Introduction
Auto-aggressive effector T cells have been implicated in the development of cell-mediated autoimmune diseases including Multiple Sclerosis and type 1 diabetes. More specifically, IFN-γ-producing Th1 cells have long been associated with the pathogenesis of many organ-specific autoimmune diseases as IFN-γ was found to be present at the site of tissue inflammation at the peak of disease and Th1 cells were able to transfer disease. However, it was later found that IFN-γ-deficient mice were not resistant but highly susceptible to many organ-specific autoimmune diseases, raising the possibility that effector T cells other than Th1 cells were responsible for inducing autoimmunity. With the discovery of IL-17-producing Th17 cells, this puzzle is beginning to unravel. Emerging data suggest that Th17 cells are highly auto-pathogenic and can induce tissue inflammation and autoimmune disease. This raises the issue of what role, if any, Th1 cells play in the induction of tissue inflammation and autoimmune disease. Do they inhibit autoimmune tissue inflammation and is this the reason why IFN-γ−/− mice have exacerbated disease or do they cooperate with Th17 cells to promote tissue inflammation? Akin to Th1 and Th2 cells, Th17 cells are differentiated by specific cytokines, activate a distinct set of transcription factors, and are characterized by a unique cytokine signature, which includes IL-17, IL-21 and IL-22. In this review, we will discuss the respective roles of the Th1 and Th17 subsets in the context of autoimmune inflammatory responses.
1-Th1/Th2 differentiation and function
Mosmann and colleagues initially proposed that CD4+ T cells can be subdivided into 2 independent subsets, T helper 1 (Th1) and T helper 2 (Th2), each characterized by distinct effector functions and specific cytokine profiles [1]. Th1 cells produce large quantities of interferon (IFN)-γ and are predominantly involved in the clearance of intracellular pathogens through the activation of macrophages and induction of immunoglobulin class switching to complement-fixing antibodies. Th2 cells are characterized by the production of interleukin 4 (IL-4), IL-5, IL-13 and IL-25 and participate in the elimination of extracellular pathogens and parasites through the induction of immunoglobulin class switching to IgG1 and IgE, respectively. Th1 cells are also involved in cell-mediated and delayed-type hypersensitivity responses while Th2 cells have been associated with allergic responses.
The differentiation of effector Th cells is initiated by combined signals from the TCR, co-stimulatory molecules, cytokine receptors and lineage-specific transcription factors. The differentiation of Th1 cells is initiated by activation of T cells in the presence of IFN-γ. This leads to the activation of STAT-1 and the Th1 specific transcription factor T-bet. T-bet induces IFN-γ production and allows responsiveness to IL-12 via the expression of the IL-12Rβ2 chain. Engagement of IL-12 receptor by IL-12 induces the phosphorylation of STAT-4 which further cooperates with T-bet to transactivate the IFN-γ gene. A positive feedback loop then ensues wherein the increased IFN-γ further up-regulates T-bet and thus strengthens Th1 commitment. The differentiation of Th2 cells is induced by IL-4 and governed by the Th2 specific transcription factor GATA-3. Engagement of the IL-4 receptor leads to the phosphorylation of STAT-6 which binds to the IL-4 promoter and further induces IL-4 production, thus establishing a positive feedback loop to increase Th2 differentiation.
2-Th1 cells and organ-specific autoimmunity
Th1 cells have been implicated in the development of organ-specific autoimmune diseases [2]. More specifically, in organ-specific T cell driven autoimmune diseases, Th1 cells were described to be the pathogenic subset whereas Th2 cells were reported to exert inhibitory effects [3]. Several observations led to this conclusion: 1) The adoptive transfer of lines or clones exhibiting a Th1 phenotype can induce experimental autoimmune encephalomyelitis (EAE), an autoimmune disease of the central nervous system (CNS), 2) Th1 specific cytokines such as IFN-γ are present in CNS inflammatory lesions at the peak of EAE but decrease during remission; 3) Th cells invading the CNS at the peak of EAE express IFN-γ [4–6] and 4) treatment of mice with IL-12 aggravates collagen-induced arthritis (CIA), another organ-specific autoimmune disease [7]. The fact that mice deficient for the transcription factors important for the differentiation of Th1 cells, such as T-bet and STAT-4, are resistant to the development of EAE further supports an important role for Th1 cells and Th1-associated cytokines in organ specific autoimmunity. Indeed, in T-bet deficient mice, Th1 cells cannot be generated and these mice are resistant to the induction of several organ-specific autoimmune diseases [8, 9]. In some but not all instances, the protection from disease in T-bet−/− mice is associated with a significant up-regulation of Th2-like cytokines (IL-4, IL-5 or IL-10).
However, the concept of Th1 cells being exclusively responsible for driving autoimmune tissue damage was challenged when it became clear that a reduction in IFN-γ signaling (using either IFN-γ deficient mice or by blocking IFN-γ) did not protect mice from EAE or CIA but rather resulted in more severe disease [10, 11]. Similar results were obtained in mice deficient in molecules critically involved in the differentiation and stabilization of the Th1 phenotype such as IL-12p35 and IL-12Rβ2 [12–16]. Furthermore, exaggerated Th2 responses were not detected in these studies, implying the involvement of another type of effector T cell.
3- Discovery of IL-23, a member of the IL-12 family of heterodimeric cytokines
IL-12, a key cytokine in the development of Th1 cells, is a heterodimeric cytokine composed of two subunits, p35 and p40. Our understanding of the role of Th1 cells in autoimmunity was further challenged when it was shown that IL-12p35−/− mice were susceptible to EAE or CIA whereas IL-12p40−/− mice were resistant to the development of EAE. [12,17–19]. This problem was resolved when it was shown that IL-23 was comprised of a unique p19 subunit and the p40 chain of IL-12. Interestingly, loss of IL-12p40 or IL-23p19 resulted in complete resistance to EAE suggesting that it is IL-23 and not IL-12 that is necessary for the induction of EAE and potentially other autoimmune diseases. These results further supported the concept that IL-12 and Th1 cells per se were dispensable for the development of autoimmunity and specifically EAE.
Later it was found that IL-23 could drive the expansion of an IL-17-producing T cell population subsequently termed Th17 cells, which could induce more severe EAE upon adoptive transfer than IL-12-driven Th1 cells [20]. Furthermore, when IL-23 is not available to maintain and expand a population of already primed Th17 cells, EAE is markedly attenuated [18, 21]. In addition, the resident microglial cells and infiltrating macrophages during an autoimmune reaction in the CNS produce IL-23 [18], suggesting that IL-23 may function in situ to sustain a population of pathogenic Th17 cells. Together, these observations established strong, although to some extent indirect, evidence that Th17 cells were necessary for the development of EAE. Thus, it was proposed that IL-23 is not only required to shape a stable Th17 population in the secondary lymphoid tissue but also to maintain a pathogenic Th17 population at the site of inflammation.
4- Differentiation of Th17 cells
IL-23 was first thought to be a key mediator in the induction of Th17 cells mainly because it was shown to expand Th17 cells in vitro. Furthermore, p19 deficient mice have a profound defect in Th17 cells [19]. However, since IL-23 receptor is not expressed on naïve T cells, IL-23 cannot act on naïve T cells to induce their differentiation into Th17 cells. Indeed, three independent studies recently demonstrated that the combination of the acute phase protein IL-6 together with TGF-β induces the differentiation of Th17 cells from naïve T cells both in vitro and in vivo [22–24]. Once induced, Th17 cells produce large quantities of IL-17, IL-22, IL-21 and express IL-23 receptor.
IL-17 was first cloned in 1993 [25] and is the founding member of the IL-17 family of cytokines, which contains IL-17A (also called IL-17), IL-17B, IL-17C, IL-17D, IL-17E (also called IL-25) and IL-17F [26, 27]. Both IL-17A and IL-17F are produced by a variety of cell types including subsets of CD4+ T cells, CD8+ T cells, γδ-T cells, NK cells and neutrophils [28]. IL-17 production has also been associated with memory CD4+ T cells. IL-17A and IL-17F have pro-inflammatory properties and act on a broad range of cell types to induce cytokines (IL-6, IL-8, GM-CSF, G-CSF), chemokines (CXCL1, CXCL10), and metalloproteinases. IL-17A and IL-17F are also key cytokines for the recruitment, activation and migration of neutrophils [29]. Furthermore, IL-17 can activate osteoclasts and therefore induce bone resorption [30].
Th17 cells are also the highest producers of IL-21, a member of the IL-2 family of cytokines. IL-21 also plays an important role in Th17 development in that IL-21 together with TGF-β further amplifies Th17 differentiation. In fact IL-6 or IL-21 can induce Th17 cells to produce more IL-21 [31–33] and disruption of the IL-21 pathway results in reduced Th17 differentiation [32, 33]. Altogether, current data suggest that IL-6 can induce IL-21 production from IL-17-producing T cells and IL-21 then functions in an autocrine self-amplification loop to increase the Th17 response. Furthermore, both IL-6 and IL-21 can up-regulate the expression of IL-23 receptor on Th17 cells.
The maintenance of already committed Th17 cells in vitro requires IL-23 [24] which supports a role for IL-23 not in the differentiation but rather in the expansion/stabilization of Th17 cells. However, the mechanisms by which IL-23 induces expansion/stabilization of Th17 cells are not clear. Therefore, there are three distinct steps in the development of Th17 cells: induction, amplification and stabilization, in which three distinct cytokines (IL-6, IL-21 and IL-23) are operational.
Analogous to Th1 and Th2 development, Th17 development relies on the action of a lineage specific transcription factor, the orphan nuclear receptor RORαt [34]. The loss of RORαt in T cells abrogates the development of myelin specific Th17 cells and the development of EAE in mice immunized with myelin antigens [34]. A recent study further demonstrated that the orphan nuclear receptor RORα acts synergistically with RORαt to promote Th17 differentiation [35]. Consistent with the role of IL-6 in the differentiation of Th17 cells, STAT-3 has also been shown to be crucial in the generation of Th17 cells [36, 37].
5- Regulation of Th17 cells
An interesting aspect in the regulation of Th17 cells is their reciprocal developmental relationship with induced Foxp3+ Tregs. Foxp3 is a transcription factor specifically expressed in Tregs and necessary for their function [38–40]. In vitro, it is clear that TGF-β induces Foxp3 in naïve T cells and is necessary for the maintenance of already existing Tregs. We suggested that there is a reciprocal relationship between Foxp3+ Tregs and Th17 cells, in that TGF-β induces Foxp3+ T cells and IL-6 acts as a switch factor that re-directs T cell differentiation from the “default” Treg pathway into the Th17 pathway. Interestingly, IL-2, which signals through STAT-5 and is an important growth factor for Th1 and Th2 cells and is indispensable for the maintenance of Foxp3+ Tregs in the peripheral immune compartment, inhibits the differentiation of Th17 cells [21]. Accordingly, IL-2 deficient and STAT-5 deficient CD4+ T cells exhibit a strong propensity to differentiate into Th17 cells [41]. Similarly, retinoic acid potentiates Treg differentiation and inhibits Th17 cells [42]. All of these observations are in line with the hypothesis that Tregs and Th17 cells are related both in their induction and differentiation. At a molecular level, emerging data suggest that the lineage specific transcription factors Foxp3 (Tregs) and RORαt (Th17) bind to each other and may antagonize each other’s function.
The reciprocal relationship between Th17 and Tregs is relevant in vivo since IL-6 deficient mice have an increased proportion of Foxp3+ Tregs in the peripheral repertoire [43]. Moreover, Th17 differentiation is defective in Il6−/− mice and the fraction of Foxp3+ Tregs in the CD4+ T cell compartment further increases after immunization with myelin antigen in complete Freund’s adjuvant. Thus, the resistance of Il6−/− mice to EAE is due to an immune response in which antigen-specific Tregs are expanded at the expense of the generation of pro-inflammatory Th17 cells [43]. Similarly, CD4−DNTGFβRII mice, which express a dominant negative mutant of the TGF-β receptor [21] do not develop a competent Th17 response and are resistant to the development of EAE. On the other hand, transgenic mice which express TGF-β under the IL-2 promoter produce TGF-β upon ex vivo stimulation and develop Th17 cells in vivo under inflammatory conditions and elevated concentrations of IL-6. The increased number of Th17 cells in these animals resulted in the exacerbation of EAE [22]. These data support that there is a reciprocal developmental pathway between Foxp3+ Tregs and Th17 cells and that IL-6 might be a major switch factor for the generation/expansion of Tregs and Th17 cells. At present, it is not yet resolved whether the high fraction of Foxp3+ Tregs in Il6−/− mice is due to expansion of already existing natural Tregs or de novo conversion of Tregs in the peripheral immune compartment.
Th1 and Th2 cells cross-inhibit each other’s differentiation. Similarly, the hallmark cytokines of Th1 and Th2 cells, IFN-γ and IL-4, inhibit the IL-23-driven expansion of Th17 cells [44, 45]. T-bet can also negatively regulate Th17 cells [46, 47]. Moreover, IL-27, a heterodimeric cytokine composed of Epstein–Barr virus-induced gene 3 (EBI3) and a p28 chain, has been demonstrated to be a negative regulator of Th17 cell development. IL-27 belongs to the IL-12 family of heterodimeric cytokines and is produced by dendritic cells and macrophages. IL-27 signals through a receptor complex composed of the IL-27 receptor chain (IL-27R, also called WSX-1 or TCCR) and the gp130 chain of the IL-6 receptor [48, 49]. The absence of IL-27-mediated signaling enhances the generation of Th17 cells, increases the number of IL-17-expressing T cells in tissue infiltrates, and exacerbates neuroinflammation. Hence, IL-27 receptor deficient mice exhibit increased immunopathology in EAE and chronic Toxoplasmosis [50, 51]. IL-25 (IL-17E), which is produced by myeloid cells and by Th2 cells, also down-regulates Th17 responses. Indeed, Il25−/− mice are highly susceptible to EAE due to an enhanced Th17 response. Conversely, treatment with recombinant IL-25 or IL-25 delivered by a viral vector system is sufficient to suppress EAE in wild type mice [52]. Although IL-25 is produced by activated Th2 cells, resident cells of the innate immune system, such as microglia, are believed to be the major source of IL-25 in the CNS. IL-25 inhibits Th17 responses indirectly by inducing IL-13, which decreases the production of IL-23, IL-1 and IL-6 in antigen presenting cells [52]. Most likely, the identification of new cytokines as well as the description of new functions for already known cytokines will further improve our understanding of the regulation of Th17 cells.
6- Th1, Th17 and autoimmunity
Since IL-17 was recognized to be increased in human autoimmune diseases like Multiple Sclerosis [53, 54], rheumatoid arthritis [55], and psoriasis [56] as well as in animal models of autoimmunity, much attention has been focused on defining the role of Th17 cells in the pathogenic process of tissue inflammation [57]. Indeed, in the last 3 years the importance of Th17 cells in the pathogenesis of organ-specific autoimmune inflammation has been demonstrated in different animal models. In fact, Th17 cells have been shown to be more potent than Th1 cells in inducing disease [20]. While the relative role of Th1 and Th17 cells in disease is currently a matter of debate, the most likely scenario is that both cell types are involved. However, to date, it is not clear how Th1 and Th17 cells interact with each other and promote autoimmune tissue injury. In the natural course of EAE, we have observed that both Th1 and Th17 cells infiltrate the CNS together with Foxp3+ Treg cells, but it was clear that the Th17 cells in the CNS peaked earlier than the Th1 cells [43]. Interestingly, we have found that CNS-derived Treg cells are not effective in controlling effector T cells recovered from the site of tissue inflammation [20]. However, effector T cells recovered during the recovery phase are suppressible by CNS-derived Tregs [43]. Since Th1 effector cells dominate just prior to the recovery phase of disease, it is an intriguing hypothesis that Th1 effector populations might be more easily controlled by Tregs than Th17 effector cells. Therefore, the sequential change in the composition of T effector populations may promote efficient Treg-mediated suppression in the target organ. Based on these data and the observations that we have made, we speculate that such a pattern of T helper cell dynamics drives immunopathology and immunoregulation in organ-specific autoimmunity.
A further wrinkle in the Th1 vs Th17 debate is the observation that a considerable population of cells secreting both IL-17 and IFN-γ can consistently be detected in vivo in the inflamed CNS. This observation may indicate the existence of a transitional cell that expresses both IFN-γ and IL-17 in vivo. Some reports propose that lineage commitment of T helper cells may be occurring in the CNS and that CNS-derived antigen presenting cells might have the capacity to differentially activate and possibly further differentiate Th17 cells, Th1 cells and Treg cells depending on the phase of EAE [58].
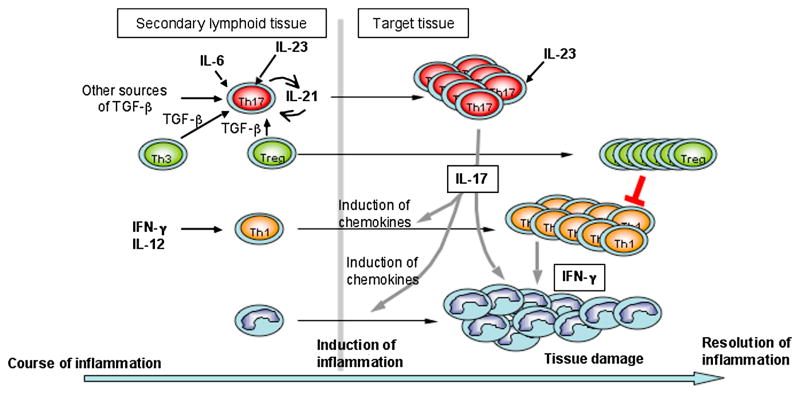
A working model for the role of Th1 and Th17 cells in the induction of organ-specific autoimmune disease is that Th17 cells constitute the first wave of effector T cells migrating to the CNS and orchestrate the recruitment of further waves of effector T cells, particularly Th1 cells. In support of this model, IL-17 is an inducer of MCP-1 [44], a chemokine that plays a prominent role in the recruitment of mononuclear cells to the CNS [59]. Furthermore, through the induction of IP-10, Th17 cells might drive the migration of Th1 cells that express the IP-10 receptor CXCR3 [60] into inflamed tissue. Thus there may be a specific temporal sequence of various T helper cell subsets infiltrating the CNS (Figure 1). Support for this idea comes from studies of the T cell response against Mycobacterium tuberculosis. Using specific vaccination strategies against infection with M. tuberculosis, it has been recognized that the elicitation of an early Th17 response is essential in promoting a delayed and sustained Th1 response that finally controls the pathogen [61].
Figure 1.
In summary, Th17 cells orchestrate tissue inflammation by inducing proinflammatory cytokines (IL-6, IL-1 and TNFα) and chemokines which recruit Th1 cells to the target tissue. Although functional Treg cells accumulate in the target tissue, they are not efficacious due to the overwhelming amounts of pro-inflammatory cytokines, such as IL-6, which protect effector T cells from the suppressive effects of Treg cells. Therefore, boosting Treg cells together with controlling tissue inflammation may provide the most effective strategy for controlling tissue inflammation and treating autoimmune disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16(1):34–8. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson LB, Kuchroo VK. Manipulation of the Th1/Th2 balance in autoimmune disease. Curr Opin Immunol. 1996;8(6):837–42. doi: 10.1016/s0952-7915(96)80013-6. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Nun A, Cohen IR. Vaccination against autoimmune encephalomyelitis (EAE): attenuated autoimmune T lymphocytes confer resistance to induction of active EAE but not to EAE mediated by the intact T lymphocyte line. Eur J Immunol. 1981;11(11):949–52. doi: 10.1002/eji.1830111119. [DOI] [PubMed] [Google Scholar]
- 5.Pettinelli CB, McFarlin DE. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2- T lymphocytes. J Immunol. 1981;127(4):1420–3. [PubMed] [Google Scholar]
- 6.Renno T, et al. TNF-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J Immunol. 1995;154(2):944–53. [PubMed] [Google Scholar]
- 7.Germann T, et al. Characterization of the adjuvant effect of IL-12 and efficacy of IL-12 inhibitors in type II collagen-induced arthritis. Ann N Y Acad Sci. 1996;795:227–40. doi: 10.1111/j.1749-6632.1996.tb52672.x. [DOI] [PubMed] [Google Scholar]
- 8.Bettelli E, et al. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200(1):79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neurath MF, et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J Exp Med. 2002;195(9):1129–43. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferber IA, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156(1):5–7. [PubMed] [Google Scholar]
- 11.Matthys P, et al. Anti-IL-12 antibody prevents the development and progression of collagen-induced arthritis in IFN-gamma receptor-deficient mice. Eur J Immunol. 1998;28(7):2143–51. doi: 10.1002/(SICI)1521-4141(199807)28:07<2143::AID-IMMU2143>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.Gran B, et al. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169(12):7104–10. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 13.Gutcher I, et al. Interleukin 18-independent engagement of interleukin 18 receptor-alpha is required for autoimmune inflammation. Nat Immunol. 2006;7(9):946–53. doi: 10.1038/ni1377. [DOI] [PubMed] [Google Scholar]
- 14.Krakowski M, Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26(7):1641–6. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 15.Tran EH, Prince EN, Owens T. IFN-gamma shapes immune invasion of the central nervous system via regulation of chemokines. J Immunol. 2000;164(5):2759–68. doi: 10.4049/jimmunol.164.5.2759. [DOI] [PubMed] [Google Scholar]
- 16.Zhang GX, et al. Role of IL-12 receptor beta 1 in regulation of T cell response by APC in experimental autoimmune encephalomyelitis. J Immunol. 2003;171(9):4485–92. doi: 10.4049/jimmunol.171.9.4485. [DOI] [PubMed] [Google Scholar]
- 17.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest. 2002;110(4):493–7. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 19.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198(12):1951–7. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veldhoen M, et al. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7(11):1151–6. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 22.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 23.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 24.Veldhoen M, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Rouvier E, et al. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150(12):5445–56. [PubMed] [Google Scholar]
- 26.Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71(1):1–8. [PubMed] [Google Scholar]
- 27.Moseley TA, et al. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14(2):155–74. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 28.Aggarwal S, et al. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278(3):1910–4. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 29.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–76. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Kotake S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103(9):1345–52. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8(9):967–74. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 32.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448(7152):484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–3. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 34.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 35.Yang XO, et al. T Helper 17 Lineage Differentiation Is Programmed by Orphan Nuclear Receptors RORalpha and RORgamma. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathur AN, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178(8):4901–7. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 37.Yang XO, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282(13):9358–63. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 38.Khattri R, et al. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4(4):337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 39.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 40.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 41.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–81. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 43.Korn T, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13(4):423–31. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(1):1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 46.Rangachari M, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203(8):2009–19. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mathur AN, et al. T-bet is a critical determinant in the instability of the IL-17-secreting T-helper phenotype. Blood. 2006;108(5):1595–601. doi: 10.1182/blood-2006-04-015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5(7):521–31. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 49.Pflanz S, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16(6):779–90. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 50.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7(9):929–36. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 51.Stumhofer JS, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7(9):937–45. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 52.Kleinschek MA, et al. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204(1):161–70. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lock C, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8(5):500–8. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 54.Matusevicius D, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5(2):101–4. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 55.Aarvak T, et al. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J Immunol. 1999;162(3):1246–51. [PubMed] [Google Scholar]
- 56.Teunissen MB, et al. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111(4):645–9. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 57.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13(2):139–45. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 58.Deshpande P, King IL, Segal BM. Cutting edge: CNS CD11c+ cells from mice with encephalomyelitis polarize Th17 cells and support CD25+CD4+ T cell-mediated immunosuppression, suggesting dual roles in the disease process. J Immunol. 2007;178(11):6695–9. doi: 10.4049/jimmunol.178.11.6695. [DOI] [PubMed] [Google Scholar]
- 59.Fife BT, et al. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192(6):899–905. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu L, et al. Severe disease, unaltered leukocyte migration, and reduced IFN-gamma production in CXCR3−/− mice with experimental autoimmune encephalomyelitis. J Immunol. 2006;176(7):4399–409. doi: 10.4049/jimmunol.176.7.4399. [DOI] [PubMed] [Google Scholar]
- 61.Khader SA, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8(4):369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]