Abstract
Objective
Women who become pregnant during the conduct of biomedical HIV prevention trials are taken off the study product for safety reasons. High pregnancy rates can compromise statistical integrity in these trials. The comprehensive contraceptive curriculum developed for the Centre for the AIDS Programme of Research in South Africa (CAPRISA) 004 trial was evaluated for its ability to enhance contraceptive uptake, reduce pregnancy rates, and preserve statistical integrity.
Methods
Contraceptive- and pregnancy-related eligibility criteria were specified in the protocol. We enrolled women who opted for a non-barrier method of contraceptive and provided hormonal contraceptives on-site at no cost. At each monthly study visit, we provided pregnancy prevention counselling and performed pregnancy testing. Study product was withheld on pregnancy diagnosis, but women continued with monthly follow-up.
Results
Contraceptive usage was high throughout the study with 100% uptake at baseline and 94.71% use after a mean of 18 months follow-up at exit. Injectable progestins, particularly medroxyprogesterone acetate, remained the preferred choice of contraceptive. After 30 months of follow-up, 54 pregnancies were reported out of 889 participants, giving a pregnancy incidence rate of 3.95 per 100 woman-years (95% CI: 2.96 to 5.17). Of all pregnancies, 2/3 (64.81%) resulted in a full-term live birth, while 18.52% and 11.11% pregnancies culminated as miscarriage and terminated pregnancies, respectively. There were no congenital anomalies in the early neonatal period. Pregnancies resulted in 1.56% of woman-years of study follow-up lost due to temporary product withdrawal.
Conclusion
The CAPRISA 004 contraceptive curriculum was an effective strategy for maintaining low pregnancy rates, thereby minimizing product withdrawal and loss of follow-up time.
INTRODUCTION
In 2009, there were an estimated 33.3 million Human Immunodeficiency Virus (HIV) infected people globally (1). A distinctive feature of this epidemic in the 21st century is its increasing burden on young women. In sub-Saharan Africa, women aged 15–24 years are as much as eight times more likely to be HIV positive than men of the same age (2). While condoms are an effective HIV prevention strategy, many women are unable to successfully negotiate their use with their male partners, making an HIV prevention method that women can initiate and control a priority. Since 1990, various women-initiated candidate biomedical microbicides have been tested in effectiveness trials (3) to assess their impact on preventing HIV infection, and several more trials are underway or planned (4).
All biomedical microbicide trials, irrespective of the product being investigated, share common challenges. As these trials enroll mostly women within the reproductive age group, one of the greatest challenges has been the high pregnancy rates, ranging from 21 to 76 per 100-person-years (5-10). Women who become pregnant during clinical trials of experimental drugs must be taken off the product for safety reasons. Frequent and prolonged product withdrawal can compromise statistical integrity in these trials (6).
Although all efforts are made to limit exposure to experimental drugs during pregnancy, some women may use study product during the first weeks of pregnancy. Therefore it is important to monitor the safety of the study product on pregnancy rates and outcomes. While little is known about the safety of tenofovir gel when used during pregnancy, the oral formulation, Tenofovir disoproxil fumarate (Viread), which has been widely used by HIV-infected individuals for treatment, is designated as a pregnancy category B drug in the United States of America (USA), and has a reassuring teratogenic profile. Congenital anomaly rates at 2.72/100 live births are comparable to those in the Center for Disease Control’s (CDC) population-based birth defects surveillance system in the USA, and to the rates of other antiretroviral drugs in the Antiretroviral Pregnancy Registry (11).
With these challenges in mind, we hypothesized that an intensive comprehensive contraceptive curriculum is an effective strategy for enhancing contraceptive uptake, reducing pregnancy rates, minimizing inadvertent in utero drug exposure and thereby maintaining statistical integrity and fetal safety in biomedical HIV prevention trials. Here we provide an analysis of contraceptive choices, pregnancy rates and outcomes from the CAPRISA 004 tenofovir get trial.
MATERIALS AND METHODS
CAPRISA 004, a phase IIb placebo-controlled, double-blind randomized clinical trial , was conducted to assess safety and effectiveness of 1% vaginal tenofovir gel to reduce HIV acquisition in women at one rural (Vulindlela) and one urban (eThekwini) clinic in South Africa (12). A total of 889 non-pregnant HIV uninfected women between the ages of 18 and 40 were eligibly enrolled and followed-up for an average of 18 months (range: 12 to 30 months), 611 from the rural site and 278 from the urban site. The goal was to enroll up to 1,250 women over approximately 18 months and to continue follow-up until 92 incident infections were observed. This number of events was expected to provide 90% power to detect a 50% effect (using a two-sided alpha=0.05 significance level test). Participants were accrued over 19 months from May 2007 to January 2009. Women were randomly assigned in equal proportions to one of two study arms; tenofovir gel and placebo gel within 30 days of the screening visit. To facilitate blinding, each subject was randomly assigned within one of six different groups (designated by an alphanumeric variable e.g., A, B, C, D, E and F) in a 1:1:1:1:1:1 allocation ratio. Three groups corresponded to the placebo and three to tenofovir gel. This analysis includes all women enrolled into CAPRISA 004 and included in the intention-to-treat analysis.
The main outcome of this analysis was pregnancy incidence rates. Other outcomes included contraceptive uptake and adherence, pregnancy outcomes, and time off study product. We developed tools and aids to support the trial’s comprehensive contraceptive curriculum, which were used from the screening visit until study termination. We used these tools to train clinical staff on contraceptive counselling for the trial participants and to guide clinical staff with regards to all pregnancy-related procedures including study product withdrawal and resumption.
Clinical staff conducted urine pregnancy tests (QuickVue One-Step hCG Urine Test Quidel Corporation, San Diego, USA) at screening and pregnant women were excluded. As part of eligibility criteria, we assessed a participant’s contraceptive needs and pregnancy intentions over study duration. Only women who were not planning to become pregnant sometime over the duration of the study and agreed to use a non-barrier method of contraception for the duration of the study were offered enrolment into the study. Other non-pregnancy or contraceptive related exclusions are provided elsewhere (13). We repeated pregnancy tests at enrolment if more than 21 days had lapsed between the screening and enrolment visits.
We provided hormonal contraceptives on-site at no cost, including progesterone containing injectables (depot-medroxyprogesterone acetate (DMPA) and norethisterone enanthate (NET-EN) and combined oral contraceptives (COC). Women were referred to the nearest family planning institution per referral guidelines issued by the South African Department of Health (DOH) if they opted for a non-hormonal method such as an intra-uterine device (IUD) or tubal ligation (TL). Contraceptive choice was recorded on a family planning card and an individual contraceptive history log that was updated at each study visit and was retained in the participant binder. Barrier methods including both male and female condoms were provided at each study visit but were promoted principally as an HIV risk-reduction method.
Trained clinical study staff provided individual contraceptive counselling as part of routine monthly follow-up procedure. Contraceptive counselling was individualised and tailored according to each woman’s needs. We used a contraceptive log to indirectly assess compliance with a method. To enhance continual supply, participants’ contraceptive visits were scheduled to coincide with their regular monthly study visits whenever possible. Participants who chose to receive their contraception from a government family planning clinic were required to bring their signed DOH family planning cards to each study visit for study data collection on use of contraceptive methods.
Urine pregnancy testing was conducted monthly. Participants testing positive for pregnancy continued with monthly follow-up visits but study product was temporarily withheld. A woman continued to have monthly pregnancy tests for the first three months of pregnancy to exclude unrecognized early preclinical pregnancy loss. Blood βhCG quantification was done to confirm ambiguous urine results. Product was resumed following a live birth or once the chemical tests (urine and/or blood) reverted to negative following pregnancy end.
On pregnancy cessation, the clinical staff collected data on pregnancy complications and documented pregnancy outcomes. Pregnant women presented their babies once in the early postpartum period to the study clinician for assessment of abnormalities, if any. Case reports of each pregnancy were discussed during the Protocol Safety Review Team (PSRT) meetings. PSRT comprised the principal investigators, the study Obstetrician, the study and site clinicians, project directors, and an independent clinician. This team met regularly —initially every 3 months then every 2 months to review blinded safety information from the study.
The trial (NCT00441298) was reviewed and approved by the University of KwaZulu-Natal’s Biomedical Research Ethics Committee (BREC ref: E 111/06), the South Africa Medicines Control Council (MCC ref: 20060835) and Family Health International’s Protection of Human Subjects Committee (PHSC ref: 9946). Prior to enrolment into the study, written informed consent was obtained from each participant.
Outcome measures of this analysis included contraceptive uptake and use patterns, pregnancy rates and pregnancy outcomes, and time off study product. Contraception and pregnancy data were entered onto standardized case report forms at the study sites and were faxed into the CAPRISA Data Management Centre, using the DataFax system (Clinical DataFax Systems Inc., Ontario, Canada). Data on contraceptive method at each visit starting from screening, pregnancy diagnosis date, date of study product withdrawal and resumption, and pregnancy outcomes were captured and entered into the study database. Pregnancy outcomes documented included miscarriages, terminations of pregnancy, stillbirths, preterm live births with and without congenital anomalies, and full-term live births with and without congenital anomalies. Adverse maternal outcomes were also documented as adverse events.
For the first part of the analysis, contraceptive method groups were defined according to baseline method used, and did not take into account method switching, discontinuation, missed visits or contraceptives obtained from other external sources. We calculated incidence rates of pregnancy per 100 woman-years (w-y) of observation were for each contraceptive category considering method used up to the time of index pregnancy, thereby excluding pregnancy periods. This latter part of the analysis considered method switching, discontinuation, and missed study visits. Time to pregnancy, in days, was calculated as the midpoint between the date of the first positive pregnancy test and the date of the previous negative pregnancy test. For women who had a pregnancy outcome with no pregnancy testing or women with several missed visits before a positive pregnancy test, the pregnancy start date was defined as 14 days after the last normal menstrual period. For women who did not become pregnant during the study, the censoring date was the last date with a negative pregnancy test on or before study termination date. Duration of time on study (in months) was calculated from randomization to estimated date of pregnancy or date of withdrawal, termination from study whichever occurred first. In women with two pregnancy diagnoses during study follow-up period, only the first pregnancy was included in Kaplain Meier analysis. Univariable and multivariable Cox proportional hazards analysis was used to examine baseline factors associated with incident pregnancy.
Statistical analysis was done using SAS (version 9.1.3; SAS Institute Inc., Cary, NC, USA). All statistical tests were two-sided and done at 5% level of significance. Fisher’s exact or Chi-square tests were used for testing associations between categorical data. A Poisson distribution was assumed for 95% confidence intervals (CI) of pregnancy rates (14) and incidence rate ratios. Unpaired t-test/Wilcoxon rank sum two-sample tests were performed where appropriate.
RESULTS
Of 2,160 women screened, 51 were screened out for pregnancy related indications: 36 were already pregnant and 15 were planning to become pregnant sometime during study duration. Twenty-six women were not on an eligible method of contraception. Nine-hundred ninety-eight women were excluded for other non-pregnancy or contraceptive related indications. After randomization, a 196 women were excluded from the analysis for ineligibility based on pre-enrolment criteria (12). A total of 889 non-pregnant women were eligibly enrolled in the CAPRISA 004 trial and were included in this analysis (Figure 1).
Figure 1.
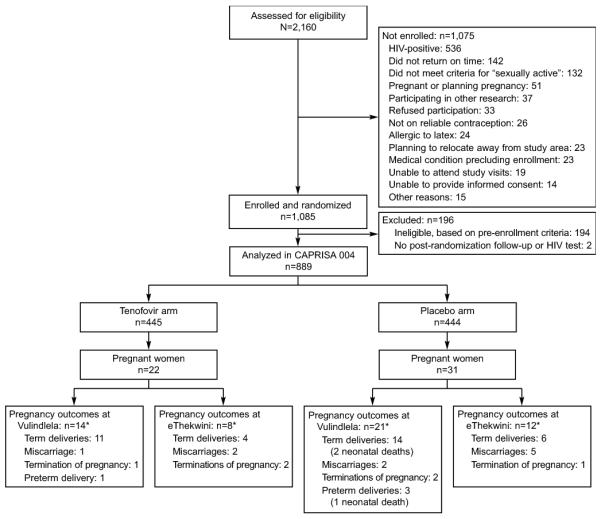
Screening, enrolment, and pregnancy outcomes after 30 months of follow-up in the Centre for the AIDS Programme of Research in South Africa (CAPRISA) 004 tenofovir gel trial by study arm and site. *There were 55 pregnancy outcomes from 53 pregnancies. One woman was pregnant twice, and one gave birth to twins.
Women from the urban site were older, more likely to have a stable partner, and had a lower monthly income. Women at the rural site reported fewer lifetime sexual partners (2.1 vs 6.0; p < 0.001) and had younger partner(s) in the 30 days prior to enrolment (26.4 vs 39.6; p < 0.001) (13). The mean age of the participants was 23.9 years. Three-quarters of the women were multiparous, almost all were in stable relationships, few reported having a casual partner in the 30 days prior to enrolment, and 71% reported inconsistent condom use (Table 1).
Table 1.
Baseline demographic characteristics and sexual behaviour by study arm
| Variable | All | Tenofovir Arm n(%) |
Placebo Arm n(%) |
p-value |
|---|---|---|---|---|
| Site | 0.77 | |||
| eThekwini site | 278 | 137 (30.8) | 141 (31.8) | |
| Vulindlela site | 611 | 308 (69.2) | 303 (68.2) | |
| Mean age,(sd) | 23.88 (5.11) | 24.17 (5.26) | 23.59 (4.94) | 0.13 |
| Marital Status | ||||
| Married | 50 | 26 (5.84) | 24 (5.41) | 0.92 |
| Other | 56 | 29 (6.52) | 27 (6.08) | |
| Stable | 783 | 390 (87.64) | 393 (88.51) | |
| Highest education | ||||
| No Schooling | 4 | 2 (0.45) | 2 (0.45) | 0.30 |
| Primary Schooling Complete | 10 | 6 (1.35) | 4 (0.90) | |
| Primary School Not Complete | 27 | 19 (4.27) | 8 (1.80) | |
| High School Complete | 301 | 150 (33.71) | 151 (34.01) | |
| High School Not Complete | 483 | 238 (53.48) | 245 (55.18) | |
| Tertiary Education Complete | 18 | 11 (2.47) | 7 (1.58) | |
| Tertiary Education Not Complete |
46 | 19 (4.27) | 27 (6.08) | |
| Number of children | ||||
| 0 | 197 | 90 (20.23) | 107 (24.10) | 0.42 |
| 1 | 470 | 238 (53.48) | 232 (52.25) | |
| 2 | 147 | 73 (16.40) | 74 (16.67) | |
| 3 | 50 | 30 (6.74) | 20 (4.51) | |
| 4+ | 25 | 14 (3.15) | 11 (2.48) | |
| Sex acts in past 30 days | ||||
| 0 | 22 | 11 (2.47) | 11 (2.48) | 0.13 |
| 1-5 | 360 | 186 (41.80) | 174 (39.19) | |
| 6-10 | 299 | 134 (30.11) | 165 (37.16) | |
| 11+ | 208 | 114 (25.62) | 94 (21.17) | |
| Casual partner in past 30 days | ||||
| 0 | 826 | 412 (92.58) | 414 (93.24) | 0.96 |
| 1 | 43 | 23 (5.17) | 20 (4.51) | |
| 2+ | 20 | 10 (2.25) | 10 (2.25) | |
| Condom use during sex | ||||
| Always | 259 | 128 (28.76) | 131 (29.51) | 0.81 |
| Occasionally | 630 | 317 (71.24) | 313 (70.50) | |
| Contraception | ||||
| Injectable | 730 | 359 (80.67) | 371 (83.56) | 0.29 |
| Oral | 138 | 73 (16.40) | 65 (14.64) | |
| Tubal ligation | 20 | 13 (2.92) | 7 (1.58) | |
| Hysterectomy | 1 | 0 (0.0) | 1 (0.23) |
All women were using a non-barrier method of contraception at the time of enrolment into the study; 730 (82.1%) chose an injectable form of contraceptive, 138 (15.5%) chose COCs and 21 (2.4%) had undergone female sterilization (20 tubal ligations and one hysterectomy) (Table 2). DMPA was a most popular injectable method, used by more women than NET-EN throughout the follow-up period. Overall, method switching was uncommon and contraceptive use remained high throughout the study. At study exit, 47 (5.3%) of participants reported not using any contraceptive method.
Table 2.
Contraceptive method uptake at baseline and use at study exit by site
| Vulindlela (N=611) |
eThekwini N=2(78) |
Total(N=889) | ||||
|---|---|---|---|---|---|---|
| Baseline N (%) |
Exit N (%) |
Baseline N (%) |
Exit N (%) |
Baseline N (%) |
Exit N (%) |
|
| DMPA | 442 (72.34) |
435 (71.19) |
140 (50.36) | 119 (42.81) |
582 (65.47) | 554 (62.32) |
| NET-EN | 66 (10.80) | 73 (11.95) | 82 (29.50) | 76 (27.34) | 148 (16.65) | 149 (16.76) |
| COC | 89 (14.57) | 63 (10.31) | 49 (17.63) | 55 (19.78) | 138 (15.52) | 118 (13.27) |
|
Tubal
Ligation |
13 (2.13) | 13 (2.13) | 7 (2.52) | 7 (2.52) | 20 (2.25) | 20 (2.25) |
|
Hysterectom
y |
1 (0.16) | 1 (0.22) | 0 (0.0) | 0 (0.0) | 1 (0.11) | 1 (0.11) |
| No method | 0 (0.0) | 26 (4.26) | 0 (0.0) | 21 (7.55) | 0 (0.0) | 47 (5.29) |
| All methods | 611 (100.0) |
585 (95.74) |
278 (100.0) | 257 (92.45) |
889 (100.0) |
842 (94.71) |
DMPA = depot-medroxyprogesterone acetate
NET-EN = norethisterone enanthate
COC = combined oral contraceptive
At the end of 30 months of study follow-up period, the overall pregnancy incidence rate was 3.95 per 100 woman-years (95% CI: 2.96, 5.17), somewhat higher in the urban than the rural cohorts (IRR: 0.75, 95% CI: 0.41 to 1.37, z-test p=0.30,). Although not significantly different, pregnancy rates were slightly lower in the tenofovir gel arm than the placebo arm (z-test p=0.18, Table 3). Pregnancy rates remained somewhat static around 4 per 100 w-y from beginning through to study completion (Figure 2).
Table 3.
Pregnancy rates by study arm and study site
| Variable | Vulindlela (N = 34) |
eThekwini (N = 19) |
Total (N = 53) |
||
|---|---|---|---|---|---|
| Tenofovir | Placebo | Tenofovir | Placebo | ||
| Number of pregnancies | 14 | 20 | 8 | 11 | 53 |
|
Pregnancy incidence per
100 woman-years |
2.90 | 4.30 | 4.07 | 5.62 | 3.95 |
|
| |||||
|
Incidence rate
ratio |
0.67 | 0.72 | 0.69 | ||
|
| |||||
|
Confidence interval (p-
value) |
0.31,1.40 (0.25) | 0.25, 1.98 (0.49) | 0.38, 1.23 (0.18) | ||
Figure 2.
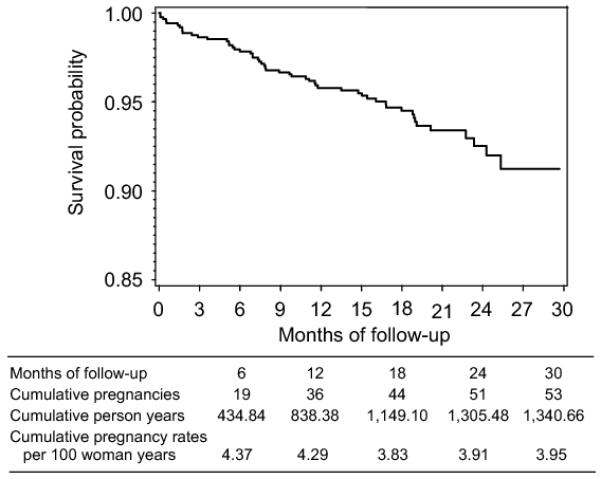
Kaplan Meier survival curve for pregnancy in the Centre for the AIDS Programme of Research in South Africa (CAPRISA) 004 trial.
By study end, 54 pregnancies were observed among 53 women who had 55 outcomes between them (Figure 1). One participant was pregnant twice and another had a twin pregnancy. Participants who became pregnant had similar baseline characteristics as those who did not become pregnant except for level of education: 97.2 % had a high school education versus 88.2% in the non-pregnant.
Among the 54 pregnancies; 10 (18.52%) resulted in miscarriages (including four probable chemical and spurious pregnancies), six (11.11%) were terminated pregnancies (including one non-invasive mole), 35 (64.81%) resulted in full-term live deliveries (two early neonatal deaths), and four (7.41%) resulted in pre-term deliveries (one early neonatal death) (Figure 1). All three babies with diagnosed fetal distress syndrome were in the placebo arm. There were no stillbirths. All early neonatal deaths were in the placebo arm. There were no congenital abnormalities observed.
Of the 39 viable pregnancies; three delivered by caesarean section (two in the tenofovir arm and one in the placebo arm), one had postpartum haemorrhage and one had premature rupture of membranes. The latter two were allocated to the tenofovir gel arm.
As expected, pregnancy rates based on category of contraceptive method used at baseline were 19 times higher among COC users than among injectable contraceptive users with no distinct differences between DMPA and NET-EN users (Table 4). However, taking method switching and discontinuation into consideration, the pregnancy rates were lowest amongst DMPA users (0.46 per 100 w-y, 95% CI: 0.13, 1.18) followed by NET-EN (1.75 per 100 w-y, 95% CI: 0.48, 4.49) and COC (11.47 per 100 w-y, 95% CI: 7.27, 17.22) users.
Table 4.
Participant profile, number of pregnancies and pregnancy rates by contraceptive method at baseline
| Variable | DMPA (N=582) |
NET-EN (N=148) |
COC (N=138) |
Other (N=21) |
Total |
|---|---|---|---|---|---|
| Age groups | |||||
| 18-24 | 398 (68.38) | 105 (70.95) | 74 (53.62) | 2 (9.52) | 579 |
| 25-29 | 112 (19.24) | 29 (19.59) | 32 (23.19) | 4 (19.05) | 177 |
| 30-34 | 44 (7.56) | 10 (6.76) | 22 (15.94) | 9 (42.86) | 85 |
| 35+ | 28 (4.81) | 4 (2.70) | 10 (7.25) | 6 (28.57) | 48 |
| Median (IQR) | 22 (20-26) | 22 (20-25) | 24 (20-29) | 33 (29- 35) |
|
| Parity N (%) | |||||
| 0 | 91 (15.64) | 51 (34.46) | 55 (39.86) | 0 (0.0) | 197 |
| 1 | 349 (59.97) | 71 (47.97) | 50 (36.23) | 0 (0.0) | 470 |
| 2 | 100 (17.18) | 20 (13.51) | 23 (16.67) | 4 (19.05) | 147 |
| 3 | 29 (4.98) | 3 (2.03) | 8 (5.80) | 10 | 50 |
| 4+ | 13 (2.23) | 3 (2.03) | 2 (1.45) | 7 (33.33) | 25 |
| Pregnant N (%) | |||||
| Negative | 570 (97.94) | 145 (97.97) | 100 (72.46) | 21 (100.0) |
836 |
| Positive | 12 (2.06) | 3 (2.03) | 38 (27.54) | 0 (0.0) | 53 |
| Pregnancy incidence rate per 100 woman years (95% CI) |
1.34 (0.69- 2.35) |
1.37 (0.28- 3.99) |
19.73 (13.96- 27.08) |
||
| Pregnancy rate * | |||||
| Pregnancy incidence rate per 100 woman years (95% CI) |
0.46 (0.13- 1.18) |
1.75 (0.48- 4.49) |
11.47 (7.27- 17.22) |
at time of index pregnancy accounting for method switching
Pregnancies occurred in women who either missed study visits thus discontinuing method use (22/53, 41.5%), or did not miss study visits but were on COC’s (19/53, 35.9%), or switched contraceptive methods (12/53, 22.6%) (Figure 3).
Figure 3.
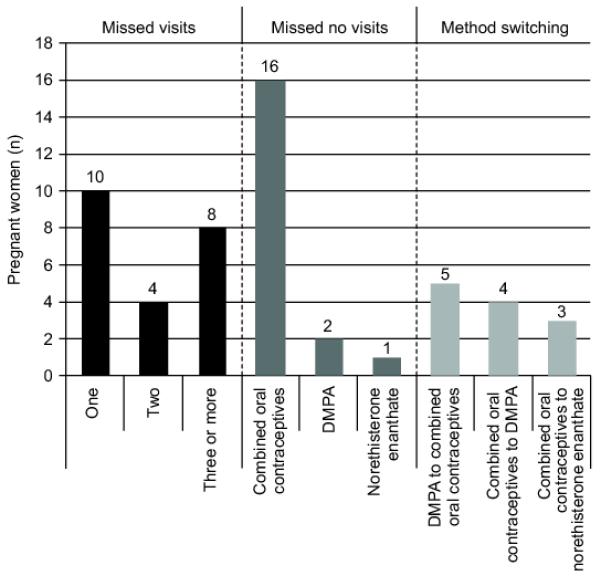
Clinic attendance and contraceptive use patterns amongst women who became pregnant (N=53) in the Centre for the AIDS Programme of Research in South Africa (CAPRISA) 004 trial. DMPA, depot medroxyprogesterone acetate.
Three women who became pregnant also became HIV-infected during the study, all in the placebo arm. Two of these pregnancies were diagnosed prior to HIV seroconversion. Method of contraception was the only baseline factor that was significantly associated with pregnancy in both the univariable and multivariable proportional hazard models. The adjusted hazard ratio (HR) for COC use was 15.91 (95% CI: 8.03, 31.52; p<0.0001; Table 5). Inconsistent condom use in the last 30 days was also a significant predictor of pregnancy, with an HR of 2.05 (1.04, 4.04) in the multivariable model.
Table 5.
Baseline predictors of pregnancy in Cox regression models
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Site | ||||
| Vulindlela | Ref | Ref | ||
| eThekwini | 1.33(0.76-2.34) | 0.32 | 1.9(0.97-4.03) | 0.06 |
| Age groups | ||||
| 18-24 | Ref | Ref | ||
| 25-29 | 1.19(0.62-2.31) | 0.60 | 0.86(0.43-1.72) | 0.66 |
| 30-34 | 1.61(0.74-3.48) | 0.23 | 0.85(0.34-2.12) | 0.73 |
| 35+ | N/A | |||
| Type of contraception | ||||
| DMPA | Ref | Ref | ||
| NET-EN | 1.01(0.29-3.59) | 0.98 | 0.86(0.24-3.11) | 0.81 |
| Oral | 14.51(7.58-27.78) | <0.000 1 |
15.91(8.03-31.52) | <0.0001 |
| Marital status | ||||
| Other | Ref | Ref | ||
| Married | 1.1(0.07-17.52) | 0.95 | 1.54(0.09-25.97) | 0.77 |
| Stable | 3.57(0.49-25.87) | 0.20 | 3.59(0.47-27.62) | 0.22 |
| Education | ||||
| Primary school | Ref | Ref | ||
| High school | 2.45(0.34-17.74) | 0.38 | 0.78(0.11-5.83) | 0.81 |
| Tertiary | 5.31(0.65-43.18) | 0.12 | 0.76(0.08-6.92) | 0.81 |
| Number of previous live births | ||||
| Previous live births (per 1 live birth increase) |
0.71 (0.51-0.995) | 0.0466 | 0.95(0.65-1.40) | 0.81 |
| Sex acts in last 30 days | ||||
| Sex acts in last 30 days (per 1 act increase) |
1.0 (0.98-1.03) | 0.73 | 1.01 (0.98-1.05) | 0.53 |
| Condom use in last 30 days | ||||
| Always | Ref | Ref | ||
| Inconsistent | 1.29 (0.69-2.42) | 0.42 | 2.05 (1.04-4.04) | 0.04 |
HR = hazard ratio; CI = confidence interval
N/A = not applicable
Ref = reference statistic
Total person-time of observation was 1340.7 w-y. Time off product due to pregnancies was 20.9 w-y (1.6% of total person-time), mostly attributable to the full-term births. The median time between date of last product use and pregnancy detection was 14 days (range 0 to 92).
DISCUSSION
The high contraceptive use throughout follow-up in this trial resulted in a low overall pregnancy rate of 3.95 per 100 woman-years. This pregnancy rate was lower compared to 17.7 per 100 woman-years that was observed in the CAPRISA 050/051 microbicide preparedness study, a study conducted without the implementation of the contraceptive curriculum (15). The observed pregnancy rate in the CAPRISA 004 trial was also lower than previous microbicide studies, even after procedures were revised to dispense contraceptives at the study sites (16). The low pregnancy rate observed in the CAPRISA 004 trial suggests that the contraceptive curriculum was effective in reducing pregnancy rates. On-site method provision and study visits synchronized with injectable administration further enhanced compliance with the method. Most importantly, CAPRISA 004 was the first trial to include an eligibility criterion requiring effective contraceptive initiation, and provide effective methods on-site. Future biomedical prevention trials should consider including a comprehensive contraception strategy in order to effectively reduce pregnancy rates.
Although not statistically significant, pregnancy incidence rates were higher in the tenofovir gel arm. While tenofovir gel is not known to reduce probability of conception, there needs to be vigilance in evaluating this association in future tenofovir trials.
Pregnancies that did occur in the CAPRISA 004 trial were mainly among COC users. The pregnancy rate was more than 10-fold higher in COC users than DMPA users, and COC use was the most important predictor of pregnancy in this trial, a finding consistent with findings in the HPTN 039 study (17). Inadvertent pregnancies in pill users are not uncommon, and often involve pill-taking errors, although many factors may contribute to ‘pill failure’ (18). Data from the US indicates that pregnancy rates within the first year of perfect method use are the same for COCs and DMPA at 0.3%. However, by 12 months the pregnancy rate with typical use for COC is 8% compared to 3% for DMPA (19). The COC pregnancy rate was higher still in this trial.
Pregnancies occurred with higher frequency amongst women who missed one or more study visits or switched methods. For future microbicide studies, researchers need to prioritize these women and develop a strategy of timely identification and providing targeted care/counseling in dealing with women who miss method replenishment dates and carefully manage timing of switching in order to minimize pregnancies.
Exposure to tenofovir gel during pregnancy was limited (median 14 days) and was not associated with adverse outcomes in this trial. Most pregnancies resulted in a full-term live birth. The three neonatal deaths in this study were all in the placebo arm. Additionally, no congenital anomalies were noted in the 39 babies that were delivered alive (both pre-term and full-term) and assessed in the early post-partum period. Potential teratogenecity was minimized by optimizing frequency of pregnancy testing, using a highly sensitive testing assay and thus reducing the period of potential drug exposure. . These findings, albeit following very short exposure to the drug and small sample size, are in keeping with those of the Tenofovir Antiretroviral Pregnancy Registry, which shows no evidence of higher rates of congenital anomalies following systemic exposure in patients on treatment (11). Furthermore, these findings are similar to non-human studies in which there were no demonstrable teratogenic effects up to 5 years after birth, post exposure to chronic low dose tenofovir in pregnancy (20). Due to high HIV seroconversion rates late in pregnancy, the safety of continuous gel use throughout pregnancy needs to be evaluated further.
Product withdrawal due to pregnancy accounted for only 1.6% of total person-time in CAPRISA 004 with negligible statistical impact. It has been shown that frequent pregnancy testing and using highly sensitive pregnancy tests to diagnose pregnancy in HIV prevention trials may lead to many false positive or chemical pregnancies. Consequently, women may be withdrawn from product for unnecessary prolonged periods of time thus compromising the power of the study (21). To overcome this limitation, CAPRISA 004 successfully implemented a strategy to minimise false positive or chemical pregnancies. Additionally, women continued study follow-up after a pregnancy diagnosis until the pregnancy outcome was established; allowing for timely product resumption upon pregnancy end.
The short duration of exposure to drug in utero made it difficult to assess the impact of tenofovir gel on pregnancy outcomes. In addition, high contraceptive use rates and low pregnancy rates in CAPRISA 004 may have been due to self selection of women not intending to fall pregnant into the study rather than the impact of the contraceptive curriculum per se thus limiting generalizability of the results.
Strengths of this analysis include good contraceptive data; accurate and frequent testing for the pregnancy outcome; excellent retention; and large enough study sample size to yield an adequate number of pregnancy outcomes.
The comprehensive contraceptive curriculum, including pre-specified contraceptive and pregnancy-related eligibility criteria developed for the CAPRISA 004 tenofovir gel trial was an effective strategy for enhancing contraceptive usage and reducing pregnancy rates in a microbicide trial. The pregnancy rate was low and no safety concerns arose with the use of tenofovir gel. As is the case in general use, injectable methods were more effective in preventing pregnancy than COCs. If the safety and effectiveness of tenofovir gel are confirmed, contraceptive use must be a prominent component of future prevention trials. Additionally, safety studies on prolonged tenofovir gel use throughout pregnancy should be prioritized as HIV prevention in pregnancy is important to reduce maternal morbidity and mortality, mother-to-child transmission, and infant mortality.
Acknowledgements
The authors thank the women enrolled in the trial for their willingness to participate and to remain in follow up over the study duration. The support of their communities is also gratefully acknowledged. The authors also thank the members of the CAPRISA Research Support Group at the CAPRISA Vulindlela and eThekwini Clinical Research Sites; the staff at the CAPRISA eThekwini and Vulindlela Clinical Research Sites in implementing this trial; and CAPRISA Data Management and Statistics staff for management and quality assurance of case report forms.
Sources of financial support The CAPRISA 004 tenofovir gel trial was supported by the Centre for the AIDS Programme of Research in South Africa (CAPRISA), the United States Agency for International Development (USAID), FHI (co-operative agreement # GPO-A-00-05-00022-00, contract # 132119), and the Technology Innovation Agency (formerly known as LIFElab), a biotechnology centre of the South African Department of Science and Technology. CONRAD provided support with product manufacturing and packaging and Gilead Sciences provided tenofovir used in the production of gel. The Columbia University-Southern African Fogarty AIDS International Training and Research Programme (AITRP), funded by the Fogarty International Center, National Institutes of Health (grant# D43TW00231), supported the training of clinical trial staff, including Dr Sengeziwe Sibeko.
Quarraisha Abdool Karim is the Co-Principal investigator of the HPTN Prevention Leadership Group (NIH/NIAID U01 AI068619), and Salim S Abdool Karim was the Protocol Chair of the HPTN 035 trial, which was supported by the National Institutes of Health (grant # U01AI46749 and U01AI068633).
Footnotes
Financial Disclosure The other authors did not report any potential conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.UNAIDS . UNAIDS Report on the global AIDS Epidemic 2010. Joint United Nations Programme on HIV/AIDS; Geneva: [Accessed 11 February 2011]. 2010. Available from: http://www.unaids.org/globalreport/ [Google Scholar]
- 2.UNAIDS . Report on the global AIDS Epidemic Update. Joint United Nations Programme on HIV/AIDS; Geneva: [Accessed 31 October 2008]. 2008. http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp. 2008. [Google Scholar]
- 3.Padian NS, McCoy SI, Balkus JE, Wasserheit JN. Weighing the gold in the gold standard: challenges in HIV prevention research. Aids. 2010;24(5):621. doi: 10.1097/QAD.0b013e328337798a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AVAC [Accessed February 2011]];Ongoing and planned PreExposure Prophylaxis (PrEP) trials (Feb 2011) 2011 Available from: http://www.avac.org/ht/a/GetDocumentAction/i/3113.
- 5.Raymond EG, Taylor D, Cates W, Jr., Tolley EE, Borasky D, Cancel A, et al. Pregnancy in effectiveness trials of HIV prevention agents. Sexually transmitted diseases. 2007 Dec;34(12):1035–9. doi: 10.1097/OLQ.0b013e3180e90586. [DOI] [PubMed] [Google Scholar]
- 6.Lagakos SW, Gable AR, editors. Methodological Challenges in Biomedical HIV Prevention Trials. National Academy of Sciences; 2008. [Google Scholar]
- 7.Smart T. [cited June 2006];Microbicides 2006: High rates of pregnancy pose challenges for microbicide trials. 2006 Available from: http://www.aidsmap.com/en/news/3F76DCE3-83FA-4523-A920-5CB0829DD33A.asp.
- 8.Van Damme L, Govinden R, Mirembe FM, Guedou F, Solomon S, Becker ML, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. The New England journal of medicine. 2008;359(5):463. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 9.Peterson L, Nanda K, Opoku BK, Ampofo WK, Owusu-Amoako M, Boakye AY, et al. SAVVY®(C31G) Gel for Prevention of HIV infection in Women: A Phase 3, Double-Blind, Randomized, Placebo-Controlled Trial in Ghana. PLoS One. 2007;2(12):1312. doi: 10.1371/journal.pone.0001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldblum PJ, Adeiga A, Bakare R, Wevill S, Lendvay A, Obadaki F, et al. SAVVY vaginal gel (C31G) for prevention of HIV infection: a randomized controlled trial in Nigeria. PLoS ONE. 2008;3(1):e1474. doi: 10.1371/journal.pone.0001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puoti M, Brown RS, Jr, Goodwin D, Zhang S, Fagan E. Tenofovir disoproxil fumarate in pregnancy: findings from the antiretroviral pregnancy registry. Dig Liver Dis. 2009;41:A39–40. [Google Scholar]
- 12.Karim Q Abdool, Karim SS Abdool, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karim Q Abdool, Kharsany A, Frohlich JA, Baxter C, Yende N, Mansoor LE, et al. Recruitment of high risk women for HIV prevention trials: baseline HIV prevalence and sexual behavior in the CAPRISA 004 tenofovir gel trial. Trials. 2011;12 doi: 10.1186/1745-6215-12-67. doi:10.1186/745-6215-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCullagh P, Nelder JA. Generalized Linear Models. Chapman and Hall, Inc.; London, England: 1983. [Google Scholar]
- 15.Karim Q Abdool, Kharsany AB, Frohlich JA, Werner L, Mashego M, Mlotshwa M, et al. Stabilizing HIV prevalence masks high HIV incidence rates amongst rural and urban women in KwaZulu-Natal, South Africa. Int J Epidemiol. 2010 Nov 3; doi: 10.1093/ije/dyq176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skoler-Karpoff S, Ramjee G, Ahmed K, Altini L, Plagianos MG, Friedland B, et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. The Lancet. 2008;372(9654):1977–87.13. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 17.Reid SE, Dai JY, Wang J, Sichalwe BN, Akpomiemie G, Cowan FM, et al. Pregnancy, Contraceptive Use, and HIV Acquisition in HPTN 039: Relevance for HIV Prevention Trials Among African Women. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2010;53(5):606. doi: 10.1097/QAI.0b013e3181bc4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser IS, Jansen RP. Why do inadvertent pregnancies occur in oral contraceptive users? Effectiveness of oral contraceptive regimens and interfering factors. Contraception. 1983 Jun;27(6):531–51. doi: 10.1016/0010-7824(83)90019-7. [DOI] [PubMed] [Google Scholar]
- 19.Trussell J. Contraceptive failure in the United States. Contraception. 2004;70(2):89–96. doi: 10.1016/j.contraception.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Van Rompay KKA, Durand-Gasselin L, Brignolo LL, Ray AS, Abel K, Cihlar T, et al. Chronic administration of tenofovir to rhesus macaques from infancy throughout adulthood and pregnancy: summary of pharmacokinetics, biological and virological effects. Antimicrobial agents and chemotherapy. 2008;52(9):3144–3160. doi: 10.1128/AAC.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schreiber CA, Sammel M, Hillier SL, Barnhart KT. A Little Bit Pregnant: Modeling How the Accurate Detection of Pregnancy Can Improve HIV Prevention Trials. American Journal of Epidemiology. 2009;169(4):515. doi: 10.1093/aje/kwn345. [DOI] [PMC free article] [PubMed] [Google Scholar]


