Abstract
Using in vitrotooth germ cultures and analysis by confocal microscopy, ameloblasts treated with sodium fluoride were found to have elevated amounts of filamentous actin. Because this response is reduced by inhibitors of the Rho/ROCK signaling pathway, we generated mice that express dominant negative RhoA (RhoADN) in ameloblasts for in vivo analysis. Expression of the EGFP-RhoADN fusion protein was evaluated by RT-PCR and immunohistochemistry, and teeth were analyzed by scanning electron microscopy. The 3 strains expressed at either low (TgEGFP-RhoADN-8), intermediate (TgEGFP-RhoADN-2), or high (TgEGFP-RhoADN-13) levels, and the molar teeth from the 3 strains had enamel hypoplasia and surface defects. We conclude that RhoADN expressed in ameloblasts interferes with normal enamel development through the pathway that is induced by sodium fluoride.
Key Words: Enamel, Transgenic mice, RhoA, Ameloblasts
Introduction
During dental development, a single layer of inner enamel epithelial cells undergoes a remarkable change in cell shape in preparation for the secretion of enamel extracellular matrix. These cells develop into tall ameloblasts with cellular extensions called Tomes’ processes, which function during enamel matrix secretion. Following generation of the enamel layer, the ameloblasts shorten and reorganize during the transition stage; they then enter maturation, where they change histologically from ruffle-ended to smooth-ended at the location where Tomes’ processes have retracted. These cells reduce the enamel protein content and increase the mineral content so that the enamel layer can develop into the hardest tissue in the body. Finally, the cells shorten further and adhere to the enamel surface until just before eruption of the tooth into the oral cavity [Smith, 1979]. This remarkable change in both structure and function of ameloblast cells during development is associated with changes in the cytoskeletal framework. Filamentous actin (F-actin) has been localized using phalloidin stain [Chalfie, 1995] at junctional complexes and the Tomes’ process [Nishikawa and Kitamura, 1986; Nishikawa and Josephsen, 1987; Nishikawa et al., 1988.
In many cell types, activation of the RhoA signaling pathway is associated with an increase in F-actin [Ridley and Hall, 1992; Maekawa et al., 1999]. Actin monomers associate to form F-actin filaments often detected in structures that are associated with cellular movement and alterations in shape. Sodium fluoride elevates F-actin through activation of the RhoA signaling pathway in fibroblasts as well as in ameloblasts in cultured murine teeth [Vincent and Settleman, 1999; Li et al., 2005]. When RhoA activity is limited using inhibitors of downstream ROCK (Rho kinase) such as Y-27632, the elevation of F-actin in teeth treated with NaF is reduced, indicating a role of RhoA in regulation of the ameloblast cytoskeleton [Li et al., 2005].
To test the role of RhoA in vivo, transgenic mice were generated that express dominant negative RhoA (RhoADN) in ameloblasts as the N19 dominant negative mutation decreases the activity of endogenous RhoA [Qiu et al., 1995]. Ameloblasts are thought to move in sliding rows in order to generate decussation patterns in enamel through effects of F-actin at junctional complexes [Nishikawa, 1990; Smith and Nanci, 1995]. The hypothesis tested was that mice that express this transgene can reveal the effects of inhibition of this signaling pathway in vivo.
Materials and Methods
Tooth Germ Cultures
First molar teeth were dissected from wild-type (WT) mice, cultured, and treated. Frozen sections were stained with phalloidin and evaluated by confocal microscopy as described [Li et al., 2005], except that the NaF concentration was reduced to 500 μM. Some teeth were preincubated with Y-27632 as previously described or with 20 μM HA1077 (Sigma, Milwaukee, Wisc., USA) for 30 min prior to treatment with NaF or NaCl.
Generation of the Expression Vector and Transgenic Mice
The plasmid used previously to generate transgenic mice that express various amelogenins by ameloblasts [Gibson et al., 2007; Pugach et al., 2010] was altered by removal of the signal sequence for secretion and by insertion of the enhanced green fluorescent protein (EGFP) gene fused to the RhoADN coding sequence from pcDNA3-EGFPR-T19N (Addgene, Cambridge, Mass., USA). The DNA sequence was verified, and the plasmid was digested to release the insert which was injected at the University of Pennsylvania Transgenic Core Facility. The 3 founder mice were mated separately to WT mice to generate 3 strains. All work was approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Molecular Analysis of Mice
Each mouse was analyzed by PCR of tail DNA using G929 [Chen et al., 2003] and G1108 5′TGAACAGCTCCTCGCCCTTGCTC. Molar teeth were dissected from postnatal day 3 (PN3) mice, and RNA was isolated. First-strand cDNA was synthesized using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, Calif., USA) for RT-PCR with 3′ primers specific for EGFP (G1109 5′AGTCGTGCTGCTTCATGTGGT) or RhoA (G1112 5′ATCACCAACAATCACCAGTTTC). β-Actin primers were as described [Yuan et al., 1996].
Immunohistochemistry
Mandibles were fixed and embedded in paraffin. Sections stained for the reporter protein using anti-GFP (Abcam, Inc., Cambridge, Mass., USA) and a Vectastain ABC kit (Vector Laboratories, Burlingame, Calif., USA) indicated the localization of transgenic protein in molars. WT sections and transgenic sections lacking primary antibody served as controls.
Scanning Electron Microscopy
First molars from 8-week-old WT and transgenic mice were sectioned mesiodistally and etched for 30 s with 10% HCl to reveal decussation patterns using scanning electron microscopy (SEM). To examine molar surfaces, mandibles from 8-week-old mice were sputter coated and analyzed by SEM at 20 kV (JEOL JSM T330A; JEOL, Peabody, Mass., USA).
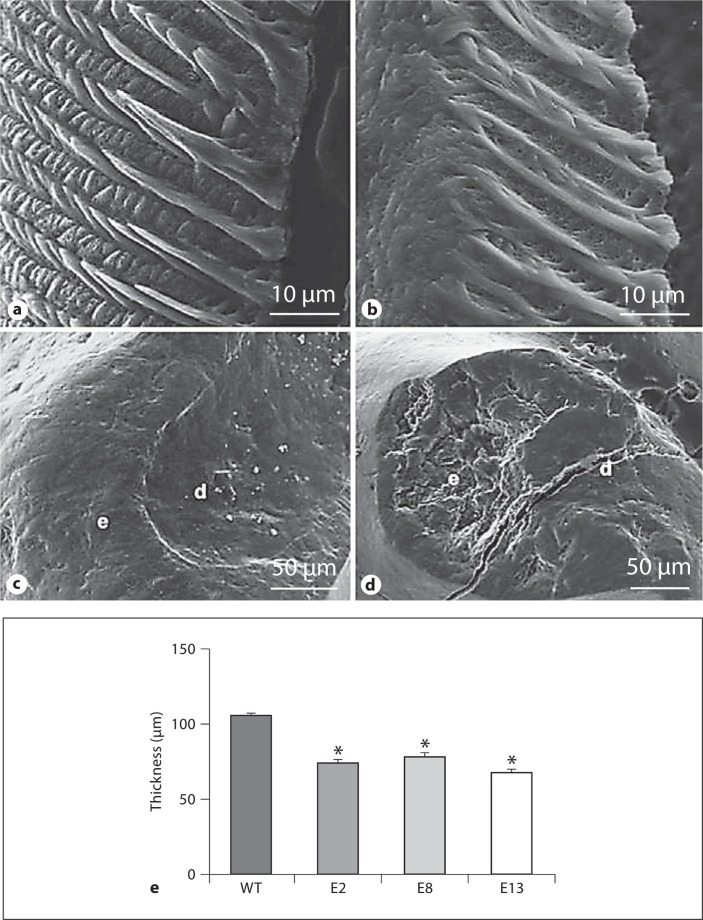
WT and transgenic first molar enamel thicknesses were measured in mesiodistal longitudinal sections. Sections were etched for 30 s with 10% HCl and imaged by SEM (n = 3). The enamel thickness of the distal face of the central cusp was measured at 10 locations via analysis by ImageJ [Rasband, 1997–2009]. The asterisk indicates a significant difference compared to the WT (p < 0.05) using ANOVA.
Results
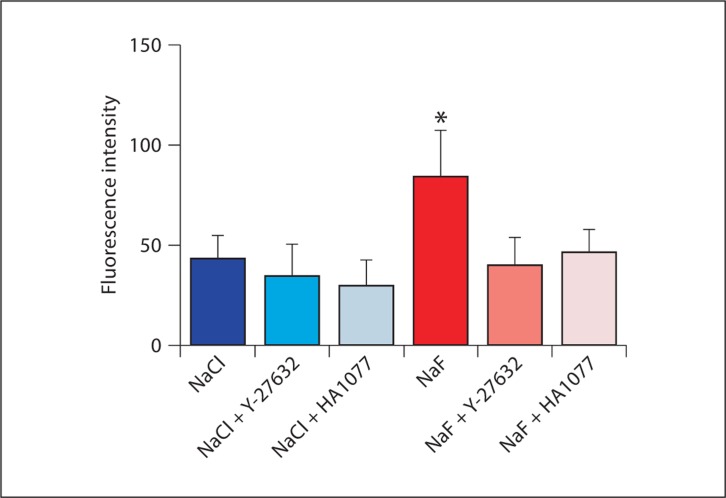
WT molar teeth from PN1.5–2 mice were dissected and incubated in the presence of NaF or NaCl as had been done previously [Li et al., 2005] but with 1/8 of the concentration of halide. Pretreatment with the previously tested ROCK inhibitor Y-27632 or with the slightly more specific ROCK inhibitor HA1077 [Davies et al., 2000] indicated that inhibition of ROCK abolished F-actin elevation by fluoride using either reagent as measured by confocal microscopy (fig. 1).
Fig. 1.
Treatment of WT PN1.5–2 tooth germs with 500 μM NaF or NaCl in culture plus ROCK inhibitor pretreatment. Molar teeth were treated for 30 min with NaF or NaCl with or without pretreatment with ROCK inhibitors Y-27632 or HA1077. Teeth were sectioned and stained with phalloidin; ameloblasts were analyzed by confocal microscopy for fluorescence intensity. ∗ Statistically significant difference compared to any of the other treatments using ANOVA, with p < 0.001.
In order to test the in vivo importance of this pathway, a plasmid was generated for the expression of a fused EGFP-RhoADN protein under the control of amelogenin gene regulatory sequences. Three strains of transgenic mice were generated, i.e. TgAmelxEGFP-RhoADN- 2, TgAmelxEGFP-RhoADN-8, and TgAmelxEGFP-RhoADN-13, and each transgenic mouse was genotyped by PCR of tail DNA.
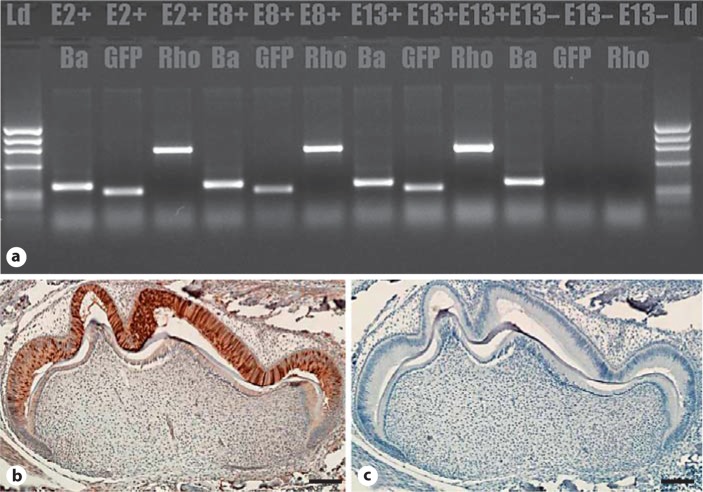
RNA analysis by RT-PCR indicated molar expression of EGFP and RhoADN by the transgenic mice (fig. 2a), which is in agreement with the results of Western blot using anti-GFP or anti-RhoA antibodies (not shown). In addition, Western blot analysis showed that TgEGFP-RhoADN-13 had the highest level of expression, strain TgEGFP-RhoADN-8 had the lowest, strain TgEGFP-RhoADN-2 was intermediate, and in strain TgEGFP-RhoADN-13 transgenic and endogenous RhoA levels were similar.
Fig. 2.
Transgene expression in molars of transgenic mice. a RT-PCR using primers to detect β-actin control, GFP reporter, and RhoADN in the 3 strains and nontransgenic mice. E2+, E8+ and E13+ are transgene and β-actin positive; E13– is transgene negative but β-actin positive. Immunohistochemistry of the PN3 TgEGFP-RhoADN-13 first molar using antibody to GFP (b) and control lacking primary antibody (c). Ba = β-Actin; E2+ = TgEGFP-RhoADN-2; E8+ = TgEGFP- RhoADN-8; E13+ = TgEGFP-RhoADN-13; E13– = transgene negative or WT. Scale bars = 100 μm.
Phalloidin staining and histological analysis of ameloblasts from PN2–3 mice did not reveal an obvious morphological anomaly in transgenic teeth (not shown). Expression of the transgene was apparent at PN2 (not shown) and elevated at PN3 (fig. 2b, c). Controls lacking primary antibody or WT sections were negative for stain. We hypothesized that there could be an effect of RhoADN in molars from slightly older mice, but an initial analysis of the decussation patterns of sectioned and etched molar teeth did not indicate an obvious anomaly in strain TgEGFP-RhoADN-13 (high expressor; fig. 3a, b) or either of the other strains (data not shown). SEM analysis of the transgenic molars revealed similar enamel pitting defects in all 3 strains at ages 2, 4 and 8 weeks, and a typical view is shown for strain TgEGFP-RhoADN-8 (low expressor; fig. 3d) compared to the WT (fig. 3c). In addition, the molar enamel layer was significantly thinner in all 3 transgenic strains (fig. 3e).
Fig. 3.
SEM of transgenic and WT teeth. a, b WT and TgEGFP-RhoADN-13 (high expressor) molar decussation patterns from the mesial side of the 1st molar. c, d Surface of WT and TgEGFP-RhoADN-8 first molars, indicating pitting defects in the transgenic molar cusp. e = Enamel; d = dentin. e Molar enamel thickness measurements for WT and transgenic mice. E2, E8 and E13 are as described in figure 2. ∗ Significantly difference from the WT (p < 0.05).
Discussion
Previously we showed that the Y-27632 inhibitor of the RhoA/ROCK pathway interferes with the ability of 4 mM NaF to double F-actin in ameloblasts using tooth organ cultures. Because reported effects of NaF include ameloblast-specific alterations [Smith et al., 1993; Robinson et al., 2004], these experiments were repeated with an additional ROCK inhibitor and lower levels of NaF and NaCl, and the results were similar to those at the higher concentration of NaF. We therefore hypothesized that Rho/ROCK may also be involved in the ameloblast shape changes that occur normally during the ameloblast developmental stages.
Three lines of transgenic mice are described that express a transgenic EGFP-RhoADN fusion protein in ameloblasts during the secretory stage of enamel development. The transgenic protein was detected in ameloblasts at PN2, a time when phalloidin staining of ameloblasts for the F-actin component of the cytoskeleton revealed a normal staining pattern. Amelogenin proteins are expressed normally until at least PN4 in these transgenic mice (not shown).
Transgene expression is elevated at PN3 and PN4, and using SEM of 2-, 4-, and 8-week-old molars, a pitting defect was observed in the molar cusp enamel at all ages analyzed and in all transgenic strains regardless of the level of transgene expression. The 2-week-old mice still had a layer of mucosa covering the molars, so the defect is considered to be developmental rather than due to attrition because of mastication. Although in an initial analysis decussation patterns appeared to be relatively normal in defect-free areas, enamel hypoplasia was observed in molars of the 3 strains.
RhoA and ROCK mRNA levels were recently reported to be elevated in WT rat first molars at PN5 [Biz et al., 2010], and we have observed a similar elevation in murine molars (not shown). Because the Rho dissociation inhibitor RhoGDI, which sequesters Rho to control its status, decreases shortly after birth [Hatakeyama et al., 2009], this pathway fluctuates exactly as the ameloblast cells undergo changes in structural/functional relationships.
In conclusion, disruption of the RhoA pathway leads to hypoplastic enamel with surface defects; this is consistent with an important role for RhoA during the secretory stage. Because the Rho/ROCK pathway is elevated in the presence of sodium fluoride, RhoA signaling may be important both developmentally and in conditions in which the fluoride ion interferes with its normal activity.
Acknowledgements
This work was supported by the National Institutes of Health, by NIDCR grants R21-DE0176110, R01-DE011089 (C.W.G.), and F32-DE019968 (M.K.P.), and by the Cheung Family Scholarship (L.P.).
Glossary
Abbreviations used in this paper
| EGFP | enhanced green fluorescent protein |
| F-actin | filamentous actin |
| PN | postnatal day |
| RhoADN | dominant negative RhoA |
| ROCK | Rho kinase |
| RT-PCR | reverse transcriptase-polymerase chain reaction |
| SEM | scanning electron microscopy |
| WT | wild type |
References
- Biz M.T., Marques R., Crema V.O., Moriscot A.S., dos Santos M.F. GTPases RhoA and Rac1 are important for amelogenin and DSPP expression during differentiation of ameloblasts and odontoblasts. Cell Tissue Res. 2010;340:459–470. doi: 10.1007/s00441-010-0961-0. [DOI] [PubMed] [Google Scholar]
- Chalfie M. Green fluorescent protein. Photochem Photobiol. 1995;62:651–658. doi: 10.1111/j.1751-1097.1995.tb08712.x. [DOI] [PubMed] [Google Scholar]
- Chen E., Yuan Z.A., Wright J.T., Hong S.P., Li Y., Collier P.M., Hall B., D’Angelo M., Decker S., Piddington R., Abrams W.R., Kulkarni A.B., Gibson C.W. The small bovine amelogenin LRAP fails to rescue the amelogenin null phenotype. Calcif Tissue Int. 2003;73:487–495. doi: 10.1007/s00223-002-0036-7. [DOI] [PubMed] [Google Scholar]
- Davies S.P., Reddy H., Caivano M., Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson C.W., Yuan Z.A., Li Y., Daly B., Suggs C., Aragon M.A., Alawi F., Kulkarni A.B., Wright J.T. Transgenic mice that express normal and mutated amelogenins. J Dent Res. 2007;86:331–335. doi: 10.1177/154405910708600406. [DOI] [PubMed] [Google Scholar]
- Hatakeyama J., Fukumoto S., Nakamura T., Haruyama N., Suzuki S., Hatakeyama Y., Shum L., Gibson C.W., Yamada Y., Kulkarni A.B. Synergistic roles of amelogenin and ameloblastin. J Dent Res. 2009;88:318–322. doi: 10.1177/0022034509334749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Decker S., Yuan Z.A., DenBesten P.K., Aragon M.A., Jordan-Sciutto K., Abrams W.R., Huh J, McDonald C., Chen E., MacDougall M., Gibson C.W. Effects of sodium fluoride on the actin cytoskeleton of murine ameloblasts. Arch Oral Biol. 2005;50:681–688. doi: 10.1016/j.archoralbio.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Maekawa M., Ishizaki T., Boku S., Watanabe N., Fujita A., Iwamatsu A., et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- Nishikawa S., Fujiwara K., Kitamura H. Formation of the tooth enamel rod pattern and the cytoskeletal organization in secretory ameloblasts of the rat incisor. Eur J Cell Biol. 1988;47:222–232. [PubMed] [Google Scholar]
- Nishikawa S., Josephsen K. Cyclic localization of actin and its relationship to junctional complexes in maturation ameloblasts of the rat incisor. Anat Rec. 1987;219:21–31. doi: 10.1002/ar.1092190106. [DOI] [PubMed] [Google Scholar]
- Nishikawa S., Kitamura H. Localization of actin during differentiation of the ameloblast, its related epithelial cells and odontoblasts in the rat incisor using NBD-phallacidin. Differentiation. 1986;30:237–243. doi: 10.1111/j.1432-0436.1986.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Nishikawa S., Tsukita S., Tsukita S., Sasa S. Localization of adherens junction proteins along the possible sliding interface between secretory ameloblasts of the rat incisor. Cell Struct Funct. 1990;15:245–249. doi: 10.1247/csf.15.245. [DOI] [PubMed] [Google Scholar]
- Pugach M.K., Li Y., Suggs C., Wright J.T., Aragon M.A., Yuan Z.A., Simmons D., Kulkarni A.B., Gibson C.W. The amelogenin C-terminus is required for enamel development. J Dent Res. 2010;89:165–169. doi: 10.1177/0022034509358392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu R.G., Chen J., McCormick F., Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci USA. 1995;92:11781–11785. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband, W.S. (1997–2009) ImageJ, US National Institutes of Health. http://rsb.info.nih.gov/ij/
- Ridley A.J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Robinson C., Connell S., Kirkham J., Brookes S.J., Shore R.C., Smith A.M. The effect of fluoride on the developing tooth. Caries Res. 2004;38:268–276. doi: 10.1159/000077766. [DOI] [PubMed] [Google Scholar]
- Smith C.E. Ameloblasts: secretory and resorptive functions. J Dent Res. 1979;58(Spec Issue B):695–707. doi: 10.1177/002203457905800221011. [DOI] [PubMed] [Google Scholar]
- Smith C.E., Nanci A. Overview of morphological changes in enamel organ cells associated with major events in amelogenesis. Int J Dev Biol. 1995;39:153–161. [PubMed] [Google Scholar]
- Smith C.E., Nanci A., DenBesten P. Effects of chronic fluoride exposure on morphometric parameters defining the stages of amelogenesis and ameloblast modulation in rat incisors. Anat Rec. 1993;237:243–258. doi: 10.1002/ar.1092370212. [DOI] [PubMed] [Google Scholar]
- Vincent S., Settleman J. Inhibition of RhoGAP activity is sufficient for the induction of Rho-mediated actin reorganization. Eur J Cell Biol. 1999;78:539–548. doi: 10.1016/S0171-9335(99)80019-3. [DOI] [PubMed] [Google Scholar]
- Yuan Z.A., Collier P.M., Rosenbloom J., Gibson C.W. Analysis of amelogenin mRNA during bovine tooth development. Arch Oral Biol. 1996;41:205–213. doi: 10.1016/0003-9969(95)00119-0. [DOI] [PubMed] [Google Scholar]