Abstract
Objective
To describe the natural history of genital warts and vulvar intraepithelial neoplasia (VIN) in women with human immunodeficiency virus (HIV).
Methods
A cohort of 2,791 HIV infected and 953 uninfected women followed for up to 13 years had genital examinations at 6-month intervals, with biopsy for lesions suspicious for VIN.
Results
The prevalence of warts was 4.4% (5.3% for HIV seropositive women and 1.9% for seronegative women, P < 0.0001). The cumulative incidence of warts was 33% (95% C.I. 30, 36%) in HIV seropositive and 9% (95% C.I. 6, 12%) in seronegative women (P < 0.0001). In multivariable analysis, lower CD4 lymphocyte count, younger age, and current smoking were strongly associated with risk for incident warts. Among 501 HIV seropositive and 43 seronegative women, warts regressed in 410 (82%) seropositive and 41 (95%) seronegative women (P = 0.02), most in the first year after diagnosis. In multivariable analysis, regression was negatively associated with HIV status and lower CD4 count as well as older age. Incident VIN of any grade occurred more frequently among HIV seropositive than seronegative women: 0.42 (0.33 – 0.53) vs 0.07 (0.02 – 0.18)/100 person-years (P < 0.0001). VIN2+ was found in 58 women (55 with and 3 without HIV, P < 0.001). Two women with HIV developed stage IB squamous cell vulvar cancers.
Conclusion
While genital warts and VIN are more common among HIV seropositive than seronegative women, wart regression is common even in women with HIV, and cancers are infrequent.
Introduction
Women infected with human immunodeficiency virus (HIV) face an increased risk of human papillomavirus (HPV) infections and resulting lower genital tract lesions (1–4). Cervical lesions, including cervical cancer, have been studied extensively, but HPV infections also can cause vulvar warts, vulvar intraepithelial neoplasia (VIN), and vulvar cancer; these lesions have been less well studied in women with HIV. The prevalence of HPV-related vulvar lesions is higher in HIV seropositive compared to seronegative women. Jamieson and colleagues reported that HIV seropositive women had more vulvar, vaginal, and perianal lesions than seronegative women, with an incidence of 2/100 person-years for HIV seropositive women (5). The Women’s Interagency HIV Study (WIHS) has also shown that vulvar lesions are more prevalent among women with HIV than those not HIV infected, and antiretroviral therapy appears to ameliorate this risk (6, 7). We set out to use longer-term follow-up data from the WIHS to reassess the incidence of warts and VIN and to study the regression of warts and correlates of regression, including the effect of wart treatment.
Materials and Methods
This analysis was part of the WIHS, an ongoing multicenter cohort study of the natural history of HIV infection and related health conditions among HIV seropositive women and at-risk HIV-uninfected comparison women. The protocols, recruitment processes, procedures, and baseline results of the WIHS have been previously described (8). WIHS enrollment began October 1, 1994 at 6 study consortia and over time enrolled 3,766 women, with expansion during 2001–2002 (9). WIHS enrollees are similar to U.S. women with HIV (8). Women from the two enrollment periods were combined for analysis where no significant outcome differences between them were found. Written informed consent for study was obtained after local human subjects committees approved. Follow up continues, but this analysis includes information obtained before October 1, 2008.
Every six months, participants had histories taken by trained interviewers, followed by a physical examination that included a gynecologic exam. Lesions identified by inspection were documented using a vulvar diagram divided into seven subsites. According to study-wide protocol, genital lesions were biopsied if not typical warts, as determined by examining clinicians, provided patients consented. Histology results were interpreted locally and were not centrally reviewed. HIV status was established by Western blot, and women who seroconverted during follow-up were excluded from this analysis.
Contingency tables were constructed to summarize selected baseline characteristics of HIV seropositive and seronegative women diagnosed with vulvar warts or VIN. Chi-square or Fisher’s exact tests were used to contrast these characteristics by HIV seropositivity.
The prevalence of one or more vulvar warts was calculated by HIV serostatus at each visit. A multivariate generalized estimating equation (GEE) model for binary data (10) was used to compare wart prevalence in HIV seropositive and seronegative women, while adjusting for repeated observations of the same women. Results in the GEE model reflected not only repeated visits over time but also repeated observations of each patient by vulvar region, with warts documented using a vulvar diagram divided into seven subsites.
All multivariate models in this study adjusted for age, smoking, and the number of sexual partners in the prior 6 months. Temporal trends were assessed by incorporating the number of study visits since enrollment as a time-updated variable.
We studied the incident detection of vulvar warts in the subset of women who at enrollment had no warts present. Subsequent incident events were evaluated by subsite, such that women who had an incident wart at one site could have an incident wart occur at another but not the same subsite. Multivariate Cox analysis was done using the Wei, Lin, Weissfeld method to assess the hazard ratios for associations with wart incidence while adjusting for repeated observations involving the same women (11, 12).
We additionally examined the incidence of any wart across all subsites, one observation per woman. Differences in incidence rates by HIV serostatus were studied univariately using Chi-square tests based on normal approximation of incidence rate difference (13). The cumulative risk of any wart was then studied by including both prevalent and incident cases and using life table methods (14), with the log-rank test used to assess differences by HIV serostatus.
The spontaneous clearance of warts was defined as absence of a previously diagnosed wart at a given subsite during clinical exams for two consecutive visits. These analyses used similar WLW and life table methods as described above for incident detection, with the addition that these multivariate WLW models adjusted for whether the lesions were incident or prevalent at baseline.
Vulvar biopsy, VIN, and VIN2+ incidence was examined first by Chi-square test and then by multivariate analyses conducted using Cox models. We also examined the highest grade of VIN detected at any visit using Fisher’s exact test to compare results in HIV seropositive versus seronegative women.
Results
Of the 3766 women in WIHS (2791 HIV seropositive, 975 seronegative), we excluded 22 HIV seroconverters and 127 women missing data on baseline wart status. Women with HIV were more likely than uninfected women to have data on wart status missing (109 (3.9%) vs 18 (1.9%), P = 0.003). Of the remaining 3617 women, 160 (142 HIV seropositive, 18 seronegative) had genital warts at enrollment, for a prevalence rate of 4.4/100 women (5.3/100 for HIV seropositive women and 1.9/100 for seronegative women, P < 0.0001). The baseline prevalence in the 1994–5 subcohort was 4.9/100 women (5.6/100 for HIV seropositive and 2.4/100 for seronegative women). Baseline prevalence in the 2001–2 subcohort was lower at 3.4/100 women (P = 0.04) with 4.5 cases/100 women for HIV seropositive and 1.3/100 for seronegative women. For both subcohorts and overall, differences in wart prevalence between HIV seronegative and seropositive women were significant (all P < 0.005). Demographic and medical characteristics of women with warts are presented in Table 1.
Table 1.
Demographic and medical characteristics characteristics at the time of first diagnosis with vulvar warts, vulvar intraepithelial neoplasia (VIN) of any grade, VIN 2 or a more severe lesion (VIN2+). All P-values by Fisher exact test except where noted.
| Characteristic | Vulvar Warts | Any VIN | VIN2+ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HIV1+ (N = 612) | HIV− (N = 57) | P-value | HIV+ (N = 111) | HIV− (N = 5) | P-value | HIV+ (N = 55) | HIV− (N = 3) | P-value | |
| Age (years) | 0.0012 | 0.40 | 0.67 | ||||||
| <30 | 100 (16) | 21 (37) | 20 (18) | 2 (40) | 10 (18) | 1 (33) | |||
| 30–34 | 136 (22) | 6 (11) | 15 (14) | 0 (0) | 10 (18) | 0 (0) | |||
| 35–39 | 157 (26) | 14 (25) | 23 (21) | 2 (40) | 11 (20) | 1 (33) | |||
| 40–44 | 111 (18) | 12 (21) | 24 (22) | 1 (20) | 10 (18) | 1 (33) | |||
| >=45 | 108 (18) | 4 (7) | 29 (26) | 0 (0) | 14 (25) | 0 (0) | |||
| Ethnicity | 0.832 | 0.11 | 0.17 | ||||||
| White | 77 (13) | 8 (14) | 12 (11) | 2 (40) | 8 (15) | 2 (67) | |||
| Hispanic | 140 (23) | 15 (26) | 43 (39) | 0 (0) | 22 (40) | 0 (0) | |||
| Black | 380 (62) | 32 (56) | 53 (48) | 3 (60) | 23 (42) | 1 (33) | |||
| Other | 15 (2) | 2 (4) | 3 (3) | 0 (0) | 2 (4) | 0 (0) | |||
| Smoking | 0.132 | 0.45 | 0.76 | ||||||
| Never smoked | 145 (24) | 7 (12) | 30 (28) | 0 (0) | 12 (22) | 0 (0) | |||
| Former smoker | 101 (17) | 10 (18) | 18 (17) | 1 (20) | 10 (19) | 0 (0) | |||
| Current smoker | 361 (59) | 40 (70) | 60 (56) | 4 (80) | 32 (59) | 3 (100) | |||
| Intravenous drug use in prior 6 months | 0.35 | 1.00 | 1.00 | ||||||
| Yes | 34 (6) | 1 (2) | 6 (6) | 0 (0) | 2 (4) | 0 (0) | |||
| No | 573 (94) | 56 (98) | 102 (94) | 5 (100) | 52 (96) | 3 (100) | |||
| # of male sexual partners in prior 6 months | 0.012 | 0.01 | 0.32 | ||||||
| 0 | 174 (29) | 9 (16) | 40 (38) | 0 (0) | 16 (31) | 0 (0) | |||
| 1 | 332 (56) | 30 (53) | 56 (53) | 2 (40) | 31 (60) | 2 (67) | |||
| 2 | 54 (9) | 11 (19) | 7 (7) | 2 (40) | 3 (6) | 1 (33) | |||
| >=3 | 36 (6) | 7 (12) | 2 (2) | 1 (20) | 2 (4) | 0 (0) | |||
| CD4 (cells/cmm) | |||||||||
| >500 | 114 (19) | NA3 | 16 (15) | NA | 2 (4) | NA | |||
| 200–500 | 245 (42) | NA | 45 (42) | NA | 27 (51) | NA | |||
| <200 | 227 (39) | NA | 47 (44) | NA | 24 (45) | NA | |||
| HIV RNA level (/cmm) | |||||||||
| <=4000 | 226 (38) | NA | 50 (46) | NA | 17 (31) | NA | |||
| 4001–20000 | 105 (18) | NA | 20 (18) | NA | 15 (27) | NA | |||
| 20001–100000 | 134 (23) | NA | 23 (21) | NA | 9 (16) | NA | |||
| >100000 | 128 (22) | NA | 16 (15) | NA | 14 (25) | NA | |||
Human immunodeficiency virus
By Pearson’s chi-square test. All others by Fisher’s exact test.
Not applicable
After excluding these prevalent cases and 310 women (223 HIV seropositive, 87 seronegative) with no follow-up, 3147 women (2317 HIV seropositive, 830 seronegative) were included in an analysis of the incidence of vulvar warts. These included 2145 (1673 HIV+, 472 HIV−) recruited in 1994–5 and 1002 (644 HIV+, 358 HIV−) recruited in 2001–2. Of these, 479 women (15%) were diagnosed with incident vulvar warts, including 441 (19%) HIV seropositive and 38 (5%) seronegative women..
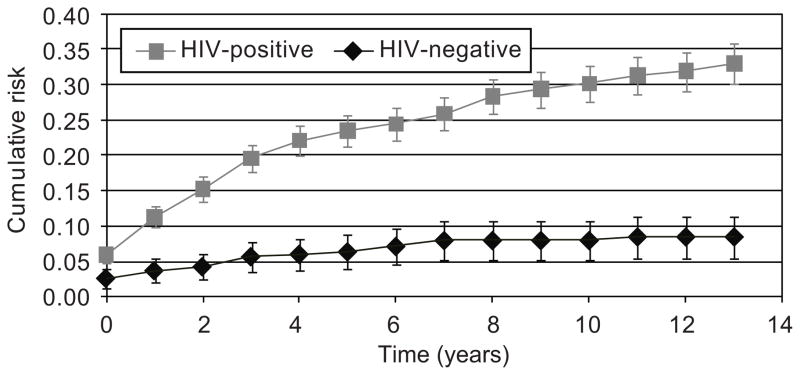
The annual incidence of warts per 100 person-years was 3.1 (95% C.I. 2.8, 3.5) for HIV seropositive women and 0.6 (95% C.I. 0.4, 1.0) for seronegative women in the 1994–5 cohort (P < 0.0001) and 2.5 (95% C.I 2.0, 3.2) and 0.6 (95% C.I. 0.3, 1.1) for seropositive and seronegative women in the 2001–2 cohort (P < 0.0001). Incidence rates fell over time for women in both cohorts (P < 0.0001 for the combined cohorts). In addition, Fig. 1 shows the cumulative risk of genital warts in the combined cohorts. After up to 13 years of follow-up in the 1994–5 cohort, the cumulative incidence of genital warts was 33% (95% C.I. 30, 36%) in HIV seropositive and 9% (95% C.I. 6, 12%) in seronegative women (P < 0.0001). After up to 6 years of follow-up in the 2001–2 cohort, the cumulative incidence of genital warts was 17% (95% C.I. 14, 20%) in HIV seropositive and 5% (95% C.I. 3, 7%) in seronegative women (P < 0.0001).
Fig. 1.
Cumulative risk of genital warts among human immunodeficiency virus (HIV) seropositive and seronegative women in the original cohort. P < .001 for differences between groups across all visits.
In multivariable analysis, lower CD4 lymphocyte count, younger age, and current but not former smoking were strongly correlated with incident genital warts (Table 2). The number of recent sexual partners was not significantly associated with wart incidence.
Table 2.
Results of multivariable analysis of risk factors for diagnosis of incident genital warts.
| Hazard Ratio (HR) | 95% Lower CL | 95% Upper CL | p-value | ||
|---|---|---|---|---|---|
| CD4+ | HIV− (ref) | <.0001* | |||
| >500 | 2.72 | 1.60 | 4.62 | <.0001 | |
| 200–500 | 6.67 | 3.99 | 11.14 | <.0001 | |
| <200 | 16.58 | 9.85 | 27.91 | <.0001 | |
| Age | <30 (ref) | ||||
| 30–34 | 0.82 | 0.58 | 1.16 | 0.26 | |
| 35–39 | 0.70 | 0.48 | 1.02 | 0.06 | |
| 40–44 | 0.53 | 0.36 | 0.78 | 0.002 | |
| >45 | 0.38 | 0.26 | 0.58 | <.0001 | |
| Smoking | Never (ref) | ||||
| Former | 0.99 | 0.70 | 1.40 | 0.95 | |
| Current | 1.80 | 1.37 | 2.38 | <.0001 | |
P for trend.
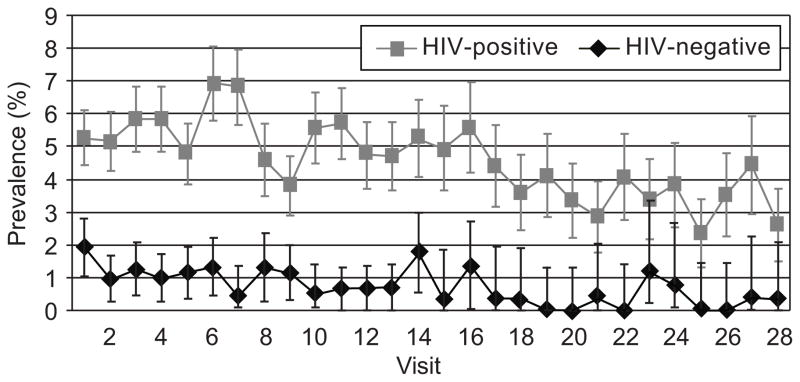
We also examined trends in the burden of warts over time. As shown in Fig. 2, the visit-specific prevalence of genital warts was higher among HIV seropositive women at all visits (P < 0.0001). Fig. 2 also shows that for both HIV seropositive and seronegative women, prevalence fell over time (P < 0.0001).
Fig. 2.
Visit-specific point prevalence of genital warts among human immunodeficiency virus (HIV) seropositive and seronegative women. P < .001 for differences between groups across all visits.
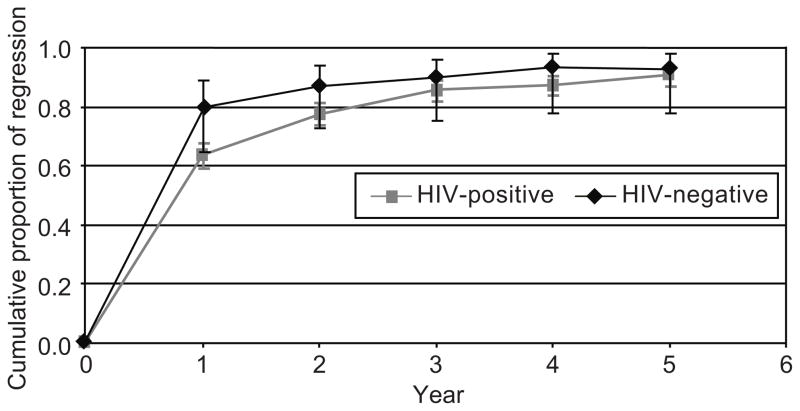
We explored the likelihood of spontaneous regression among 669 women (612 HIV seropositive, 57 seronegative) with prevalent and incident warts. After excluding 65 women (59 HIV seropositive, 6 seronegative) whose warts were treated and 60 women (52 HIV seropositive, 8 seronegative) with inadequate follow-up, among the remaining 544 women (501 HIV seropositive, 43 seronegative) followed for up to five years, 451 (83%) regressed (410 (82%) HIV seropositive, 41 (95%) seronegative, P = 0.02). As shown in Fig. 3, most regression occurred in the first year after diagnosis in both groups, although regression continued to occur with ongoing follow-up, especially among women with HIV. In multivariable analysis including both incident and prevalent lesions (Table 3), wart regression was less likely in HIV seropositive women, especially those with lowest CD4 counts and higher HIV RNA levels (P for trend in stratum with CD4 counts <200/cmm < 0.0001). Regression was not linked to smoking or number of sexual partners (data not shown). When the multivariable analysis was carried out distinguishing prevalent from incident warts, prevalent warts were less likely to clear than incident warts (P < 0.0001), though the effects of CD4 count and HIV viral load were similar for both prevalent and incident warts.
Fig. 3.
Cumulative likelihood of spontaneous regression of vulvar warts over time after excluding treated women (P = .004 for human immunodeficiency virus [HIV]-positive compared with HIV-negative women).
Table 3.
Results of multivariable analysis of risk factors for regression of genital warts.
| Hazard Ratio (HR) | 95% Lower CL | 95% Upper CL | p-value | ||
|---|---|---|---|---|---|
| CD4+ by HIV Viral Load | HIV− (ref) | 1.00 | |||
|
| |||||
| CD4>500, VL<=4,000 | 0.79 | 0.52 | 1.22 | 0.29 | |
| CD4>500, VL: 4,001–20,000 | 0.72 | 0.41 | 1.25 | 0.24 | |
| CD4>500, VL: 20,001–100,000 | 0.63 | 0.36 | 1.09 | 0.10 | |
| CD4>500, VL>100,000 | 0.68 | 0.15 | 2.95 | 0.60 | |
|
| |||||
| CD4:200–500, VL<=4,000 | 0.73 | 0.48 | 1.11 | 0.14 | |
| CD4:200–500, VL: 4,001–20,000 | 0.60 | 0.38 | 0.95 | 0.03 | |
| CD4:200–500, VL: 20,001–100,000 | 0.78 | 0.49 | 1.23 | 0.28 | |
| CD4:200–500, VL>100,000 | 0.69 | 0.41 | 1.18 | 0.18 | |
|
| |||||
| CD4<200, VL<=4,000 | 0.63 | 0.40 | 0.99 | 0.047 | |
| CD4<200, VL: 4,001–20,000 | 0.40 | 0.24 | 0.68 | 0.001 | |
| CD4<200, VL: 20,001–100,000 | 0.46 | 0.28 | 0.73 | 0.001 | |
| CD4<200, VL>100,000 | 0.37 | 0.23 | 0.59 | <.0001 | |
|
| |||||
| Age | <30 (ref) | ||||
|
| |||||
| 30–34 | 1.09 | 0.82 | 1.44 | 0.55 | |
|
| |||||
| 35–39 | 1.37 | 1.06 | 1.77 | 0.02 | |
|
| |||||
| 40–44 | 0.98 | 0.71 | 1.34 | 0.89 | |
|
| |||||
| >45 | 1.18 | 0.89 | 1.56 | 0.24 | |
Of 669 women (612 HIV seropositive, 57 seronegative) diagnosed with warts during follow-up extending up to 14 years, 77 treatments were used for 63 (9%) women, (57 (9%) HIV seropositive; 6 (10%) seronegative, P = 0.81). These treatments included excision by scalpel or electrosurgical loop (23, 30%); laser or other ablation (24, 31%); combined laser and excision (2, 3%); topical therapies including trichloroacetic acid (17, 22%), imiquimod (5, 6%), 5-fluorouracil (4, 5%), and podophyllin (2, 3%). Among these women, 51 had one treatment, 10 had two treatments, and two had three treatments.
After excluding 2 women with inadequate documentation of wart status, 39 (64%) of the remaining 61 treated women were free of warts at the next visit (34/56 or 61% among HIV seropositive women and 5/5 or 100% among seronegative women, P = 0.15). Among the 39 women whose lesions had cleared by the first post-treatment visit, treatment effects were durable in 100% among HIV seronegative women but waned with time among seropositive women, with same-site wart-free survival of 88% at 12 months, 65% at 3 years, and 56% at 5 years. However, long-term follow-up was not available for many women, and rates did not differ by HIV serostatus at any of these time points.
Of 373 vulvar biopsies from 213 women (199 HIV seropositive, 14 HIV seronegative), results for two biopsies from two women were missing. Multiple biopsies from the same visit were assessed according to the most severe result, leaving 332 results from the 213 women, with 2 excluded for missing result.
Women with HIV were more likely to have vulvar lesions biopsied: the vulvar biopsy rate was 0.79 (95% C.I. 0.66–0.94)/100 person-years for HIV seropositive women with HIV and 0.17 (0.08–0.32) for seronegative women (P < 0.0001). In multivariable analysis, risk factors for biopsy included HIV serostatus and CD4 lymphocyte count (compared to HIV seronegative women, H.R. 2.3, 95% C.I. 1.1, 4.9 for women with HIV and CD4 counts >500/cmm, H.R. 4.0, 95% C.I. 2.0, 8.0 for those with CD4 counts 200–500/cmm, H.R. 10.0, 95% C.I. 5.0, 20.0 for those with CD4 counts <200/cmm, P for trend < 0.0001) and vulvar treatment within six months (H.R. 9.38, 95% C.I. 1.3, 67.8, P = 0.03).
The higher vulvar biopsy rate among women with HIV appeared to be due to a truly higher risk for lesions rather than to a lower threshold for biopsy, as the distribution of the histologic severity of biopsies did not differ by HIV status. Table 4 shows the results of the highest grade biopsy for each woman.
Table 4.
Highest grade of vulvar intraepithelial neoplasia (VIN) found in 213 women undergoing biopsy. For the comparison of HIV seropositive and seronegative women, P = 0.44 by Fisher’s exact test. N (column percent).
| HIV+ | HIV− | Total | |
|---|---|---|---|
| Normal/Benign | 6733.67 | 857.14 | 75 |
| VIN1 | 8140.70 | 321.43 | 84 |
| VIN2 | 168.04 | 17.14 | 17 |
| VIN3 | 3316.58 | 214.29 | 35 |
| Cancer | 21.01 | 00.00 | 2 |
| Total | 199 | 14 | 213 |
P = 0.44 (Fisher’s exact test p-value).
Table 1 shows the demographic and medical characteristics at the time of diagnosis for 116 women (111 with and 5 without HIV) found to have VIN or cancer; HIV seropositive women had fewer sexual partners than seronegative women.
Incident VIN of any grade occurred more frequently among HIV seropositive than seronegative women: 0.42 (0.33 – 0.53) vs 0.07 (0.02 – 0.18)/100 person-years (P < 0.0001). In multivariable analysis, only HIV serostatus/CD4 lymphocyte count was correlated with incident VIN (compared to HIV seronegative women, H.R. 3.7, 95% C.I. 1.2, 11.4 for women with HIV and CD4 counts >500/cmm, H.R. 5.4, 95% C.I. 1.9, 15.7 for those with CD4 counts 200–500/cmm, H.R. 16.3, 95% C.I. 5.6, 47 for those with CD4 counts <200/cmm, P for trend < 0.0001).
VIN2+ was found in 58 women (55 with and 3 without HIV, P < 0.001). Demographic factors from the time of diagnosis are presented in Table 1; no significant differences in VIN2+ were evident between HIV seropositive and seronegative women, though the small number of seronegative women may have been limiting.
Incident VIN2+ was much less common than VIN of any grade but was more frequent among HIV seropositive than seronegative women. The incidence of VIN2+ was 0.18/100 person-years (95% C.I. 0.12–0.26) for women with HIV and 0.03/100 person-years (95% C.I. 0.004–0.12) for HIV seronegative women (P = 0.01).
In multivariable analysis, being HIV seropositive and having lower CD4 lymphocyte count was associated with VIN2+ (compared to HIV seronegative women, H.R. 0.6, 95% C.I. 0.1, 6.3, P = 0.64 for women with HIV and CD4 counts >500/cmm, H.R. 5.5, 95% C.I. 1.2, 25.2, P = 0.03 for those with CD4 counts 200–500/cmm, H.R. 16.3, 95% C.I. 3.6, 73.4, P = 0.0003 for those with CD4 counts <200/cmm). In a separate multivariable model, age, ethnicity, smoking, and the number of sexual partners in the six months before diagnosis did not distinguish women with VIN2+ from those with genital warts and those with VIN1 (not shown). In an additional multivariable model including only women with HIV, risk for VIN2+ was higher among women with a clinical diagnosis of genital warts when CD4 counts were lower (compared to women with CD4 >500/cmm, O.R. for VIN2+ vs warts was 6.3, 95% C.I. 1.5, 27.1, P = 0.01 when CD4 = 200–500/cmm and 5.6, 95% C.I. 1.3, 23.8, P = 0.02 when CD4 <200/cmm). Similarly, the likelihood that biopsies were diagnosed as VIN2+ rather than VIN1 was higher among HIV-infected women with lower CD4 counts (compared to women with CD4 >500/cmm, OR for VIN2+ vs VIN1 was 21.5, 95% C.I. 2.5, 185.5, P = 0.01 when CD4 = 200–500/cmm and 21.8, 95% C.I. 2.4, 195.1, P = 0.01 when CD4 < 200/cmm).
Of 114 women with VIN, 41 (38 HIV seropositive, 3 seronegative), had 54 vulvar treatments. Therapies included ablation (24, 44%), excision (21, 39%), topical therapies (7, 13%), and both excision and ablation (2, 4%). Since repeat biopsy was often not performed, we could not define treatment success rates.
Two women in our HIV seropositive group developed incident stage IB vulvar squamous cell cancers, the first in 1996 and the second in 2002. Both had prior Paps showing atypical or low grade squamous cells. The former patient had a lesion detected several months before diagnosis. The latter had a wart excised in 1995 and was followed without lesions until 2000, when an ulcer was managed as infectious until biopsy the following year showed VIN3 and local excision showed invasion. Both cancers were treated surgically without adjuvant therapy and although the first patient developed a recurrence in 2002 and later an anal carcinoma managed with resection, both were alive without vulvar cancer recurrence in 2010.
Discussion
As we and others have shown, HIV infection increases women’s risk for genital warts and VIN (1–7). In addition to providing longer follow-up, showing that a third of HIV-infected women in our long-term cohort developed genital warts at some point during up to 13 years of observation, our results confirm and expand on prior work. We found a clear dose response but no threshold CD4 count for risk, indicating that even marginal immunosuppression increases susceptibility to HPVs that cause warts. Nevertheless, HIV seropositive women’s experience of vulvar disease is dynamic. HIV infection delays spontaneous clearance and may impair the effectiveness of treatments for genital warts, though differences by HIV status did not reach significance. Most warts among these women resolve spontaneously or respond to therapy. Only the most profound immunosuppression (CD4 <200/cmm) had a detectable impact on the relatively high rate of spontaneous regression. Women with HIV and relatively high CD4 levels can be observed or treated with reasonable expectation of ultimate wart clearance, while women with lower CD4 counts may benefit from earlier, more aggressive, and repeated treatment. As reported by de Panfilis and colleagues (15), women with HIV and genital warts should be counseled that recurrence can be a troubling but manageable complication of HIV infection, with 46% of women in their study who were treated for genital warts recurring within five years. In that study and ours, incidence rates fell with time and were lower among older women. Smoking is a modifiable risk factor for incident warts, and smoking cessation should be especially encouraged for HIV seropositive smokers with genital warts.
While we found that VIN2+ was also more frequent in women with than those without HIV, it remained relatively uncommon. Intact immunity is important not only in the control of initial HPV infections that cause warts and VIN1 but also in the control of precancers. There may be a threshold effect, as the incidence of VIN2+ was increased only for women with CD4 counts <500/cmm.
With only two incident vulvar cancers, we could not study invasive cancers. However, Simard and colleagues found that vulvovaginal cancer risk does not appear to rise until years after HIV diagnosis, suggesting that immunosuppression must be prolonged to allow progression of preinvasive lesions to cancer (16). Dedes and coworkers found that vulvar cancers developed despite therapy in 2/20 (10%) HIV seropositive women with VIN2+ followed at least two years(17). Ongoing surveillance for recurrent VIN and new cancer is important for early diagnosis, and the finding that both our cancers were preceded for months to years by visible lesions underscores the importance of liberal biopsy. Given the high regression rate of warts in our study, the finding that these diagnoses were delayed despite a protocol requiring biopsy of all lesions except apparent warts suggests persistent warts merit biopsy.
We have previously shown in this cohort that some cervicovaginal HPV infections in women with HIV reflect reactivation of latent HPV rather than new infections (18). In this study, the number of recent sexual partners did not affect the incidence of new genital warts. This also suggests that new warts in HIV-infected women may represent not new HPV infections but rather activation of extant HPV infections, possibly from immunosuppression, from sexual or other trauma, or from these and other factors in combination.
Our study was limited by several factors. Our results should be generalizable to most women with HIV, although lesions found after less regular follow-up may be more advanced, more likely VIN2+, and less likely to regress. While study-wide protocol recommended biopsy for persistent lesions, this did not occur in all cases, owing both to patients declining biopsy and to clinicians with a low index of suspicion. However, 75% of women who had biopsies done were found to have either no VIN or VIN1, which has minimal neoplastic potential, and only two incident cancers developed throughout follow-up, suggesting that failure to detect high grade VIN was uncommon. In addition, diagnosis of genital warts was by clinical inspection alone; some skin tags, keratoses, and other abnormalities might have been misdiagnosed as genital warts. The effect of this would have been to weaken observed associations; colposcopy surveillance and more liberal biopsy might have identified additional risk factors and trends. Further, while participants were asked about genital treatments at each visit, faulty recall might have caused us to misclassify some treatment-based regression as spontaneous if treatments outside WIHS were not reported. Similarly, we relied on biopsy to confirm VIN, and since repeat biopsy was often not performed, we could not define successful treatment rates. The small number of women with VIN2+, especially in the HIV seronegative group, precluded multivariable analysis of factors potentially contributing to development of VIN2+. We lacked sufficient numbers to compare the efficacy of various treatments for warts and VIN. We did not have access to reasons why some women with warts were treated and others followed. A lower regression rate for warts in HIV seropositive women may in part reflect undiagnosed VIN. Finally, HPV typing of wart and VIN specimens was not performed, and we could not assess HPV type distribution or the role of HPV type in determining regression and response to treatment among women with genital warts and VIN.
Although many vulvar lesions resolve, Conley and associates have reported that vulvar cancers can develop despite surveillance and treatment of VIN (19). Vulvar inspection should be a routine part of interval examination for women with HIV, since surveillance and treatment of VIN2+ is an important component of cancer control. All lesions except typical warts should be biopsied, and high grade VIN should be treated unless terminal comorbid conditions mean life expectancy is less than the potential 1–2 year transition time to cancer (17). Although most genital warts are caused by HPV types 6 and 11, which usually do not progress to cancer, a substantial minority of warts are associated with high-risk HPV types and may pose a risk for cancer (20). Persistent warts should be biopsied. The 82% spontaneous regression rate for warts in HIV seropositive women, with a lower rate of regression after one year, suggests that apparent genital warts in women with HIV can be followed without biopsy or treatment for at least 12 months before biopsy or excision, which both treats warts and identifies unsuspected VIN. Our finding that only two cases of incident invasive vulvar cancer developed in our HIV infected cohort during up to 13 years of observation despite an increased risk for VIN2+ suggests that vulvar surveillance and treatment can be effective in preventing vulvar cancer among women with HIV. Women who develop invasive vulvar cancer should receive standard therapy, which can be curative.
Acknowledgments
The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Analysis was funded through R01-CA-085178.
Footnotes
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange).
Clinical Trial Registration: ClinicalTrials.gov, www.clinicaltrials.gov, NCT00000797.
Financial Disclosure: Dr. D’Souza has received research funding from and served on an advisory panel for Merck, Inc. The other authors did not report any potential conflicts of interest.
Contributor Information
L. Stewart Massad, Washington University School of Medicine, St. Louis, MO
Xianhong Xie, Albert Einstein College of Medicine, Bronx, NY
Teresa Darragh, University of California, San Francisco, CA
Howard Minkoff, Maimonides Medical Center, State University of New York, Downstate, Brooklyn, NY
Alexandra M. Levine, City of Hope Medical Center, Duarte, CA, and Keck School of Medicine, University of Southern California, Los Angeles, CA
D. Heather Watts, Eunice K. Shriver National Institute of Child Health and Human Development, Bethesda, MD.
Rodney L. Wright, Albert Einstein College of Medicine, Bronx, NY
Gypsyamber D’Souza, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD
Christine Colie, Georgetown University School of Medicine, Washington, DC
Howard D. Strickler, Albert Einstein College of Medicine, Bronx, NY
References
- 1.Palefsky JM, Minkoff H, Kalish LA, Levine A, Sacks HS, Garcia P, et al. Cervicovaginal human papillomavirus infection in Human Immunodeficiency Virus-1 (HIV)-positive and high-risk HIV-negative women. J Natl Cancer Inst. 1999;91:226–36. doi: 10.1093/jnci/91.3.226. [DOI] [PubMed] [Google Scholar]
- 2.Cubie HA, Seagar AL, Beattie GJ, Monaghan S, Williams ARW. A longitudinal study of HPV detection and cervical pathology in HIV infected women. Sex Transm Inf. 2000;76:256–61. doi: 10.1136/sti.76.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strickler HD, Burk RD, Fazzari M, Anastos K, Minkoff H, Massad LS, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus (HIV) positive women. J Natl Cancer Inst. 2005;97:577–86. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 4.Massad LS, Seaberg EC, Wright RL, Darragh T, Lee YC, Colie C, et al. Squamous cervical lesions in women with Human Immunodeficiency Virus: long-term follow up. Obstet Gynecol. 2008;111:1388–93. doi: 10.1097/AOG.0b013e3181744619. [DOI] [PubMed] [Google Scholar]
- 5.Jamieson DJ, Paramsothy P, Cu-Uvin S, Duerr A. Vulvar, vaginal, and perianal intraepithelial neoplasia in women with or at risk for Human Immunodeficiency Virus. Obstet Gynecol. 2006;107:1023–8. doi: 10.1097/01.AOG.0000210237.80211.ff. [DOI] [PubMed] [Google Scholar]
- 6.Massad LS, Silverberg MJ, Springer G, Minkoff H, Hessol N, Palefsky JM, et al. Effect of antiretroviral therapy on the incidence of genital warts and vulvar neoplasia among women with the human immunodeficiency virus. Am J Obstet Gynecol. 2004;190:1241–8. doi: 10.1016/j.ajog.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 7.Dolev JC, Maurer T, Springer G, Glesby MJ, Minkoff H, Connell C, et al. Incidence and risk factors for verrucae in women. AIDS. 2008;22:1213–9. doi: 10.1097/QAD.0b013e3283021aa3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barkan SE, Melnick SL, Martin-Preston S, Weber K, Kalish LA, Miotti P, et al. The Women’s Interagency HIV Study. Epidemiol. 1998;9:117–25. [PubMed] [Google Scholar]
- 9.Bacon M, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diag Lab Immunol. 2005;12:1013. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 11.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distribution. J Am Stat Assoc. 1989;84:1065–73. [Google Scholar]
- 12.Xue X, Gange SJ, Zhong Y, Burk RD, Minkoff H, Massad LS, et al. Marginal and mixed-effects models in the analysis of human papillomavirus natural history data. Cancer Epidemiol Biomarkers Prev. 2010;19:159–69. doi: 10.1158/1055-9965.EPI-09-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahai H, Khurshid A. Statistics in epidemiology: Methods, techniques, and applications. Boca Raton, FL: CRC Press; 1996. [Google Scholar]
- 14.Berkson J, Gage R. Calculation of survival rates for cancer. Mayo Clin Proc. 1950;25:270–86. [PubMed] [Google Scholar]
- 15.de Panfilis G, Melzani G, Mori G, Ghidini A, Graifemberghi S. Relapses after treatment of external genital warts are more frequent in HIV-positive patients than in HIV-negative controls. Sex Trans Dis. 2002;29:121–5. doi: 10.1097/00007435-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Simard EP, Pfeiffer RM, Engels EA. Spectrum of cancer risk late after AIDS onset in the United States. Arch Intern Med. 2010;170:1337–45. doi: 10.1001/archinternmed.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dedes KJ, Beneder C, Samartzis N, Muller, Fink D, Fehr MK. Outcome of treated anogenital intraepithelial neoplasia among human immunodeficiency virus-infected women. J Reprod Med. 2008;53:947–51. [PubMed] [Google Scholar]
- 18.Strickler HD, Burk RD, Fazzari M, Anastos K, Minkoff H, Massad LS, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus (HIV) positive women. J Natl Cancer Inst. 2005;97:577–86. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 19.Conley LJ, Ellerbrock TV, Bush TJ, Chiasson MA, Sawo D, Wright TC. HIV-1 infection and risk of vulvovaginal and perianal condylomata acuminate and intraepithelial neoplasia: A prospective cohort study. Lancet. 2002;359:108–13. doi: 10.1016/S0140-6736(02)07368-3. [DOI] [PubMed] [Google Scholar]
- 20.Garland SM, Steben M, Sings HL, James M, Lu Shuang, Railkar R, et al. Natural history of genital warts: Analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199:805–14. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]