., Myers T. E., Gallup G. G., Jr. (2003). Contagious yawning: the role of self-awareness and mental state attribution. Brain Res. Cogn. Brain Res. 17, 223–227 10.1016/S0926-6410(03)00109-5 [DOI] [PubMed] [Google Scholar]
- Provine R. R. (1986). Yawning as a stereotyped action pattern and releasing stimulus. Ethology 72, 109–122 10.1111/j.1439-0310.1986.tb00611.x [DOI] [Google Scholar]
- Provine R. R., Tate B. C., Geldmacher L. L. (1987). Yawning: no effect of 3–5% CO2, 100% O2, and exercise. Behav. Neural Biol. 48, 382–393 10.1016/S0163-1047(87)90944-7 [DOI] [PubMed] [Google Scholar]
- Senju A., Maeda M., Kikuchi Y., Hasegawa T., Tojo Y., Osanai H. (2007). Absence of contagious yawning in children with autism spectrum disorder. Biol. Lett. 3, 706–708 10.1098/rsbl.2007.0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoup-Knox M. L., Gallup A. C., Gallup G. G., Jr., McNay E. C. (2010). Yawning and stretching predict brain temperature changes in rats: support for the thermoregulatory hypothesis. Front. Evol. Neurosci. 2:108. 10.3389/fnevo.2010.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]