Peripheral blood cytopenias result from a wide variety of infectious diseases and may significantly complicate the prognosis and outcome of those diseases. Infection with Gram-negative, LPS-negative members of the Anaplasmataceae family, including Anaplasma phagocytophilum and Ehrlichia muris, results in bi- or pancytopenia. The specific mechanisms responsible for the cytopenias are unknown.
Viruses and bacteria have profound yet often discrepant effects on bone marrow (BM) and circulating numbers of blood cells. Multiple line-age cytopenias (bi- and pancytopenia) are a frequent sequel of viral infections [1]. Different viruses can directly infect BM stromal cells or haematopoietic stem cells and/or modulate cytokine production by accessory cells. Infections with Gram-negative, LPS-positive bacteria result in an endotoxin-mediated, acute phase, proinflammatory cascade of events that result in granulocyte efflux from the BM, peripheral neutrophilia and BM hyperplasia [2]. In contrast, gram-negative, LPS-negative bacteria, including members of the Anaplasmataceae family, elicit a very different, poorly-characterised, cascade of events. Haematologic alterations in both natural and experimental infection include a marked thrombocytopenia, moderate leukopenia (lymphopenia and neutropenia) and mild to moderate, non-regenerative anaemia [3,4]. Cytopenias vary from profound and irreversible (for example, dogs with chronic E. canis infection) to moderate and transient. Paradoxically, although Anaplasma family organisms display cell tropism for specific haematopoietic cell lineages, infection, regardless of aetiologic agent, host species or cell target, is characterised by bicytopenia or, occasionally, pancytopenia. This suggests the potential for more global haematopoietic disturbances. We hypothesised that the cytopenias may be due in part to bacteria-induced haematopoietic suppression.
C57/Bl6 mice were infected intraperitoneally with E. muris and samples were collected on days 0–60 post-infection. C3H/HeN mice were infested with A. phagocytophilum-infected Ixodes scapularis nymphal ticks and sampled on days 0–21 post-infection. Blood samples were collected for a complete blood cell analysis and quantitative PCR for pathogen detection (A. phagocytophilum p44 DNA). BM was collected for haematopoietic colony-forming assays, cytology, histology and quantitative PCR. Cultured BM supernatants from A. phagocytophilum infected mice were collected for cytokine analysis.
Thrombocytopenia and anaemia (bicytopenia) developed in both E. muris and A. phagocytophilum-infected mice. Leukopenia was variable. A. phagocytophilum infection induced significant decreases in BM proliferation and differentiation from day 4 to day 21 post-infection, concurrent with the cytopenias (Fig. 1). E. muris infection resulted in a similarly dramatic decrease in BM proliferation and differentiation on days 8 to 14 post-infection. Decreases in BM haematopoietic production included all lineages. Haematopoietic colony forming assays are supplemented with cytokines and assess haematopoietic stem cell/progenitor cell activity in the absence of stromal cells. As such, findings support infection-induced alterations in stem cell differentiation, proliferation or mobilisation. Both thrombocytopenia and myelosuppression were more severe in mice infected with A. phagocytophilum via tick bite compared with mice infected intraperitoneally in previous studies. Thrombocytopenia did not correlate with pathogen burden in blood, as assessed by quantitative PCR, but was strongly correlated with BM colony numbers in A. phagocytophilum-infected mice. This temporal association suggests that rapid changes in BM may be contributing to alterations in circulating platelet numbers. Cytologic evaluation of BM revealed infection-induced lymphocyte depletion and a reciprocal granulocytic hyperplasia in both infection models, similar to kinetics noted in other models of inflammation [5]. In addition, A. phagocytophilum infection resulted in BM production of myelosuppressive chemokines, including the murine interleukin-8 homologues, macrophage inflammatory protein-2 and KC. Findings are compatible with previous work that demonstrated a predominance of myelosuppressive chemokines in human BM after A. phagocytophilum infection [6]. Anaplasma family pathogens appear to perturb normal haematopoiesis, resulting in shifts in BM cell populations and transient failure of haematopoietic colony formation, which may contribute to peripheral blood cytopenias.
Fig. 1.
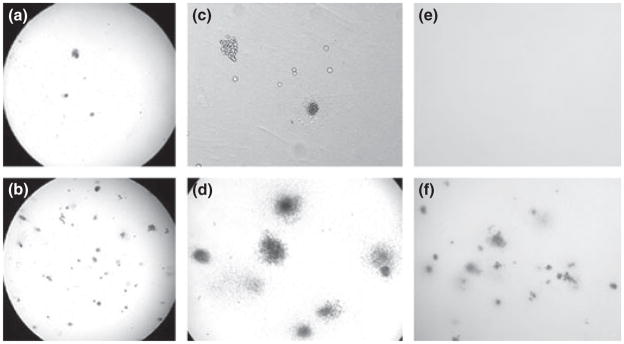
Bone marrow was collected from Anaplasma phagocytophilum-infected and uninfected mice on days 0–21 after tick-borne infection. An aliquot of BM was plated in duplicate and cultured in complete MethoCult media for 7–10 days (StemCell Technologies) according to the manufacturer’s instructions. The colonies were counted and scored as granulocytic/monocytic, mixed or erythroid. Depicted are representative images of assays on day 7 after A. phagocytophilum infection. (a) Infected mouse, low power. (b) Uninfected mouse, low power. (c) Infected mouse with increased macrophages, high power. (d) Uninfected mouse, high power. (e) Infected mouse, high power. (f) Uninfected mice infested with ticks, high power.
Footnotes
The authors have declared no potential conflicts.
References
- 1.Moses A, Nelson J, Bagby GC., Jr The influence of human immunodeficiency virus-1 on hematopoiesis. Blood. 1998;91:1479–1495. [PubMed] [Google Scholar]
- 2.Quesenberry P, Morley A, Stohlman F, Jr, et al. Effect of endotoxin on granulopoiesis and colony-stimulating factor. N Engl J Med. 1972;286:227–232. doi: 10.1056/NEJM197202032860502. [DOI] [PubMed] [Google Scholar]
- 3.Bakken JS, Krueth J, Wilson-Nordskog C, et al. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–20. [PubMed] [Google Scholar]
- 4.Borjesson DL, Simon SI, Tablin F, et al. Thrombocytopenia in a mouse model of human granulocytic ehrlichiosis. J Infect Dis. 2001;184:1475–1479. doi: 10.1086/324518. [DOI] [PubMed] [Google Scholar]
- 5.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein MB, Hu S, Chao CC, et al. The agent of human granulocytic ehrlichiosis induces the production of myelosuppressing chemokines without induction of proinflammatory cytokines. J Infect Dis. 2000;182:200–205. doi: 10.1086/315641. [DOI] [PubMed] [Google Scholar]