Abstract
The ability of IL-10 producing Type 1 regulatory T cells (Tr1) to restrain the activation of effector immune cells during autoimmune responses underscores their essential role in maintaining immune tolerance. While mouse studies have demonstrated that increasing the numbers and/or function of Tr1 cells could improve the course of autoimmune diseases, the inability to generate Tr1 cells in vitro in large numbers has hampered identification of the molecular mechanisms responsible for their differentiation. Interleukin-27 (IL-27), a member of the IL-12 heterodimeric cytokine family, was identified as an important cytokine that suppresses effector TH17 cells and promotes the generation of Tr1 cells. Tr1 cells dampen autoimmunity and tissue inflammation partly through their secretion of the immunosuppressive cytokine IL-10. Here we review the molecular mechanisms involved in IL-27-induced Tr1 cell differentiation, with a focus on the role of two transcription factors, the aryl hydrocarbon receptor (AhR) and c-Maf. We also discuss how ligands that bind to AhR and affect the biology of IL-27-induced Tr1 cells can be exploited as a therapeutic approach to alleviate human autoimmune diseases.
Keywords: Type 1 regulatory T cells, differentiation, IL-27, c-Maf Protooncogene (c-Maf), aryl hydrocarbon receptor (AhR)
1. Introduction
Mosmann and Coffman originally classified CD4+ T lymphocytes into TH1 and TH2 subsets [1]. However, the repertoire of effector CD4+ T cell subsets has recently expanded to include additional effector T cell subsets like TH17 cells [2-5] and TH9 cells [6, 7]. In addition, a number of regulatory T cell subsets have been identified that suppress effector T cells, tissue inflammation and autoimmunity. Interest in regulatory T (Tregs) cells was revived with the discovery of FoxP3, a transcription factor exclusively expressed in Tregs cells, and by the evidence that a genetic loss of FoxP3 and Tregs results in loss of immunological tolerance and induction of multi-organ autoimmune disease in both mice (“scurfy”, an X-linked recessive mouse mutant)[8] and humans (Immunodysregulation, Polyendocrinopathy, and Enteropathy, X-linked syndrome IPEX)[9]. Two major classes of CD4+ regulatory T cells have been described: CD4+ FoxP3+ Tregs [10] and type 1 regulatory T cells (Tr1 cells) that secrete IL-10 and lack FoxP3 expression. Tr1 cells are additionally characterized by their high levels secretion of TGF-β and, like FoxP3+ Tregs, by their ability to suppress immune and autoimmune responses [11].
Although extensive studies have been performed to differentiate Tr1 cells from other Tregs, the lack of consensus in their phenotype and differentiation factors have complicated their study. The original protocols designed to generate Tr1 cells in vitro involved the activation of naïve T cells in an antigen-specific or TCR-mediated manner in the presence of IL-10 alone, IL-10 together with Vitamin D3, IFN-α or other immunosuppressive drugs such as dexamethasone or Rapamycin [11-14]. In humans, Tr1 cells have also been induced in vitro with anti-CD46 antibodies in the presence of IL-2 [15], however mice do not express CD46 and therefore the biological role of CD46 in differentiation of Tr1 cells could not be undertaken in mice. While many of these protocols resulted in the differentiation of IL-10-secreting cells that shared immunosuppressive functions, the inability to expand Tr1 cells in large quantities in vitro has hampered progress in understanding the biology of these cells. In this respect, different groups have tried to unravel a specific Tr1 signature gene. Cobbold et al have compared gene expression between murine Tr1 cells clones with CD4+CD25+Foxp3+ Tregs and found that the repressor of GATA-3 (ROG) was expressed specifically in Tr1 cells but not in natural Tregs [16]. However, the in vivo relevance of ROG in the biology of Tr1 cells was not further explored as ROG is also expressed in other TH cells stimulated with anti-CD3 [17]. Overall, Tr1 cells are important for the suppressing tissue inflammation and inhibiting autoimmunity, which underscores a need to identify reliable protocols to grow them in large numbers for testing their in vivo biology and identify molecular mechanisms for their generation and function.
2. Tr1 cells in transplantation and autoimmune diseases
Given the immunosuppressive functions of Tr1 cells, research has focused on developing ways to utilize Tr1 cells to alleviate inflammatory pathologies in a wide range of contexts, in particular in transplantation and autoimmune diseases [11, 14, 18]. In humans, Tr1 cells were first described in severe combined immunodeficient (SCID) patients who had developed long-term tolerance to stem cell allografts, suggesting that these cells might naturally regulate immune responses in humans [19]. Administration of IL-10 in combination with Rapamycin in vivo was also shown to induce Tr1 cells that mediated tolerance in type 1 diabetic mice after pancreatic islet transplantation [14]. Furthermore, cell therapy with alloantigen-specific Tr1 cells could promote an IL-10-dependent graft-specific tolerance in a mouse model of islet transplant, eliminating the need of an immunosuppressive treatment [20]. In humans, Tr1 cells may also play an important role in inducing transplantation tolerance, as peripheral blood mononuclear cells (PBMC) isolated from patients who underwent islet transplant and became insulin independent produced significantly higher IL-10 when compared with transplant subjects that continued to be insulin-dependent [21]. Induction of Tr1 cells was also described in patients who spontaneously developed tolerance to kidney or liver allografts [22]. Taken together, these data indicate that IL-10-producing Tr1 cells can be induced under different states of transplantation tolerance and may be naturally involved in inducing tolerance to allotransplants.
Tr1 cells have also been identified in various tissues during autoimmune inflammation. They are, for instance, found in large numbers in the intestine where they are proposed to have a protective role during colitis. Maynard et al. have suggested that Tr1 may play a major role in maintaining immune homeostasis to the intestinal microbiota [23], which is consistent with the finding that loss of IL-10 results in the development spontaneous enterocolitis in IL-10 deficient mice. The role of Tr1 cells in the prevention of autoinflammation was also demonstrated in another colitis model where the pathogenicity of transferred CD45RBhighCD4+ T cells into SCID mice could be mediated by co-transfer of murine Tr1 clones derived from CD4+ T cells expressing a transgenic T cell receptor specific for an ovalbumin (OVA) peptide. These cells inhibited colitis only in recipients that received specific antigen (OVA) in their drinking water thus demonstrating that the immune suppression relied on the antigen specific activation of Tr1 cells in vivo [11]. Similarly, in mice suffering from experimental autoimmune encephalomyelitis (EAE), the rodent model of human Multiple Sclerosis (MS), transfer of in vitro generated OVA specific Tr1 cells could prevent the development of neurological symptoms when OVA peptide was injected intracranially [12]. Tr1 cells have also been induced in vivo using the soluble peptide Myelin Basic Protein (MBP) p87-99, which could reverse ongoing EAE disease in rats immunized with MBP [24]. In addition, Meiron et al. reported that stromal cell–derived factor 1α (CXCL12), redirects the polarization of effector Th1 cells into CD4+CD25-Foxp3-IL-10high antigen-specific regulatory T cells that suppressed autoimmune inflammation in mice undergoing EAE [25]. Finally, the generation of IL-10-producing T cells was impaired in patients suffering from MS compared to healthy volunteers, suggesting that Tr1 cells may have a protective role during multiple sclerosis (MS) [26]. These studies suggest that Tr1 cells may play a crucial role in suppressing autoimmunity, not only in experimental autoimmune disease models, but also in many human autoimmune diseases by suppressing tissue inflammation and maintaining self-tolerance.
3. IL-27 induces IL-10 producing Tr1 cells
IL-27, a member of the IL-12 cytokine family, which consists of two subunits p28 and the Epstein-Barr virus-induced gene 3 (EBI3), has recently been identified as a differentiation factor for the generation of IL-10-producing Tr1 cells [27-29]. IL-27 is secreted by tolerogenic dendritic cells (DC) that were conditioned in vitro or in vivo by FoxP3+ Treg cells. These Tregs-modified DC express plasmacytoid-like markers, similar to what has been previously described for tolerogenic DCs [30].
Based on the structural homology between IL-12 and IL-27, IL-27 was initially described as a proinflammatory cytokine that could act primarily act on naïve CD4+ T-cells to induce TH1 response [31] [32, 33]. This interpretation was consistent with the ability of IL-27 to induce the transcription factor T-bet, the master TH1 transcription factor that transactivates IFN-γ and IL-12Rβ2 genes (Figure 1) [31, 34, 35]. Subsequent work from Chris Hunter's laboratory established that IL-27 was actually not a proinflammatory cytokine but in fact might suppresses excessive immune responses in vivo (reviewed in[36]) as IL-27R-deficient mice did not have a defect in inducing TH1 cells but died due to excessive immune response following Leishmania infection [37, 38]. Subsequently, three independent laboratories including ours discovered that addition of IL-27 to naïve T cells induced expansion and differentiation of CD4+ T cells that secreted high levels of IL-10 (Figure 1). Those IL-27-driven Tr1 cells proliferate poorly following TCR-mediated activation and suppress effector T cells in vitro partly through IL-10 production[39]. Additional studies revealed that Tr1 cells also suppress effector T-cells responses in a contact dependent manner, possibly through Granzyme-B-mediated lysis, since we found that in addition to IL-10, Granzyme-B is induced by IL-27 during Tr1 differentiation (Figure 2). Finally, IL-27 can also induce suppressive IL-10-producing Tr1 cells in humans from naïve CD4+ T cells [40]. Taken together, these findings provide impetus to evaluate the potential of IL-27-induced Tr1 cells in autoimmune diseases.
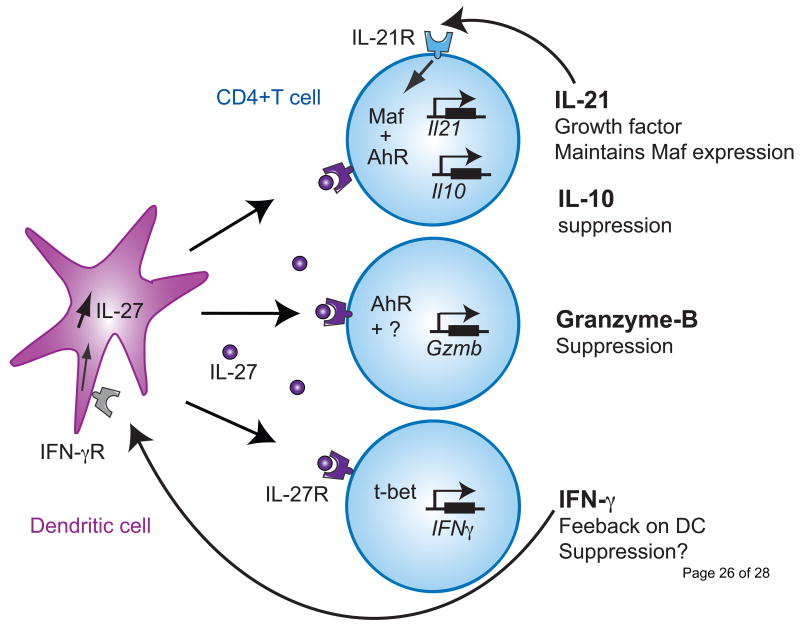
Figure 1. Molecular mechanisms governing the induction of IL-27-induced Tr1 cells.
Three different pathways are induced by IL-27 during Tr1 cell differentiation. IL-27 drives the expression of the transcription factors c-Maf and AhR, which bind together to transactivate the Il21 and Il10 promoters. IL-21 maintains Maf and AhR expression, while IL-10 is essential for the suppressive function of Tr1 cells (Upper panel). IL-27-induced AhR, alone or with an unknown cofactor, promotes Granzyme-B expression that mediates the contact-dependent suppressive activity of Tr1 cells (middle panel). Finally IL-27 promotes T-bet expression that results in IFN-γ secretion. IFN-γ then acts on DCs to enhance IL-27 expression and further supports Tr1 cell differentiation (lower panel).
Figure 2. IL-27 induces Granzyme B expression.

RNA isolated from naïve CD4+CD44loCD62LhiCD25- cells differentiated with IL-27 (25ng/ml) (Tr1) or without (TH0) in the presence of anti-CD3 and anti-CD28 antibodies (2ug/ml) was subjected to quantitative real-time PCR amplification relative to the expression of mRNA encoding β-actin to examine expression of Il10 (left panel) and Gzmb (right panel) Il10 at different time points following activation.
4. Transcriptional regulation of IL-27-induced Tr1 cells
4.1 Maf and AhR in Tr1 cell differentiation
The molecular mechanisms involved in IL-27-induced Tr1 cell differentiation are progressively being unraveled. We and others have identified an essential role of IL-21, a member of the IL-2 cytokine family that signals through the common gamma-chain receptor, in Tr1 cell differentiation [39, 41]. IL-21 secretion was induced by IL-27 and was indispensable for the development of Tr1 cells, as demonstrated by the defective generation of Tr1 cells in IL-21R deficient T cells [39]. Since the expression of IL-21 in TH17 cells is controlled by the transcription factor c-Maf [42], we have tested Maf expression during Tr1 cell differentiation and found that Maf indeed was induced at high levels in Tr1 cells (Figure 1). C-Maf indeed binds il21 promoter and transactivates il21 gene. Additional studies of the role of c-Maf during Tr1 cell differentiation using Maf deficient T cells revealed that c-Maf was absolutely critical for IL-10 secretion since IL-27-induced Tr1 differentiation was defective in c-Maf-/- mice. [39]. Interestingly, IL-21 induced by IL-27 during Tr1 cell differentiation in turn maintains Maf expression, suggesting that IL-21 signaling acts as a feed-forward loop to induce c-Maf which further acts as a growth factor for Tr1 cell development (Figure 1) (reviewed in [36]). It is noteworthy that these findings are reminiscent of results obtained in TH17 cells where IL-21 was shown to support IL-17 secretion from TH17 cells through a self-amplifying feed-forward loop [43].
Despite its crucial role as a growth factor for Tr1 cells, IL-21 on its own failed to promote Tr1 cell differentiation from naïve T cells, suggesting the existence of additional factors that are required for Tr1 cell differentiation. By undertaking gene expression profiling of mouse IL-27-induced Tr1 cells, we found that T cell activation in the presence of IL-27 not only induces Maf but also the arylhydrocarbon receptor (Ahr), a ligand-activated transcription factor that mediates cellular responses to environmental pollutants. While the involvement of AhR in host responses against the prototypical AhR ligand TCDD (Dioxin) have been well-characterized, recent findings regarding the function of the AhR in the immune system have led immunologists to investigate its role in the differentiation of CD4+ T cells. AhR was found to be expressed in both TH17 and Foxp3+ Treg cells. The activation of AhR signaling by FICZ, a putative endogenous AhR ligand, specifically induced the expression of IL-22, and supported TH17 cell differentiation [44]. Conversely, the activation of AhR signaling by TCDD enhanced the induction of Foxp3+Tregs and suppressed TH17 cells [45]. These studies support the contention that AhR, rather than being a lineage specific transcription factor, may promote CD4+ T cell differentiation based on which ligand activates it.
We have found that during Tr1 cell differentiation, AhR is activated as shown by the transcription of its target gene cyp1a1. IL-27-induced AhR expression enhanced both IL-21 and IL-10 secretion from developing Tr1 cells [46]. In the absence of AhR signaling, the ability of murine naïve CD4+ T cells activated in the presence of IL-27 to produce IL-10 and IL-21 was severely compromised. We have additionally identified AhR binding sites (xenobiotic response element, XRE) within the il10 and il21 promoters and showed that AhR could directly transactivate both promoters in Tr1 cells (Figure 1). The findings that c-Maf and AhR were concomitantly induced by IL-27 and the reported ability of the AhR to interact directly with other proteins including nuclear factors such as NF-kb [47, 48], retinoblastoma protein [49] and estrogen receptor [50] led us test whether AhR can associate with c-Maf. Our results revealed that AhR binds to c-Maf in Tr1 cells resulting in an enhanced transcriptional activity of the il10 and il21 promoters. Since AhR signaling controlled Tr1 cells generation and IL-10 secretion, we hypothesized that the immunoregulatory action of IL-27 would be abrogated in the absence of AhR signaling in vivo. Our findings indeed revealed that CD4+ T cells isolated from MOG-immunized AhRd mice, which present defective AhR signaling in vivo, showed decreased IL-10 production when treated with MOG and IL-27. While wild-type MOG specific CD4+ T cells treated with IL-27 protected mice against EAE, IL-27 treated CD4+ T cells from AhRd mice failed to do so.
4.2 Modulation of AhR signaling in Tr1 cell differentiation
We have shown that mouse Tr1 cell induction by IL-27 can be affected by AhR signaling. Naïve CD4+ T cells differentiated in vitro with IL-27 in the presence of the AhR ligand TCDD had enhanced secretion of both IL-10 and IL-21. Those results were in accordance with the work of Marshall et al., who showed that regulatory T cells generated upon TCDD administration, prevented graft-versus-host disease in a transplantation model [51]. Interestingly, in vivo TCDD-induced regulatory T cells were not only suppressive but also secreted significant amounts of IL-10 in response to polyclonal and alloantigen stimuli. In addition, these TCDD-induced CD4+ T cells expressed Granzyme B but not Foxp3, a phenotype that shares striking similarities with IL-27-induced Tr1 cells (figure 1). TCDD is highly stable in vivo and thus chronically activates AhR [52, 53]. The systemic toxicity resulting from sustained AhR activation has raised concerns regarding chronic engagement of AhR in vivo [54]. 6- formylindolo[3,2-b]carbazole (FICZ), a tryptophan derivative, is a photoproduct that is generated in response to UVB stress and leads to the transcriptional induction of cytochrome P450 through AhR activation. UVB irradiation has indeed been shown to generate FICZ in the cytosol of living cells, supporting the idea that FICZ is an endogenous ligand for AhR [55]. Further studies have shown that FICZ, unlike TCDD, is sensitive to xenobiotic-metabolizing enzymes (XMEs) and therefore only transiently activates AhR. Despite their similarity in binding to AhR, TCDD supports Foxp3+ Treg cell differentiation while FICZ enhances IL-17 and IL-22 secretion from TH17 cells [44, 56] and inhibits TGF-β-induced Treg development [45]. It has been proposed that the promotion of Treg and TH17 differentiation by AhR may result from its ability to interact with different transcriptional partners in different cellular contexts [45]. In this regard, it is noteworthy that the addition of FICZ during Tr1 cell differentiation increased both IL-10 and IL-21 from Tr1 cells in a similar manner to TCDD. While the intracellular signaling pathways accounting for these observations remain elusive, the ability of both endogenous and exogenous AhR ligands to enhance IL-27-induced Tr1 cell generation clearly underscores the major role of AhR in the Tr1 transcriptional program.
Tr1 cells may have a role in regulating autoimmune responses in humans. A number of studies have revealed that during the course of inflammatory diseases such as Multiple Sclerosis, the frequency and the functionality of Tr1 cells are severely impaired [26, 57]. In order to contemplate the use of Tr1 cells for cellular therapy of human diseases, it is thus essential to understand the pathways controlling their generation. Like in mouse, AHR was also shown to be essential for Tr1 cell differentiation in humans. The activation of human CD4+T cells with TCDD or FICZ selectively enhanced their IL-10 secretion [58]. Accordingly, functional analyses further confirmed that AHR transactivated the il10 promoter during human Tr1 cell differentiation, following addition of TCDD. Furthermore, human TCDD-treated T cells exhibited an AHR-dependent suppressive activity, indicating that TCDD-treated T cells harbor not only phenotypic but also functional properties of Tr1 cells. Consistent with results obtained in mouse Tr1 cells, the human AHR was found to interact with the transcription factor c-MAF ultimately resulting in an enhanced IL-10 secretion from Tr1 cells. While these observations are in line with the recently proposed role of AHR in mouse Tr1 cell differentiation, it is noteworthy that the activation of murine CD4+ T cells with TCDD in the absence of any differentiating cytokines was unable to drive Tr1 cell differentiation [46]. Since mouse AhR has at least a 10-fold higher sensitivity for TCDD than human AhR [59], it is unlikely that these phenotypic differences can be attributable to AhR-mediated signaling alone. In this regard, it is notable that the transcription factor c-Maf that is essential for mouse Tr1 cell differentiation is detectable in human T cells activated without IL-27. Since Maf expression is strictly dependent on IL-27 in mice, we would postulate that the observation that TCDD mediated expansion of human Tr1 cells is due to their endogenous expression of c-MAF. Indeed, overexpression of c-MAF alone only induces marginal expression of IL-10 from human CD4+ T cells, while the TCDD-driven activation of AhR combined with c-MAF led to significant IL-10 expression [58].
It has been previously shown that IL-10 was necessary but not sufficient for the differentiation and function of human Tr1 cells, and that their capacity to suppress immune responses also relied on GranzymeB and perforin [15, 60]. Interestingly, besides increasing IL-10 expression, AHR activation with TCDD induced Granzyme B in human Tr1 cells [58]. Granzyme B was indeed required for the suppressive activity of TCDD-treated T cells as illustrated by the ability of a Granzyme B inhibitor to abrogate the suppressive activity TCDD-induced human Tr1 cells. Overall, results obtained from both mouse and human studies support a major role of AhR in dictating Tr1 cell differentiation and effector functions, thereby opening avenues for therapeutic interventions.
5. Therapeutic use of Tr1 cells
Over the last few years, great progress has been made to differentiate and expand murine and human Tr1 cells ex vivo. A subset of IL-10–producing human dendritic cells (DCs), termed DC-10, were recently characterized in vivo and can be induced in vitro with IL-10. DC-10 express CD14+, CD16+, CD11c+, CD11b+ and HLA-DR+ as well as the costimulatory molecules CD40, CD80 and CD86. DC-10 have been shown to support antigen-specific IL-10–producing Tr1 cell differentiation. Importantly, monocyte-derived DC-10 could induce IL-10–producing antigen-specific Tr1 cells from naïve T cells after a single round of stimulation [61]. While these results support the development of cell therapy strategies relying on Tr1 cells (reviewed in [62]), the implementation of cell therapy protocols requires a clinical cell therapy unit with strong expertise in good manufactory practice along with highly skilled and trained personnel.
Thus, while the generation of Tr1 cells using IL-27 could be hindered by its short in vivo half-life, strategies designed to enhance IL-27 induction from DC and/or to target downstream mediators of the IL-27R pathway in Tr1 cells could enhance immunosuppressive Tr1 cell immunosuppressive functions, thereby providing an attractive alternative for the treatment of autoimmune disorders.
5.1 Affecting DC biology to support Tr1 differentiation
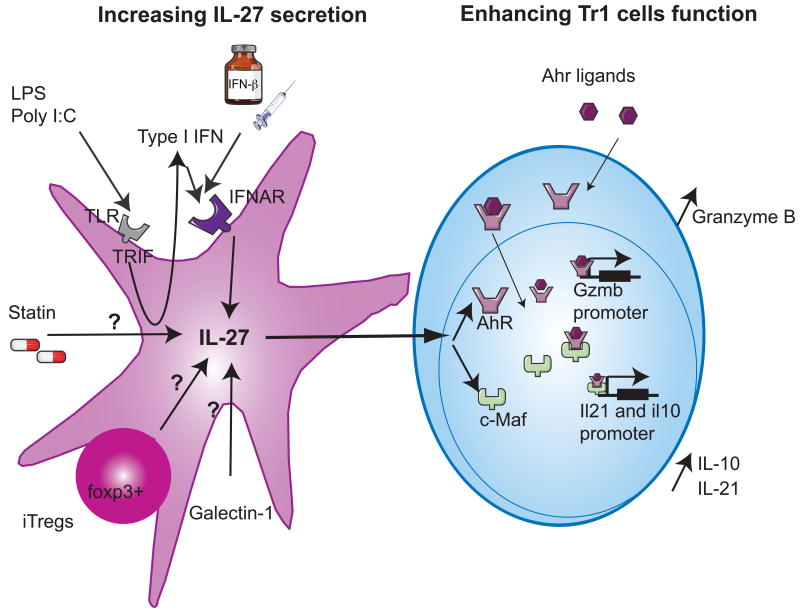
Immunosuppressive drugs have been shown to increase IL-27 production from DCs. Among the therapies implemented for treating multiple sclerosis, statins, beyond their cholesterol-lowering activity, were indeed recently ascribed to have anti-inflammatory properties. Simvastatin was for instance shown to increase IL-27 secretion from human monocytes of MS patients (Figure 3)[63], suggesting that IL-27 might contribute to the anti-inflammatory effects of this treatment. Similarly, IFN-β, a member of the type I interferon family that is approved for the treatment of MS, promotes IL-27 from DC in humans (Figure 3)[64]. The molecular mechanisms by which IFN-β increases IL-27 production have been studied using murine macrophages. It was demonstrated that IFN-β increased IL-27 production through Toll-IL-1 receptor domain– containing adaptor using IFN-β (TRIF) as illustrated by a reduced IL-27 secretion from TRIF-deficient macrophages in response to LPS-induced type I IFN. Additionally, type I interferon receptor IFNAR-/- mice were shown to be highly sensitive to EAE and this was associated with a decreased IL-27 expression in central nervous system (CNS) tissues compared to wild-type mice. Importantly, the severity of the disease in IFNAR-/- mice was alleviated by administration of recombinant IL-27 protein, suggesting that IFN-β– mediated IL-27 secretion protects the CNS from autoimmune injury. Since IFN-β-mediated IL-27 production by innate immune cells was critical for the immunoregulatory role of IFN-β in this mouse model of CNS autoimmune disease [65], it is tempting to speculate that drugs that selectively target the TRIF or type I IFN signaling pathways could increase IL-27 production (Figure 3) from innate immune cells and promote Tr1 cell differentiation.
Figure 3. Pharmacologic tools to support the differentiation of IL-27-induced Tr1 cells.
Different treatments induce IL-27 secretion from dendritic cells (left panel) that will in turn enhance the development of Tr1 cells, resulting in the resolution of autoimmune inflammation. TLR stimulation with Poly I:C or LPS will activate TRIF, leading to endogenous type I IFN secretion. Endogenous type I IFN or exogenous treatment with IFN-β signals through their receptor IFNAR and enhance IL-27 expression. Statins, Galectin-1 as well as Foxp3+ induced Tregs promote IL-27 secretion through yet unknown mechanisms. IL-27 induces c-Maf and AhR expression in Tr1 cells (right panel). AhR ligands bind to the cytoplasmic AhR and both translocate into the nucleus. These newly formed complexes enhance the expression of Granzyme B and bind to c-Maf to transactivate Il10 and Il21 promoters.
DC maturation is an essential step in the induction of T-cell responses as it leads to the upregulation of costimulatory molecules that provide essential second signals for T cell activation and differentiation [66]. Alterations in the glycan structure during DC maturation led to the hypothesis that protein-glycan interactions may affect the DC/T cell crosstalk. Ilarregui et al have reported that galectin 1, an endogenous glycan-binding protein, could support DC ability to promote immunosuppression. DC exposed to galectin-1 secreted IL-27 (Figure 3), induced T cell tolerance and abrogated neuroinflammation in an IL-10-dependent manner. In line with these findings, in DC galectin-1 expression was the highest during the peak and the resolution of EAE. While the molecular mechanisms that control the ability of galectin-1 to enhance IL-27 secretion from DC remain unclear, these findings suggest that the manipulation of this immunoregulatory circuit between galectin-1, IL-27-secreting DC and IL-10-secreting Treg/Tr1 cells could provide therapeutic tools for the treatment of autoimmune disorders [67].
5.2 Modulating the intrinsic signaling of Tr1 cells
We have recently identified the role of the AhR during Tr1 cell differentiation. Different AhR ligands have been shown to promote either T cell inflammation or tolerance [45] as illustrated by the ability of FICZ or TCDD to induce TH17 and Treg differentiation in vitro, respectively. AhR ligands, such as the polycyclic aromatic hydrocarbons, are rapidly metabolized by AhR-inducible enzymes to generate active metabolites that could produce diverse effects on the immune system. There would therefore be a strong rationale for identifying AhR ligands that differentially induce Tr1, Foxp3+Tregs or TH17 cells in order to regulate T-cell differentiation and the quality of immune responses in vivo.
In vivo immunologic effects of AhR activation are tissue- and/or ligand-specific. Indeed, AhR ligands can modulate immune response differently depending on the cytokine milieu. For example, in an inflammatory environment where IL-6 is a predominant cytokine, FICZ promotes and supports TH17 cell differentiation, as in the presence of the anti-inflammatory cytokines IL-27 and TGF-β, it promotes Tr1 cells. In this regard, AhR transcription factor can thus be regarded as a stabilizer of different CD4+ T cell phenotypes depending on the environmental context and the transcription factors it can interact with to promote T cell differentiation.
TCDD is highly toxic and can lead to hepatocellular damage, epithelial changes, cancer, birth defects, thymic involution [68], ruling out its clinical application. There is therefore a need to design non-toxic AhR ligands that could constitute potential new drugs for the therapeutic induction or inhibition of Tr1 cells (Figure 3). In this regard, it is notable that we are exposed to numerous naturally occurring AHR ligands both through endogenous biological processes and diet. Some of these compounds are converted in the gut to high-affinity AHR ligands. Indole-3-carbinol, a metabolite of glucobrassicin found in cruciferous vegetables, is a weak AHR ligand that is converted to its acid condensation product indolo-(3,2-b)-carbazole and 3,3′-diindolylmethane that binds and activates AHR with high affinity. Another AhR ligand provided through diet is resveratrol, a compound found in a large number of plant products including mulberries, peanuts, and red grapes. Resveratrol is already commercialized as a nutritional supplement in the market because of its anti-oxidant properties. Resveratrol treatment decreased the clinical signs and inflammatory responses in experimental allergic encephalomyelitis (EAE)-induced mice [69]. The authors noted an up-regulation of Foxp3 gene in splenocytes of mice treated with resveratrol and decreased production of IL-17 in the sera, compared with control mice. In a separate study, resveratrol fed orally during EAE has been shown to increase IL-10 production by T cells in the brain of EAE mice [70].
AhR ligands are also produced during different endogenous biological processes. The essential amino acid tryptophan (Trp) is metabolized and photo-oxidized into multiple AHR ligands. Kynurenine, the first breakdown product in the IDO-dependent tryptophan degradation pathway, binds and activates AhR to promote the generation of CD25+Foxp3+ Tregs but not TH17 cells [71]. Absence of the AhR in T cells prevented these ligands from inducing Foxp3+ T cells. Furthermore, the antiallergic drug Tranilast, approved for bronchial asthma, is a derivative of the tryptophan metabolite 3-hydroxyanthranilic acid. It has been shown to suppress EAE through the induction of regulatory cells [72]. Furthermore, it has been proposed that the binding and activation of AHR by Tranilast could be responsible for the generation of those Tregs [73].
6. Open questions and concluding remarks
As no lineage specific transcription factor or specific surface marker for Tr1 cells has been identified [74], the identity of Tr1 cells as a unique T cell subset is still debated. IL-10-secreting regulatory T cells could be generated from different TH cells that have undergone chronic stimulation, resulting in the disappearance of effector T-cell cytokines but the maintenance of IL-10 levels. Indeed, “regulatory IL-10-producing TH1 cells” have been described which are similar in their phenotype to IL-27 induced Tr1 cells in that IL-27 also induces IL-10 together with the transcription factor T-bet that induces IFN-γ production (Figure 1). Whereas IL-10 can suppress T cells and DC function, IFN-γ produced by IL-27 induced Tr1 cells is also critical in inhibiting immune responses. In a study by Murugaiyan et al, it was shown that it was not IL-10 but IFN-γ produced by Tr1 cells that suppressed TH17 responses [75].
While there is still ongoing debate regarding the origin of Tr1 cells, it is now established that those cell play a major role in controlling tissue inflammation and autoimmune responses. Modulation of their function and number using IL-27-promoting drugs or different AhR ligands could suppress immune inflammation through the induction of Tr1 cells. The efficacy of aminoflavones (AFP464; Tigris Pharmaceuticals Industry), that have been reported to target the AhR, are being evaluated in a phase II clinical trial in breast cancer patients (ClinicalTrials.gov: NCT01015521), suggesting that AhR ligands devoid of toxicity can be effectively utilized to promote Tr1 differentiation. Further research is now warranted to evaluate the potential of AhR targeting for the treatment of autoimmune diseases.
Acknowledgments
C.P. was supported by the Swiss National Science Foundation (SFGBM/PASMA 118720/1) and the Novartis Foundation. L.A. was supported by the European Molecular Biology Organization.
Abbreviations
- c-Maf
avian musculoaponeurotic fibrosarcoma v-maf
- Tr1 cell
type 1 regulatory T cell
- AhR
Aryl hydrocarbon receptor
- TCDD
2,3,7,8-Tetrachlorodibenzo-p-dioxin
- FICZ
6-formylindolo[3,2-b]carbazole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 3.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 8.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 9.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 10.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 11.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 12.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 14.Battaglia M, Stabilini A, Draghici E, Gregori S, Mocchetti C, Bonifacio E, et al. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes. 2006;55:40–49. [PubMed] [Google Scholar]
- 15.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 16.Cobbold SP, Nolan KF, Graca L, Castejon R, Le Moine A, Frewin M, et al. Regulatory T cells and dendritic cells in transplantation tolerance: molecular markers and mechanisms. Immunol Rev. 2003;196:109–124. doi: 10.1046/j.1600-065x.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 17.Miaw SC, Choi A, Yu E, Kishikawa H, Ho IC. ROG, repressor of GATA, regulates the expression of cytokine genes. Immunity. 2000;12:323–333. doi: 10.1016/s1074-7613(00)80185-5. [DOI] [PubMed] [Google Scholar]
- 18.Ahangarani RR, Janssens W, VanderElst L, Carlier V, VandenDriessche T, Chuah M, et al. In vivo induction of type 1-like regulatory T cells using genetically modified B cells confers long-term IL-10-dependent antigen-specific unresponsiveness. J Immunol. 2009;183:8232–8243. doi: 10.4049/jimmunol.0901777. [DOI] [PubMed] [Google Scholar]
- 19.Bacchetta R, Bigler M, Touraine JL, Parkman R, Tovo PA, Abrams J, et al. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J Exp Med. 1994;179:493–502. doi: 10.1084/jem.179.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagliani N, Jofra T, Stabilini A, Valle A, Atkinson M, Roncarolo MG, et al. Antigen-specific dependence of Tr1-cell therapy in preclinical models of islet transplant. Diabetes. 59:433–439. doi: 10.2337/db09-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huurman VA, Velthuis JH, Hilbrands R, Tree TI, Gillard P, van der Meer-Prins PM, et al. Allograft-specific cytokine profiles associate with clinical outcome after islet cell transplantation. Am J Transplant. 2009;9:382–388. doi: 10.1111/j.1600-6143.2008.02479.x. [DOI] [PubMed] [Google Scholar]
- 22.VanBuskirk AM, Burlingham WJ, Jankowska-Gan E, Chin T, Kusaka S, Geissler F, et al. Human allograft acceptance is associated with immune regulation. J Clin Invest. 2000;106:145–155. doi: 10.1172/JCI9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3-precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 24.Wildbaum G, Netzer N, Karin N. Tr1 cell-dependent active tolerance blunts the pathogenic effects of determinant spreading. J Clin Invest. 2002;110:701–710. doi: 10.1172/JCI15176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meiron M, Zohar Y, Anunu R, Wildbaum G, Karin N. CXCL12 (SDF-1alpha) suppresses ongoing experimental autoimmune encephalomyelitis by selecting antigen-specific regulatory T cells. J Exp Med. 2008;205:2643–2655. doi: 10.1084/jem.20080730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Astier AL, Meiffren G, Freeman S, Hafler DA. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest. 2006;116:3252–3257. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 28.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 29.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 30.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 31.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 32.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naïve CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 33.Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, et al. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 34.Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naïve T cells. J Interferon Cytokine Res. 2003;23:513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- 35.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naïve CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pot C, Apetoh L, Awasthi A, Kuchroo VK. Molecular pathways in the induction of interleukin-27-driven regulatory type 1 cells. J Interferon Cytokine Res. 30:381–388. doi: 10.1089/jir.2010.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Artis D, Johnson LM, Joyce K, Saris C, Villarino A, Hunter CA, et al. Cutting edge: early IL-4 production governs the requirement for IL-27-WSX-1 signaling in the development of protective Th1 cytokine responses following Leishmania major infection. J Immunol. 2004;172:4672–4675. doi: 10.4049/jimmunol.172.8.4672. [DOI] [PubMed] [Google Scholar]
- 38.Rosas LE, Satoskar AA, Roth KM, Keiser TL, Barbi J, Hunter C, et al. Interleukin-27R (WSX-1/T-cell cytokine receptor) gene-deficient mice display enhanced resistance to leishmania donovani infection but develop severe liver immunopathology. Am J Pathol. 2006;168:158–169. doi: 10.2353/ajpath.2006.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;183:2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spolski R, Kim HP, Zhu W, Levy DE, Leonard WJ. IL-21 mediates suppressive effects via its induction of IL-10. J Immunol. 2009;182:2859–2867. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 44.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 45.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 46.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim DW, Gazourian L, Quadri SA, Romieu-Mourez R, Sherr DH, Sonenshein GE. The RelA NF-kappaB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene. 2000;19:5498–5506. doi: 10.1038/sj.onc.1203945. [DOI] [PubMed] [Google Scholar]
- 48.Tian Y, Ke S, Denison MS, Rabson AB, Gallo MA. Ah receptor and NF-kappaB interactions, a potential mechanism for dioxin toxicity. J Biol Chem. 1999;274:510–515. doi: 10.1074/jbc.274.1.510. [DOI] [PubMed] [Google Scholar]
- 49.Puga A, Barnes SJ, Dalton TP, Chang C, Knudsen ES, Maier MA. Aromatic hydrocarbon receptor interaction with the retinoblastoma protein potentiates repression of E2F-dependent transcription and cell cycle arrest. J Biol Chem. 2000;275:2943–2950. doi: 10.1074/jbc.275.4.2943. [DOI] [PubMed] [Google Scholar]
- 50.Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K, et al. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423:545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- 51.Marshall NB, Vorachek WR, Steppan LB, Mourich DV, Kerkvliet NI. Functional characterization and gene expression analysis of CD4+ CD25+ regulatory T cells generated in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Immunol. 2008;181:2382–2391. doi: 10.4049/jimmunol.181.4.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miniero R, De Felip E, Ferri F, di Domenico A. An overview of TCDD half-life in mammals and its correlation to body weight. Chemosphere. 2001;43:839–844. doi: 10.1016/s0045-6535(00)00442-2. [DOI] [PubMed] [Google Scholar]
- 53.Kerger BD, Leung HW, Scott P, Paustenbach DJ, Needham LL, Patterson DG, Jr, et al. Age- and concentration-dependent elimination half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Seveso children. Environ Health Perspect. 2006;114:1596–1602. doi: 10.1289/ehp.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 2009;30:447–454. doi: 10.1016/j.it.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Fritsche E, Schafer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci U S A. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci U S A. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinez-Forero I, Garcia-Munoz R, Martinez-Pasamar S, Inoges S, Lopez-Diaz de Cerio A, Palacios R, et al. IL-10 suppressor activity and ex vivo Tr1 cell function are impaired in multiple sclerosis. Eur J Immunol. 2008;38:576–586. doi: 10.1002/eji.200737271. [DOI] [PubMed] [Google Scholar]
- 58.Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol. 11:846–853. doi: 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramadoss P, Perdew GH. Use of 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin as a probe to determine the relative ligand affinity of human versus mouse aryl hydrocarbon receptor in cultured cells. Mol Pharmacol. 2004;66:129–136. doi: 10.1124/mol.66.1.129. [DOI] [PubMed] [Google Scholar]
- 60.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 61.Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 116:935–944. doi: 10.1182/blood-2009-07-234872. [DOI] [PubMed] [Google Scholar]
- 62.Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. Tr1 cells: from discovery to their clinical application. Semin Immunol. 2006;18:120–127. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X, Jin J, Peng X, Ramgolam VS, Markovic-Plese S. Simvastatin inhibits IL-17 secretion by targeting multiple IL-17-regulatory cytokines and by inhibiting the expression of IL-17 transcription factor RORC in CD4+ lymphocytes. J Immunol. 2008;180:6988–6996. doi: 10.4049/jimmunol.180.10.6988. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Jin J, Tang Y, Speer D, Sujkowska D, Markovic-Plese S. IFN-beta1a inhibits the secretion of Th17-polarizing cytokines in human dendritic cells via TLR7 up-regulation. J Immunol. 2009;182:3928–3936. doi: 10.4049/jimmunol.0802226. [DOI] [PubMed] [Google Scholar]
- 65.Guo B, Chang EY, Cheng G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Invest. 2008;118:1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 67.Ilarregui JM, Croci DO, Bianco GA, Toscano MA, Salatino M, Vermeulen ME, et al. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10:981–991. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- 68.Marshall NB, Kerkvliet NI. Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann N Y Acad Sci. 1183:25–37. doi: 10.1111/j.1749-6632.2009.05125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh NP, Hegde VL, Hofseth LJ, Nagarkatti M, Nagarkatti P. Resveratrol (trans-3,5,4′-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol. 2007;72:1508–1521. doi: 10.1124/mol.107.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petro TM. Regulatory role of resveratrol on Th17 in autoimmune disease. Int Immunopharmacol. doi: 10.1016/j.intimp.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 71.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Platten M, Ho PP, Youssef S, Fontoura P, Garren H, Hur EM, et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310:850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- 73.Kerkvliet NI. AHR-mediated immunomodulation: the role of altered gene transcription. Biochem Pharmacol. 2009;77:746–760. doi: 10.1016/j.bcp.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nature reviews. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 75.Murugaiyan G, Mittal A, Weiner HL. Identification of an IL-27/osteopontin axis in dendritic cells and its modulation by IFN-gamma limits IL-17-mediated autoimmune inflammation. Proc Natl Acad Sci U S A. 107:11495–11500. doi: 10.1073/pnas.1002099107. [DOI] [PMC free article] [PubMed] [Google Scholar]