Abstract
Excessive systemic exposure to fluoride (F) can lead to disturbances in bone homeostasis and dental enamel development. We have previously shown strain-specific responses to F in the development of dental fluorosis (DF) and in bone formation/mineralization. The current study was undertaken to further investigate F responsive variations in bone metabolism and to determine possible relationships with DF susceptibility. Seven-week-old male mice from FVB/NJ, C57BL/6J, C3H/HeJ, A/J, 129S1/SvImJ, AKR/J, DBA/2J, and BALB/cByJ inbred strains were exposed to NaF (0 or 50 ppm as F–) in drinking water for 60 days. Sera were collected for F, Ca, Mg, PO4, iPTH, sRANKL, and ALP levels. Bone marrow cells were subjected to ex vivo cell culture for osteoclast potential and CFU colony assays (CFU-fibroblast, CFU-osteoblast, CFU-erythrocyte/granulocyte/macrophage/megakaryocyte, CFU-granulocyte/macrophage, CFU-macrophage, and CFU-granulocyte). Femurs and vertebrae were subjected to micro-CT analyses, biomechanical testing, and F, Mg, and Ca content assays. DF was evaluated using quantitative fluorescence and clinical criteria. Strain-specific responses to F were observed for DF, serum studies, ex vivo cell culture studies, and bone quality. Among the strains, there were no patterns or significant correlations between DF severity and the actions of F on bone homeostasis (serum studies, ex vivo assays, or bone quality parameters). The genetic background continues to play a role in the actions of F on tooth enamel development and bone homeostasis. F exposure led to variable phenotypic responses between strains involving dental enamel development and bone metabolism.
Key Words: Fluoride, Fluorosis, Genetics, Bone, Teeth
Introduction
Excessive systemic exposure to fluoride (F) can lead to disturbances in bone homeostasis and amelogenesis [Everett, 2010]. The latter results in dental fluorosis (DF) [Bronckers et al., 2009]. An individual's genetic background is a factor in DF susceptibility [Everett et al., 2002; Vieira et al., 2005a; Carvalho et al., 2009]. The genetic background also plays a role in the actions of F on bone and bone cells [Mousny et al., 2006; Yan et al., 2007; Chou et al., 2009]. The relationship between DF susceptibility and the effects on bone and bone cells is not well understood. The goal of this study was to investigate the range of responses to F across several inbred strains of mice and to determine any relationship between DF susceptibility and the actions of F on bone homeostasis.
Animals and Methods
Animals
Male mice from FVB/NJ (FVB), C57BL/6J (B6), C3H/HeJ (C3H), A/J, 129S1/SvImJ (129S1), AKR/J (AKR), DBA/2J (DBA), and BALB/cByJ (BALB) inbred strains were obtained from The Jackson Laboratory (Bar Harbor, Me., USA) at 6 weeks of age and were acclimated for 1 week prior to treatment with sodium F (NaF). NaF was provided in drinking water at concentrations of 0 or 50 ppm F– for 60 days. Each treatment/control group consisted of 6 mice. All animals were housed in the Division of Lab Animal Medicine facility within the Dental Research Center at the University of North Carolina at Chapel Hill (a fully AAALAC-accredited unit). Mice were fed a constant sustenance of LabDiet® 5001 (PMI® Nutrition International) containing 0.95% calcium (Ca), 0.66% phosphate (PO4), 4.5 IU/g vitamin D3, and an average [F] of 6.56 ± 0.28 μg/g, and they were allowed food and water ad libitum. Animal studies were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
DF Phenotyping
The lower incisors were assessed for DF severity. Clinical criteria (modified TF scale) and quantitative fluorescence (QF) were used to measure the severity of DF in mice [Everett et al., 2002; Carvalho et al., 2009; Everett, 2010>].
Serum and Bone Chemistry Assays
Sera were subjected to total alkaline phosphatase (ALP), Ca, PO4, magnesium (Mg), and F determinations as previously described [Yan et al., 2007]. The proximal ends of the mouse left femurs were used for elemental analysis. Instrumental neutron activation analysis (INAA) was used to quantify F, Mg, and Ca in bone [Mousny et al., 2006].
Serum Enzyme Immunoassay
Sera were subjected to enzyme immunoassays (EIAs) for the bone metabolism markers intact parathyroid hormone (iPTH) (mouse) EIA (ALPCO Diagnostics, Salem, N.H., USA) and soluble receptor activator of nuclear factor (NF)-κB ligand (sRANKL) mouse/rat EIA (ALPCO Diagnostics) as previously described [Yan et al., 2007].
Bone Marrow Cell Cultures
Osteoclast potential (OC), hematopoietic colony-forming cells (CFC) [colony-forming unit-granulocyte/erythrocyte/macrophage/megakaryocyte (CFU-GEMM), CFU-macrophage (CFU-GM), CFU-monocyte/macrophage (CFU-M), and CFU-granulocyte (CFU-G)], CFU-F, and CFU-OB assays were performed as previously described [Yan et al., 2007; Chou et al., 2009].
μCT Analysis
Static histomorphometry was performed on L4/L5 vertebrae and femurs using a Skyscan 1074HR micro-CT (Skyscan, Aartselaar, Belgium) at a resolution of 20.5 μm/pixel. Standardized scanning and image reconstruction settings were used. Hydroxyapatite phantoms (250 and 750 mg/cm3) (CIRS, Inc., Norfolk, Va., USA) were used in order to determine bone mineral densities (BMD) within regions and volumes of interest. The microarchitecture was as follows: bone volume (BV)/tissue volume (TV) (%), bone surface (BS)/BV (mm–1), trabecular thickness (mm), trabecular separation (mm), trabecular number (mm–1), medial lateral (M/L) diameter (mm), anterior posterior (A/P) diameter (mm), and cortical thickness (mm) were calculated using 3-D-based volume models or 2-D cross-sectional images through μCT analysis including the trabecular region of L4/L5 vertebrae and a 1-mm cortical region within the mid-diaphysis of the femurs as previously described [Yan et al., 2007].
Mechanical Testing
Three-point bending (mid-shaft left femur) and vertebral compression (5th lumbar vertebrae) tests were performed as previously described [Mousny et al., 2006]. The structural, material, and geometric parameters were determined from the acquired load-displacement data, the load-deformation curve, cross section data, and stress-strain data.
Statistic Analysis
SPSS version 15.0 (SPSS Inc., Chicago, Ill., USA) was used to perform statistical analyses on all parameters. Statistical analyses were performed using 2-way ANOVA to compare results between the 8 genetic strains and the 2 treatment groups, and the Bonferroni test was used post hoc. p ≤ 0.05 was considered statistically significant.
Results
DF Evaluation
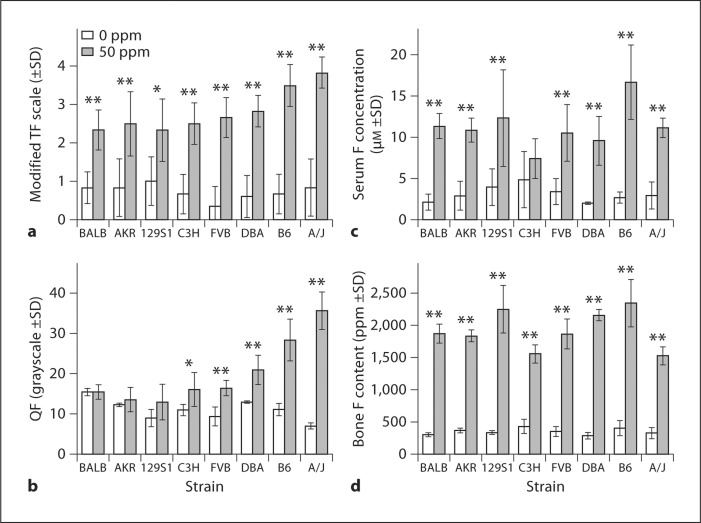
All strains developed DF following F treatment. The severity of DF showed strain-specific responses (fig. 1a, b). While baseline clinical scores did not differ between strains (p = 0.55), baseline QF did (p < 0.001). BALB had the highest baseline QF among strains. Compared to baseline (0 ppm F), the greatest increases in QF values or the severity of DF were observed in A/J (417%, p < 0.001) and B6 (157%, p < 0.001); modest increases were observed in FVB (76%, p < 0.001), DBA (62%, p = 0.001), and C3H (47%, p = 0.017), and small increases were found in 129S1 (45%, p = 0.071), AKR (10%, p = 0.334), and BALB (<1%, p = 0.893). The mean value differences with p > 0.05 are not statistically significant. Among the 8 strains investigated, A/J and B6 are the most susceptible to developing DF. 129S1, AKR, and BALB are the most resistant to DF.
Fig. 1.
a, b The severity of DF was categorized using clinical criteria and QF. a Lower incisors assessed using the modified TF scale for DF. b QF analysis of DF in lower incisors. c Serum [F] (μM) between inbred strains and F treatment. d Bone [F] content (ppm) between inbred strains and F treatment. n = 4–6; ∗ p ≤ 0.05 and ∗∗ p ≤ 0.001 comparing treated and untreated groups within each strain.
Serum Chemistries and Bone Elemental Analyses
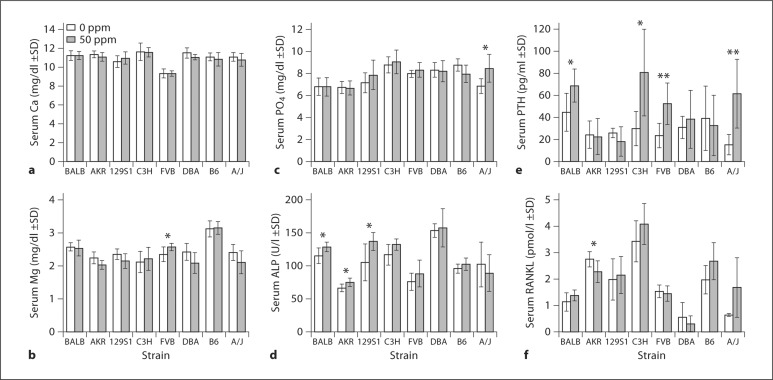
At baseline, serum F was not significantly different between all strains (p = 0.403), whereas bone F did significantly differ (p = 0.03). Serum and bone [F] increased in all strains following F exposure although differences between treated and control groups within and among strains differed greatly (fig. 1c, d). For serum F in C3H there was no significant difference between 0 and 50 ppm F (p = 0.194). The largest differences were observed in B6 (increase of 518%, p < 0.001 over baseline). At 50 ppm F, serum [F] was not significantly different (p = 1.000) between BALB (DF-resistant) and A/J (DF-susceptible) strains. All strains showed significant increases in bone [F] over baseline following F exposure. B6 demonstrated the largest increase (481%, p < 0.001) in bone F between 0- and 50-ppm treatments, whereas the increase in C3H (261%, p < 0.001) was the least among the strains investigated. At 50 ppm F, bone [F] was not significantly different (p = 0.599) between BALB (DF-resistant) and A/J (DF-susceptible) strains. There were no significant differences in serum [F] or bone [F] between both strains at baseline (0 ppm). F treatment did not alter bone Mg or Ca levels for any strain (data not shown). At baseline serum Ca, Mg, PO4, and ALP significantly differed between strains (p < 0.001). No significant differences were observed in serum Ca between the 0- and 50-ppm treatments for each strain. Serum PO4 was modestly elevated over baseline only in A/J mice (24%, p = 0.018). Serum Mg was mildly elevated over baseline only in the FVB strain (10%, p = 0.043). Serum total ALP showed significant increases over baseline following F treatment in 3 strains: 129S1 (31%, p = 0.029), AKR (13%, p = 0.032), and BALB (11%, p = 0.041).
Serum ELISA/EIA
Significant differences in baseline iPTH (p = 0.05) and sRANKL (p < 0.001) levels were observed between strains. Even though iPTH showed a wide range in mean values (15.1–44.6 pg/ml) it also demonstrated the greatest variance with SD ranging from 4.2 to 29.1 pg/ml. Following F treatment iPTH significantly increased in BALB (54.43%, p = 0.027), FVB (123.66%, p = 0.008), C3H (171.47%, p = 0.014), and A/J (308.55%, p = 0.006) (fig. 2e), whereas sRANKL decreased only in 1 strain, AKR (–17.39%, p = 0.04) (fig. 2f).
Fig. 2.
Serum Ca, Mg, PO4, ALP, iPTH, and sRANKL between strains and F treatment groups. n = 6; ∗ p ≤ 0.05 and ∗∗ p ≤ 0.001 comparing treated and untreated groups within each strain.
Ex vivo Cell Cultures
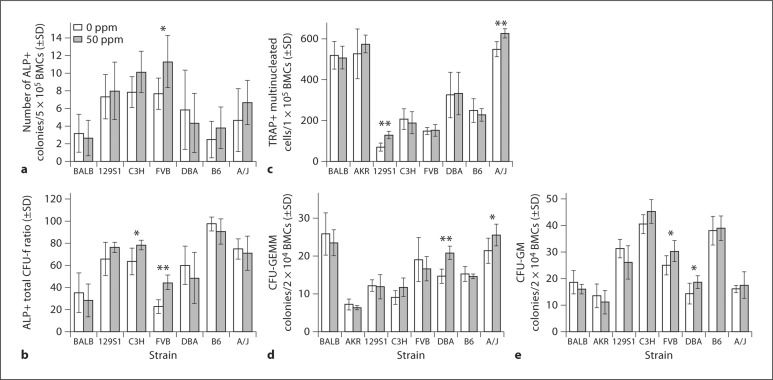
Ex vivo cell culture studies included CFU-F assays, ex vivo OC, and CFC assays. A wide range of interstrain variation for OC, CFC (CFU-GEMM and CFU-GM), and CFU-F(OB) assays existed at baseline (p < 0.001). Among the strains the greatest differences in baseline OC were between 129S1 [70/1 × 105 bone marrow cells (BMC)] and A/J (541/1 × 105 BMC). Strain-specific responses to F were observed in all ex vivo cell culture studies (fig. 3). Following F exposure, total CFU-F (ALP-positive) colonies increased over baseline only in the FVB strain (43%, p = 0.026), while the ratio of ALP-positive colonies to all CFU-F colonies increased in FVB (22%, p < 0.001) and C3H (15%, p = 0.018). OC [tartrate-resistant acid phosphatase (TRAP)-positive cells with ≥3 nuclei] increased in A/J (14%, p = 0.001) and 129S1 (84%, p < 0.001) (fig. 3c). CFU-GEMM colonies increased in A/J (20%, p = 0.039) and DBA (42%, p < 0.001) (fig. 3d). CFU-GM colonies showed an increase in FVB (21%, p = 0.034) and DBA (30%, p = 0.044) (fig. 3e).
Fig. 3.
Ex vivo BMC cultures. BMCs were cultured in osteogenic media to count the number of ALP-positive colonies/5 × 105 BMCs (a) and the ratio of ALP-positive to total CFU-F colonies (b). OC, BMCs treated with mCSF and sRANKL and TRAP-positive cells with ≥3 nuclei counted as osteoclasts/1 × 105 cells (c). CFU-GEMM and CFU-GM colonies/2 × 104 BMCs (d, e). n = 6–8; ∗ p ≤ 0.05 and ∗∗ p ≤ 0.001 between treated and untreated groups in each strain.
Bone μCT Analyses
F treatment had no significant effect on trabecular bone (vertebrae) microarchitecture (BV/TV, BS/BV, trabecular thickness, trabecular separation, or trabecular number) or BMD among the strains investigated. For cortical bone the M/L and A/P diameters were increased following F treatment in only 1 strain, i.e. 129S1, (M/L diameter: 0 ppm 1.31 ± 0.09 mm, 50 ppm 1.77 ± 0.10 mm, p = 0.001; A/P diameter: 0 ppm 0.95 ± 0.06 mm, 50 ppm 1.24 ± 0.06 mm, p = 0.002). This increase in mid-shaft dimension was not accompanied by changes in cortical thickness or cortical BMD.
Bone Mechanical Testing (Structural and Material Properties)
Cortical Bone
Three-point bending tests were performed on the left femur of each mouse to assess cortical bone quality. The structural properties of 4 out of 8 strains (B6, C3H, DBA, and AKR) were affected by the 50-ppm F treatment compared to 0-ppm controls. DBA [ultimate load (Fu): 0 ppm 21.6 ± 1.4 N, 50 ppm 16.8 ± 1.6 N, p = 0.007; bending stiffness (S): 0 ppm 184 ± 24 N/mm, 50 ppm 150 ± 6 N/mm, p = 0.026] and AKR (Fu: 0 ppm 30.8 ± 3.6 N, 50 ppm 22.8 ± 4.8 N, p < 0.001; S: 0 ppm 230 ± 14 N/mm, 50 ppm 179 ± 49 N/mm, p < 0.001) showed significant decreases in Fu and S at 50 ppm compared to the 0-ppm treatment group. B6 [energy to failure (Uf): 0 ppm 8.4 ± 5.5 mJ, 50 ppm 5.0 ± 1.8 mJ, p = 0.023)] and C3H (Uf: 0 ppm 14.46 ± 1.7 mJ, 50 ppm 11.40 ± 4.9 mJ, p = 0.042) both showed a decrease in Uf at 50 ppm relative to the 0-ppm controls. The other 4 strains showed no significant difference in bone structural properties between the 0- and 50-ppm F treatments. The material properties of 5 out of 8 strains (129S1, B6, C3H, DBA, and AKR) were differentially affected by the 50-ppm F treatment compared to 0-ppm controls. Treatment with 50 ppm F resulted in decreased ultimate stress (US) and elastic modulus (E) in 129S1 (US: 0 ppm 242.42 ± 19.08 MPa, 50 ppm 187.15 ± 15.69 MPa, p < 0.05; E: 0 ppm 0.10 ± 0.01 GPa, 50 ppm 0.07 ± 0.01 GPa, p < 0.05), decreased US in AKR (0 ppm 235.82 ± 22.22 MPa, 50 ppm 168.80 ± 40.66 MPa, p < 0.05), decreased yield stress in DBA (0 ppm 242.72 ± 20.69 MPa, 50 ppm 197.00 ± 63.80 MPa, p < 0.05), increased yield strain in B6 (0 ppm 1.71 ± 0.04%, 50 ppm 2.09 ± 0.28%, p < 0.05), and decreased toughness in C3H (0 ppm 23.36 ± 7.07 mJ/mm3, 50 ppm 16.25 ± 5.53 mJ/mm3, p < 0.05).
Vertebral Trabecular Bone
Compression testing was used to evaluate the lattice network of vertebral trabecular bone. The structural properties of the vertebrae in the BALB, 129S1, FVB, B6, and A/J strains changed variably due to F exposure. Following F exposure, FVB demonstrated significant decreases in Fu (0 ppm 32.30 ± 2.21 N, 50 ppm 20.75 ± 5.2 N, p < 0.05) and S (0 ppm 62.23 ± 7.70 N, 50 ppm 45.92 ± 12.01 N, p < 0.05). B6 showed a significant decrease in Fu (0 ppm 23.62 ± 5.40 N, 50 ppm 15.84 ± 5.12 N, p < 0.05) and displacement at failure (0 ppm 0.72 ± 0.12 mm, 50 ppm 0.54 ± 0.10 mm, p < 0.05). A/J significantly decreased in S (0 ppm 50.37 ± 19.91 N/mm, 50 ppm 29.09 ± 5.57 N/mm, p < 0.05) and there was a trend towards a decrease in Fu (0 ppm 19.12 ± 5.38 N, 50 ppm 13.5 ± 2.25 N, 0.05 < p < 0.1). BALB showed a significant decrease in Fu (0 ppm 25.17 ± 5.41 N, 50 ppm 18.34 ± 4.21 N, p < 0.05), and 129S1 experienced a significant decrease in Fu (0 ppm 39.64 ± 4.24 N, 50 ppm 30.47 ± 5.20 N, p < 0.05). The material properties of the vertebrae changed significantly yet variably in 129S1, FVB, B6, and A/J. The US decreased in 129S1 (0 ppm 17.30 ± 1.73 MPa, 50 ppm 13.20 ± 2.22 MPa, p < 0.05), B6 (0 ppm 10.94 ± 2.61 MPa, 50 ppm 6.29 ± 2.97 MPa, p < 0.05), and A/J (0 ppm 10.53 ± 3.45 MPa, 50 ppm 7.46 ± 0.78 MPa, p < 0.05). E (0 ppm 102.50 ± 14.17 MPa, 50 ppm 70.45 ± 20.45 MPa, p < 0.05) and toughness (0 ppm 1.49 ± 0.20 mJ/mm3, 50 ppm 0.98 ± 0.49 mJ/mm3, p < 0.05) decreased in FVB (p < 0.05), and E (0 ppm 103.69 ± 51.22 MPa, 50 ppm 13.20 ± 2.22 MPa, p < 0.05) decreased in A/J (p < 0.05). No significant difference was observed in geometrical parameters due to 50-ppm F exposure in any of the strains. Some correlations were observed between some parameters and F, but no fixed pattern could be followed. In the AKR strain, trabecular BMD showed significant correlation to the accumulation of bone F (r = 0.594, p = 0.042) and serum ALP (r = 0.612, p = 0.034). In the C3H strain, trabecular BMD showed significant correlation to DF (r = 0.787, p = 0.002). Significant correlation to the accumulation of bone F was observed only in FVB (Fu: r = –0.851, p < 0.001; S: r = –0.612, p = 0.034), A/J (S: r = –0.633, p = 0.049), and 129S1 (Fu: r = –0.683, p = 0.021) in mechanical tests of the vertebrae.
Discussion
Inbred mouse strains provide an opportunity to investigate environmental effects (F) superimposed upon the normal variation between strains for many of the traits examined. Differences in response to F have been demonstrated in previous studies on mice [Everett et al., 2002; Vieira et al., 2005a; Vieira et al., 2005b; Mousny et al., 2006; Yan et al., 2007]. In the current study, prolonged and continuous F exposure led to strain-dependent and variable-phenotypic responses involved in dental enamel development, bone metabolism and mechanics, serum F, and bone F. No consistent pattern was observed between DF severity and variations in bone metabolism including chemistries, ex vivo assays, and bone quality parameters. Decreased bone quality was not consistently correlated to F treatment as reduced quality was observed both in the strains with intermediate and high bone F content and in the strains with low bone F content. Thus, such changes in bone metabolism cannot simply be explained by the effect of bone F content. This indicates that high F accumulation in bone or serum may not be a predictor of altered bone metabolism. On this basis, we hypothesize that other mechanisms may be involved in bone F accumulation and its effects on bone metabolism. These may include direct interaction with the bone mineral matrix through a physicochemical mechanism [Grynpas and Omelon, 2007; Chachra et al., 2008] and/or some biological effects of F on bone cells [Farley et al., 1983; Lau and Baylink, 1998; Rodriguez and Rosselot, 2001; Oguro et al., 2003; Mousny et al., 2006; Yan et al., 2007]. DF susceptibility in mice is a complex trait and for at least 2 strains it is associated with genes on 2 chromosomes that likely have additive effects [Everett, 2010]. Multiple interacting loci are likely responsible for observed differences in DF susceptibility among the strains examined in this study. Haplotype association studies would indicate whether there are shared alleles between strains controlling DF susceptibility. Our study showed that treatment durations (3 weeks and 60 days) induce different responses regarding OC in the C3H strain. Weanling C3H mice treated with F for 3 weeks showed a robust induction of osteoclastogenesis (OC) with increases in serum RANKL and iPTH [Yan et al., 2007], whereas adult C3H mice and 60-day treatment did not show the same increase in OC. The responses of young growing mice did not forecast responses of older animals. It is possible that the induction of osteoclastogenesis (without measurable bone loss) seen previously could not be maintained because this high bone mass strain adapted to the F during the long-term treatment. However, A/J, another high bone mass strain, demonstrated increases in iPTH, RANKL, and OC in response to F. We also observed in A/J a significant increase in serum PO4 which indicated that the change in PTH might be related to PO4 homeostasis regulation since it has been reported that F may damage kidneys [Chattopadhyay et al., 2010] to induce PO4 retention; furthermore, PO4 retention leads to a chronic increase in PTH secretion [Turner et al., 2010]. This may become the initial causative factor inducing OC in bone via the PTH pathway due to F exposure. We also noticed a small but significant increase in serum Mg for FVB following F exposure. One previous report demonstrated the increase in serum Mg following F exposure and speculated it was due to more efficient absorption from the diet or F producing a bone salt that releases Mg slowly [O’Dell et al., 1973]. However, we did not observe any significant change in bone Mg content. This might be due to the regulation of the body system to keep mineral homeostasis. Thus, absorption from the diet might be one possible reason for the increased serum Mg. Changes in mechanical properties might be a result of the alterations in the mineral crystal structure due to F precipitation. Among the strains with decreased cortical bone material properties, 129S1 is the only one that demonstrated improved geometry, increased OC, and increased bone formation marker ALP, perhaps indicating increased bone remodeling. This evidence of increased cellular action further supports our idea that the effect of F on bone quality depends on the combination of the direct influence on the bone mineral matrix and biological effects on bone cells. In conclusion, prolonged and continuous F exposure leads to strain-dependent and variable-phenotypic responses involving dental enamel development and bone metabolism. No predictor of bone fluorosis can be found among DF, bone F content, and serum F. The effect of F on bone may depend on the combination of the direct influence on the bone mineral matrix and the biological effects on bone cells via hormone(s).
Acknowledgements
We gratefully acknowledge Ms. Ashley Johnson and Mr. Clay Hamrick for their technical support with the micro-CT. This work was supported by the NIH/NIDCR R01DE014853 (E.T.E.).
Glossary
Abbreviations used in this paper
| ALP | total alkaline phosphatase |
| A/P | anterior posterior |
| BMC | bone marrow cell |
| BMD | bone mineral density |
| BS | bone surface |
| BV | bone volume |
| Ca | calcium |
| CFC | hematopoietic colony-forming cell |
| CFU | colony-forming unit |
| CFU-F | colony-forming unit-fibroblast |
| CFU-G | colony-forming unit-granulocyte |
| CFU-GEMM | colony-forming unit-granulocyte/erythrocyte/macrophage/megakaryocyte |
| CFU-GM | colony-forming unit-macrophage |
| CFU-M | colony-forming unit-monocyte/macrophage |
| CFU-OB | colony-forming unit-osteoblast |
| DF | dental fluorosis |
| E | elastic modulus |
| EIA | enzyme immunoassay |
| F | fluoride |
| Fu | ultimateload |
| iPTH | intactparathyroidhormone |
| Mg | magnesium |
| M/L | mediallateral |
| NaF | sodiumfluoride |
| OC | osteoclastpotential |
| PO4 | phosphate |
| QF | quantitativefluorescence |
| S | bendingstiffness |
| sRANKL | soluble receptor activator of nuclear factor (NF)-κ B ligand |
| TRAP | tartrate-resistantacidphosphatase |
| TV | tissuevolume |
| Uf | energy to failure |
| US | ultimatestress |
References
- Bronckers A.L., Lyaruu D.M., DenBesten P.K. The impact of fluoride on ameloblasts and the mechanisms of enamel fluorosis. J Dent Res. 2009;88:877–893. doi: 10.1177/0022034509343280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho J.G., Leite A.L., Yan D., Everett E.T., Whitford G.M., Buzalaf M.A. Influence of genetic background on fluoride metabolism in mice. J Dent Res. 2009;88:1054–1058. doi: 10.1177/0022034509347249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachra D., Vieira A.P., Grynpas M.D. Fluoride and mineralized tissues. Crit Rev Biomed Eng. 2008;36:183–223. doi: 10.1615/critrevbiomedeng.v36.i2-3.40. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay, A., S. Podder, S. Agarwal, S. Bhattacharya (2010) Fluoride-induced histopathology and synthesis of stress protein in liver and kidney of mice. Arch Toxicol, E-pub ahead of print. [DOI] [PubMed]
- Chou M.Y., Yan D., Jafarov T., Everett E.T. Modulation of murine bone marrow-derived CFU-F and CFU-OB by in vivo bisphosphonate and fluoride treatments. Orthod Craniofac Res. 2009;12:141–147. doi: 10.1111/j.1601-6343.2009.01447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett, E.T. (2010) Fluoride's effects on the formation of teeth and bones, and the influence of genetics. J Dent Res, E-pub ahead of print. [DOI] [PMC free article] [PubMed]
- Everett E.T., McHenry M.A., Reynolds N., Eggertsson H., Sullivan J., Kantmann C., Martinez-Mier E.A., Warrick J.M., Stookey G.K. Dental fluorosis: variability among different inbred mouse strains. J Dent Res. 2002;81:794–798. doi: 10.1177/0810794. [DOI] [PubMed] [Google Scholar]
- Farley J.R., Wergedal J.E., Baylink D.J. Fluoride directly stimulates proliferation and alkaline phosphatase activity of bone-forming cells. Science. 1983;222:330–332. doi: 10.1126/science.6623079. [DOI] [PubMed] [Google Scholar]
- Grynpas M.D., Omelon S. Transient precursor strategy or very small biological apatite crystals? Bone. 2007;41:162–164. doi: 10.1016/j.bone.2007.04.176. [DOI] [PubMed] [Google Scholar]
- Lau K.H., Baylink D.J. Molecular mechanism of action of fluoride on bone cells. J Bone Miner Res. 1998;13:1660–1667. doi: 10.1359/jbmr.1998.13.11.1660. [DOI] [PubMed] [Google Scholar]
- Mousny M., Banse X., Wise L., Everett E.T., Hancock R., Vieth R., Devogelaer J.P., Grynpas M.D. The genetic influence on bone susceptibility to fluoride. Bone. 2006;39:1283–1289. doi: 10.1016/j.bone.2006.06.006. [DOI] [PubMed] [Google Scholar]
- O’Dell B.L., Moroni R.I., Regan W.O. Interaction of dietary fluoride and magnesium in guinea pigs. J Nutr. 1973;103:841–850. doi: 10.1093/jn/103.6.841. [DOI] [PubMed] [Google Scholar]
- Oguro A., Kawase T., Orikasa M. NaF induces early differentiation of murine bone marrow cells along the granulocytic pathway but not the monocytic or preosteoclastic pathway in vitro. In Vitro Cell Dev Biol Anim. 2003;39:243–248. doi: 10.1290/1543-706X(2003)039<0243:NIEDOM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Rodriguez J.P., Rosselot G. Sodium fluoride induces changes on proteoglycans synthesized by avian osteoblasts in culture. J Cell Biochem. 2001;83:607–616. doi: 10.1002/jcb.1255. [DOI] [PubMed] [Google Scholar]
- Turner R.T., Iwaniec U.T., Marley K., Sibonga J.D. The role of mast cells in parathyroid bone disease. J Bone Miner Res. 2010;25:1637–1649. doi: 10.1002/jbmr.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira A.P., Hanocock R., Eggertsson H., Everett E.T., Grynpas M.D. Tooth quality in dental fluorosis genetic and environmental factors. Calcif Tissue Int. 2005a;76:17–25. doi: 10.1007/s00223-004-0075-3. [DOI] [PubMed] [Google Scholar]
- Vieira A.P., Mousny M., Maia R., Hancock R., Everett E.T., Grynpas M.D. Assessment of teeth as biomarkers for skeletal fluoride exposure. Osteoporos Int. 2005b;16:1576–1582. doi: 10.1007/s00198-005-1870-z. [DOI] [PubMed] [Google Scholar]
- Yan D., Gurumurthy A., Wright M., Pfeiler T.W., Loboa E.G., Everett E.T. Genetic background influences fluoride's effects on osteoclastogenesis. Bone. 2007;41:1036–1044. doi: 10.1016/j.bone.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]