Abstract
In mammals, the female reproductive tract (FRT) develops from a pair of paramesonephric or Müllerian ducts (MDs), which arise from coelomic epithelial cells of mesodermal origin. During development, the MDs undergo a dynamic morphogenetic transformation from simple tubes consisting of homogeneous epithelium and surrounding mesenchyme into several distinct organs namely the oviduct, uterus, cervix and vagina. Following the formation of anatomically distinctive organs, the uniform MD epithelium (MDE) differentiates into diverse epithelial cell types with unique morphology and functions in each organ. Classic tissue recombination studies, in which the epithelium and mesenchyme isolated from the newborn mouse FRT were recombined, have established that the organ specific epithelial cell fate of MDE is dictated by the underlying mesenchyme. The tissue recombination studies have also demonstrated that there is a narrow developmental window for the epithelial cell fate determination in MD-derived organs. Accordingly, the developmental plasticity of epithelial cells is mostly lost in mature FRT. If the signaling that controls epithelial differentiation is disrupted at the critical developmental stage, the cell fate of MD-derived epithelial tissues will be permanently altered and can result in epithelial lesions in adult life. A disruption of signaling that maintains epithelial cell fate can also cause epithelial lesions in the FRT. In this review, the pathogenesis of cervical/vaginal adenoses and uterine squamous metaplasia is discussed as examples of such incidences.
Keywords: diethylstilbestrol (DES), adenosis, clear cell adenocarcinoma, squamous metaplasia, p63, Wnt
Development of Müllerian duct derived organs
In mammals, the majority of the FRT develops from a pair of paramesonephric or Müllerian ducts (MD) of mesodermal origin. The MDs arise as cranio-caudal invaginations of thickened coelomic epithelium (Müllerian plaque) at the upper end of the urogenital ridge on the lateral aspect of the corresponding mesonephric or Wolffian duct (WD) (Fig. 1A). The site of the invagination remains open throughout the development of the MDs and becomes the abdominal ostium of the oviduct (Fig. 1A insert). The initial segment of the MD grows caudally through urogenital ridge mesenchyme using the WD as a guide (Fig. 1B) (Grünwald, 1941). The cellular origin of the MD during caudal growth, particularly the presence/absence of cellular contribution of the WD to the MD, has been debated for decades. Some have argued that the WD simply acts as a guide for caudal MD growth (O’Rahilly, 1973), whereas others have proposed that the MD partially or completely splits off from the WD after they make intimate contact with each other (Grünwald, 1941). Recent cell fate tracing experiments in the chick and the mouse have settled the debate by demonstrating that both the epithelium and mesenchyme of the Müllerian duct arise from coelomic epithelium (Guioli et al., 2007; Orvis and Behringer, 2007), as previously suggested by the expression pattern of Amhr2 (Zhan et al., 2006). In the cell fate tracing studies, the long debated cellular contribution of the WD to the MD (Del Vecchio, 1982; Frutiger, 1969) was not detected.
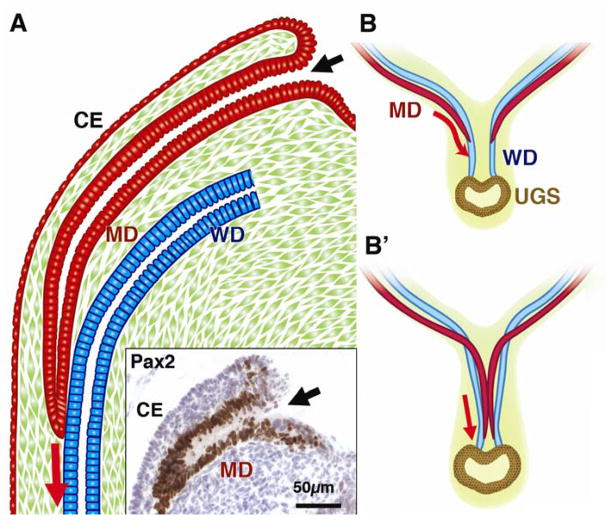
Figure 1. Development of the MD.
CE; coelomic epithelium, MD; Müllerian duct, WD; Wolffian duct, UGS; urogenital sinus
A; Formation of MD
The MD arises as an invagination of CE at the cranial end of the urogenital ridge. The insert demonstrates Pax2 immunohistochemistry on the E13.5 female mouse embryo. The Pax2 is essential for the development of the MD (Torres et al., 1995) and its expression differentiates the MDE (brown cells in the insert) from the CE. The site of infolding remains open throughout development (black arrows). The MD grows caudally through the urogenital ridge mesenchyme and the tip comes into contact with the WD within a common basement membrane. Afterwards, the tip of the growing MD maintains close contact with WD while the cranial portion is separated from the WD by intervening mesenchyme.
B; Caudal growth and fusion of the MDs
The MDs remain in contact with the WDs and use them as a guide during their caudal growth. As the MDs grow caudally, they cross over the WDs and meet in the midline to fuse with each other (B′). The caudal tips of the MDs remain separated to keep contact with the WDs (Hashimoto, 2003). Right before the MD tips reach the urogenital sinus, they finally become united and fuse with the UGS.
As the right and left MDs grow caudally, they cross the WDs ventrally to join (Fig. 1B′) and fuse with each other in the midline. Subsequently, the medial walls of the MDs degenerate forming a single canal (Fig. 2A). In females, the caudal tip of the MDs reaches the urogenital sinus (UGS), which is derived from the endoderm. The fusion of the MDs, the WDs and the UGS forms the sinovaginal bulbs, which are projections of solid epithelial cords on the dorsal wall of the UGS (Koff, 1933; Bloomfield and Frazer, 1927) (Fig. 2A). At the same time, the majority of WDs in female embryos regress due to the absence of testicular androgen, leaving fragments of WDE at the junction with the UGS. In human and mouse female embryos, a flat epithelial cord, which is called the vaginal plate in humans, is formed following the union of fused MDs with the UGS (Koff, 1933). Most textbooks indicate that the lower portion of vagina forms via the simultaneous growth and canalization of the vaginal plate (Russell, 1989; Moore and Persaud, 2002; Sadler, 2004; Gilbert, 2003; Carlson, 1999; Forsberg, 1978).
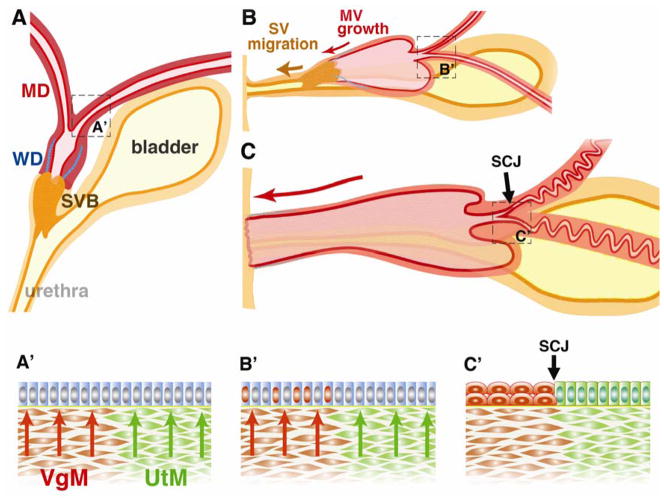
Figure 2. Formation of mouse cervix and vagina.
MD; Müllerian duct, WD; Wolffian duct, UGS; urogenital sinus, SVB; sinovaginal bulb. SV; sinus vagina, MV; Müllerian vagina, SJC; squamocolumnar junction, VgM; vaginal mesenchyme, UtM; uterine mesenchyme.
A. Late embryonic state (~E16). The MD, WD and UGS are present. The cranial portion of WD is regressed by this stage. At this stage, the MDE is uniformly undifferentiated (A′).
B. Perinatal stage. The SV moves caudally as the MV elongates caudally. During this caudal migration, the SV maintains its connection to the urethra. Around this time, ΔNp63 expression is induced in epithelial cells (red nuclei in B′) of the cervix and vagina in response to the mesenchymal induction (red arrows). By this time, the majority of the WD (blue lines) is regressed.
C. Pubertal stage. The MV reaches the posterior body wall in the neonatal stage. At puberty, the solid epithelial cord of the SV is canalized and the vaginal orifice is formed. The entire vagina is lined by epithelial cells derived from MDE. In the cervix, the SCJ is formed as a result of mesenchymal induction (C′). The residual segments of WDs may still be present in the vaginal stroma, and the amount of WDE remnants varies among adult female mice.
Classic problem; Developmental origin of the lower female reproductive tract
As described above, the MDs, WDs, and UGS are present at the site of organogenesis of the lower FRT (Fig. 2A), and the degree of contribution of these structures to the formation of the vaginal bulb and plate, as well as the adult vagina have been debated for decades. There have been four major theories for the developmental origin of vaginal epithelium. The most widely accepted has been the “UGS + MD origin” theory, in which the upper two-thirds of the vagina (Müllerian vagina) develops from the caudal portion of the MDs, and the lower portion (sinus vagina) develops from the UGS (Moore and Persaud, 2002; Sadler, 2004; Koff, 1933; Forsberg, 1963; 1973; Kobayashi and Behringer, 2003; Yin and Ma, 2005; Shapiro et al., 2000; Gilbert, 2003; Del Vecchio, 1982; Sajjad, 2010). In this theory, the vaginal bulb/plate consists solely of epithelium of UGS origin, and is thus referred to as the “sinovaginal bulb” (SVB). Accordingly, the lower vagina develops through the simultaneous growth and canalization of the UGS epithelium (UGE) (Koff, 1933; Forsberg, 1963). The shallow vagina that is present in mice and humans with complete androgen insensitivity syndrome (CAIS) or testicular feminization mutation (Tfm) provides strong support for this theory (Morris, 1953; Cunha, 1975). CAIS arises from a spontaneous mutation of the androgen receptor gene, which causes insensitivity to testosterone; as a result, the male reproductive tract, excluding the testes, does not develop normally in affected XY individuals (Quigley et al., 1995). At the same time, the MDs regress due to the production of the anti Müllerian hormone (AMH)/Müllerian inhibiting substance (MIS) by the fetal testis (Teixeira et al., 2001; Josso et al., 2001). Therefore, the short vagina present in Tfm males is believed to arise from the sinus vagina of the UGS origin (Cunha, 1975).
The alternative “MD origin” and “MD + WD origin” theories describe how the vaginal bulb/plate is derived from the MDs (Bloomfield and Frazer, 1927; Cai, 2009) or the WDs (Forsberg, 1963; Witschi, 1970; Drews, 2007), and thus, the vagina develops from either the MDs alone or the MDs plus the WDs (Hart, 1901; Bloomfield and Frazer, 1927; Mauch et al., 1985; Sánchez-Ferrer et al., 2006). In the MD + WD origin theory, the WDs contribute to the epithelium of the vagina (Hart, 1896; 1901; Forsberg, 1963; Acién, 1992; Sánchez-Ferrer et al., 2006). In contrast, the MD origin theory proposes that the WDs play a role in the downward growth of the Müllerian-derived vagina and/or in the formation of the hymen, but they do not contribute to the vaginal epithelium (Bloomfield and Frazer, 1927; Drews, 2007). In these theories, the presence of short vaginae in CAIS-affected XY individuals is considered a derivative of residual MDs and/or WDs (Forsberg, 1978; Drews et al., 2002).
Finally, the UGS origin theory suggests that the entire squamous epithelium of the cervix and vagina are originated solely from the UGE of endodermic origin (Arey, 1954; Bulmer, 1957; 1959; Ferris, 2004; Fliegner, 1994; Ulfelder and Robboy, 1976). In this view, the squamous epithelium derived from the UGS grows upward and replaces the original columnar epithelium of MD origin. However, mouse studies demonstrated that the squamous vaginal epithelium might develop from MD epithelium (MDE) (Forsberg, 1965; Cunha, 1976; Kurita et al., 2004), which questions the observations supporting the UGS origin theory. All of the theories are based upon anatomical/histological observations, with the boundaries of structures drawn subjectively. Thus, none of these theories were definitively proven or disproven until recently.
The origin of vaginal epithelium has been finally determined by a recent cell lineage tracing experiment (Kurita, 2010). In the experiment, mouse epithelial cells of embryonic WD, MD, or UGS origin were permanently labeled to express enhanced green fluorescent protein (EGFP), and the cellular origin of vaginal epithelium was determined by following the developmental cell fate of EGFP-positive cells from the embryo to the adult. The cellular origin and organogenesis process of vagina revealed by the study have refuted the four theories described above. The cell lineage tracing study agreed with the MD+UGS theory in regards to the origin of the vaginal bulb in that the vaginal bulb solely consisted of UGE. Thus the embryonic and newborn vaginae consisted of both the “Müllerian vagina” and “sinus vagina.” However, the Müllerian and sinus vaginae were never “unified” as described in the majority of textbooks. In mice, the epithelium in the sinus vagina remained solid throughout development, and it never became a part of the true vagina. Moreover, the solid epithelium of the sinus vagina never elongated. Instead, the sinus vagina connected the Müllerian vagina and urethra and migrated towards the posterior end of the body leading the caudal growth of the Müllerian vagina (Fig. 2B and C). The solid epithelial cord in the sinus vagina canalized only during the formation of the vaginal orifice at puberty (~ four-weeks-old), and thus the UGE in the sinus vagina became a part of the vulvar epithelium. Although a critical role of the WD was proposed in the MD origin and MD+WD origin theories, the WD did not appear to play a role in the development of the vagina. WDE cells were not detected in the vaginal epithelium throughout development (Kurita, 2010).
Differentiation of vaginal and uterine epithelial cells
As explained in the previous section, the entire FRT, from the oviduct to the vagina, develops from the MD. As each MD-derived organ becomes anatomically distinctive, the uniform MDE differentiates into epithelial cell types with unique morphology and functions in each organ. While the differentiation of the MDE occurs in the fetal stage of the human, mouse MDE is mostly undifferentiated at the time of birth (Kurita et al., 2005; Kurita and Nakamura, 2008). For example, ciliation of the epithelial cells in the mouse oviduct is first observed on postnatal day 5 (P5) (Komatsu and Fujita, 1978). The mouse uterine epithelium displays the first signs of morphogenesis around P4 as a complex luminal shape, and the rudimentary glands appear on ~P7 (Brody and Cunha, 1989; Kurita et al., 2001). Since it can be studied postnatally, the mechanism of MDE differentiation has been studied mainly by using the mouse as the model organism. Accordingly, this and the following sections mainly discuss the mouse studies.
Although epithelia in the uterus, cervix and vagina are derived from MDE, the simple columnar epithelium of the uterus and the stratified squamous epithelium of the cervix/vagina are profoundly different in their morphology and gene expression. For example, cytokeratin 14 (K14) is expressed in vaginal and cervical but not in uterine epithelium. Progesterone receptor (PR) is also differentially expressed in the epithelial cells of the mouse uterus versus the cervix/vagina. In mouse cervical/vaginal epithelial cells, PR is upregulated by estrogen through the estrogen receptor α (ERα) as in most progesterone target cells/tissues (Kurita et al., 2000). In contrast, PR is expressed independently of estrogen and ERα in the mouse uterine epithelium (Kurita et al., 2000). In fact, PR is down-regulated, not up-regulated, by estrogen in the mouse uterine epithelium (Kurita et al., 2000). Since this unusual pattern of PR regulation is unique for the uterine epithelial cells of rodents, estrogen-independent PR expression is an excellent marker for mouse uterine epithelial differentiation (Kurita et al., 2001).
The critical role of epithelial-mesenchymal tissue interaction in the differentiation of MDE has been established by a series of tissue recombination studies, in which the mesenchyme and epithelium isolated from the uterus and vagina of newborn mice were recombined and grafted under the subrenal capsule of syngeneic or immunodeficient mouse hosts (Cunha, 1976; Boutin et al., 1991b; Kurita et al., 2001; Kurita et al., 2004). In 1976, Cunha demonstrated for the first time that the differentiation fate of uterine and vaginal epithelial cells was exchangeable at birth, and that their organ-specific phenotypes are dictated by regional inductive cues from the surrounding mesenchyme (arrows in Fig. 2A′ and B′) (Cunha, 1976). In response to the induction by the uterine mesenchyme, vaginal epithelium differentiates into uterine epithelium containing both luminal epithelial cells and glands (Cunha, 1976; Boutin et al., 1991a). The induced uterine epithelium expresses a spectrum of differentiation markers characteristic of normal uterine epithelium such as uterine-type syndecans (Boutin et al., 1991b) and PR (Kurita et al., 2001). In the reciprocal tissue recombinants, vaginal mesenchyme induced the uterine epithelium to differentiate into a stratified squamous vaginal epithelium, which showed a mucification and cornification in response to the estrogen and progesterone (Cunha, 1976; Boutin et al., 1991a; 1992). The induced vaginal epithelium expressed vaginal differentiation markers such as the vaginal isoform of syndecan (Boutin et al., 1991b), involucrin and keratins 1, 10, 14 and 19 (Kurita et al., 2001). In the induced vaginal epithelium, PR was also expressed in the vaginal pattern (Kurita et al., 2001). Though the differentiation markers are already expressed, most cells in the vaginal and uterine epithelia from P5 mice can still trans-differentiate into either uterine or vaginal epithelium when induced by heterotypic mesenchyme (Cunha, 1976). However, the developmental plasticity of the epithelial cells is then gradually lost thereafter, and the uterine and vaginal epithelial phenotypes become irreversibly fixed. Accordingly, uterine and vaginal epithelium from seven-day-old or older mice are induced only partially to become the alternative cell type by heterotypic mesenchyme, and most adult uterine and vaginal epithelial cells cannot be re-programmed by the newborn uterine or vaginal mesenchyme to express alternative epithelial phenotypes (Kurita et al., 2001; Kurita et al., 2004). Hence, the organ specific epithelial phenotype of mouse uterine and vaginal epithelial cells is “induced” and then “stabilized” during the first week of postnatal development. As a result, a boundary known as the squamocolumnar junction (SCJ) is formed between the columnar and squamous epithelia of the uterus and cervix. In the mouse, the SCJ is located in the caudal portion of each uterine horn where the uterine and cervical epithelia meet (black arrow Fig. 2C and C′). Since cervical and vaginal epithelial cells of the mouse are indistinguishable, there is no epithelial boundary between the cervix and vagina.
p63 transcription factor in FRT development
The transcription factor p63 is the product of the mouse Trp63 or human TP63 gene, which are transcribed into isoforms containing or lacking the N-terminal transactivation domain, TA and ΔN forms, respectively. In addition, alternative splicing generates three different C-terminal sequences corresponding to the α, β and γ forms (Yang et al., 1998). p63 is highly expressed in germ cells (Petre-Lazar et al., 2007; Nakamuta and Kobayashi, 2004b; Kurita et al., 2005; Saito et al., 2006; Nakamuta and Kobayashi, 2004a; Petre-Lazar et al., 2006) and the basal cells of many epithelial tissues (Mills et al., 1999; Yang et al., 1999; Signoretti et al., 2000; Kurita and Cunha, 2001; Daniely et al., 2004). In the adult FRT, p63 is expressed in cervical and vaginal but not uterine epithelium (Kurita and Cunha, 2001; Kurita et al., 2004; Kurita et al., 2005), and the ΔNp63 is the dominant form in the cervix and vagina (Kurita et al., 2005). The MDE and its precursor coelomic cells are negative for p63, but p63 expression is induced by the cervical/vaginal mesenchyme in the early fetal stage of the human and the perinatal stage of the mouse (Fig. 2B′) (Kurita et al., 2005; Kurita and Nakamura, 2008). In developing mouse and human FRT, p63 is the first marker that is differentially expressed between uterine and vaginal/cervical epithelial cells, suggesting that the key role of p63 lies in the epithelial cell fate determination in the cervix and vagina. Indeed, the cervical/vaginal epithelium of p63 null mice differentiated into columnar uterine-like epithelium (Kurita and Cunha, 2001). The p63 null vaginal epithelium expressed PR in the absence of estrogen indicating uterine epithelial identity, demonstrating that the expression of p63 determines the developmental cell fate of MDE to become uterine epithelium (p63-negative) or cervical/vaginal epithelium (p63-positive) (Kurita et al., 2004).
When p63 is induced in the MDE by cervical and vaginal mesenchyme, the p63-positive MDE cells subsequently change their morphology and eventually transform into cervical/vaginal epithelium (Kurita et al., 2004; Kurita and Cunha, 2001). Consequently, a layer of p63 positive basal cells forms in the cervix and vagina. In the mouse, a continuous basal epithelial cell layer of cervix/vagina forms by P5 and the SCJ becomes distinctive by P14 (Kurita et al., 2005). As described above, uterine and vaginal epithelia gradually lose their ability to change in the p63-expression patterns in response to induction by heterotypic mesenchyme. Accordingly, adult vaginal epithelium maintains its original squamous phenotype and p63 expression, even when it is combined with inductive uterine mesenchyme from newborn mice (Kurita et al., 2004). This result indicates that the cervical/vaginal mesenchymal factor(s) is required only for activation and not for the maintenance of p63 expression. The developmental plasticity of uterine epithelial cells is gradually lost during postnatal development, and most uterine epithelial cells do not respond to induction by the vaginal mesenchyme (Kurita et al., 2004). However, there is a small number of epithelial cells that maintain developmental plasticity in the adult uterus as assessed by the induction of p63 expression. When the uterine epithelial cells from two-month-old virgin mice were combined with vaginal mesenchyme, p63 expression was detected in a small subpopulation of epithelial cells (< 5%) (Fig. 4C). These rare developmentally plastic cells may be stem cells and the targets of uterine squamous metaplasia (see below).
Figure 4. Uterine squamous metaplasia.
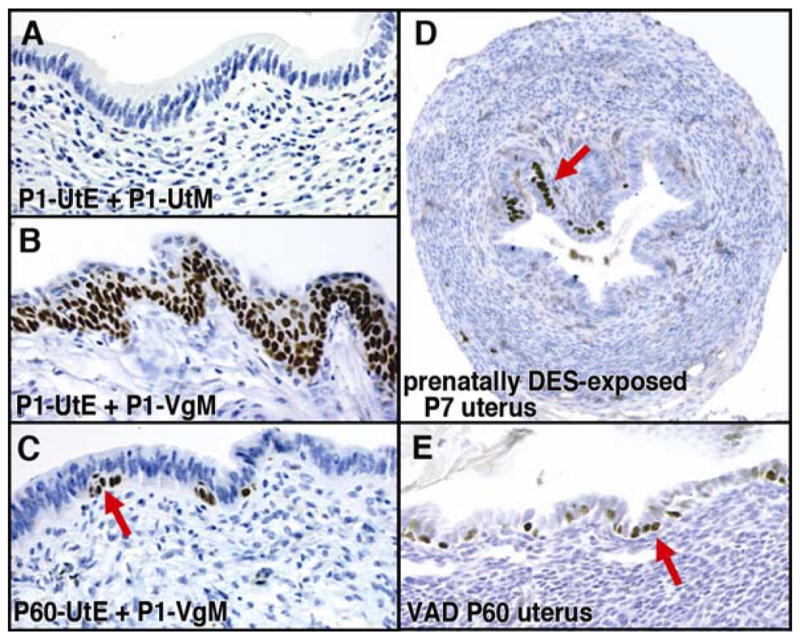
UtE; uterine epithelium, VgE; vaginal epithelium, UtM; uterine mesenchyme, VgM; vaginal mesenchyme
A– C; Tissue recombinants composed with P1 UtE + P1 UtM (A), P1 UtE + P1 VgM (B), and P60 UtE + P1 VgM (C). When the UtE from the P1 mouse is associated with a P1 UtM, it develops into simple columnar UtE, which is negative for p63 (A). The same P1 UtE can be induced to be stratified squamous VgE by P1 VgM (B). In contrast, UtE from mature mice have limited potential to transdifferentiate into VgE. UtE from two month old virgin mice remains mostly simple columnar when it is combined with P1 VgM (C). However, the bipotency of UtE is not completely lost even in the adult mice, and a small number of UtE cells can be induced to express p63 in response to the P1 VgM (C, red arrow). These developmentally plastic cells are likely to be the target of VAD-induced squamous metaplasia (E).
D. Expression of p63 in the uterus of prenatally (gestational day 14 – 17) DES-exposed mouse. The ectopic expression of p63 in the uterus was detected at P7 (red arrow). These cells are believed to develop into squamous metaplasia in a mature animal.
E. Vitamin A deficiency induces expression of p63 in the adult uterus. When female mice were fed a vitamin A deficient diet from P21, p63 positive cells were detected in the UtE by four-month-old.
DES-induced cervical/vaginal adenosis and clear cell adenocarcinoma
(a) Adenosis
Cervical and vaginal adenoses are congenital anomalies defined as the development of glandular (columnar) epithelium in cervical/vaginal epithelium, which is normally stratified squamous. Cervical/vaginal adenosis is known to be associated with in utero exposure to diethylstilbestrol (DES), a synthetic non-steroidal estrogen. DES was prescribed during the 1938–1971 for pregnant women to help prevent miscarriages. It has been estimated that more than 2 million mothers, daughters and sons were exposed to DES in the United States alone (Giusti et al., 1995). Female fetuses exposed to DES in utero (DES daughters) are at increased risk of developing clear cell adenocarcinoma (CCAC) of the cervix and vagina (Herbst and Scully, 1970; Herbst et al., 1971; Melnick et al., 1987; Verloop et al., 2010), and adenosis is believed to be the precursor of CCAC. Particularly, atypical adenosis of the tuboendometrial type has been considered as the immediate precursors of CCACs (Robboy et al., 1984). The incidences of adenosis and CCACs are directly related to the starting time of DES-treatment during pregnancy. A study demonstrated that more than a third of DES-daughters developed adenosis if DES was given before gestational week eight (Robboy et al., 1984). In the same study, adenosis was essentially absent if DES-treatment was started at week 22 or later (Robboy et al., 1984). Perinatal exposure of mice to DES also generates a spectrum of reproductive tract lesions similar to those observed in DES daughters, including cervical/vaginal adenosis (Forsberg, 1976; Plapinger and Bern, 1979; McLachlan et al., 1980). As in DES-daughters, the frequency of cervical/vaginal adenosis in the mouse is also directly related to the timing of DES exposure. When female neonatal mice were exposed to DES from P1 to 5, vaginal adenosis was detected in 75% of mice by P35, while it was detected only in 15% of mice with prenatal DES-exposure (gestation day 9 to 16) (Newbold and McLachlan, 1982). In the mouse, the first seven days of postnatal development are crucial for the MDE to establish p63 expression patterns, and thus the differentiation cell fate (Kurita et al., 2004). The developmental stage in which the human fetus is susceptible to DES-induced adenosis/CCACs also corresponds to the time period in which p63 expression is induced in the cervical/vaginal epithelium (Kurita et al., 2005). These observations suggest that DES induces cervical and vaginal adenosis by altering the expression pattern of p63, and thus the differentiation cell fate of MDE. Indeed, DES-exposure repressed expression of p63 in developing mouse cervix and vagina (Kurita et al., 2004). This inhibitory effect of DES on p63 expression is usually transient. However, when DES is present throughout the critical period for cell fate determination, the MDE cells in the cervix and vagina miss the developmental window to express p63 and consequently, the p63-negative MDE cells differentiate into uterine epithelium within cervix and vagina forming adenosis (Kurita et al., 2004).
The inhibitory effect of DES on p63 in developing cervical/vaginal epithelium was mediated via ERα (Kurita et al., 2004). A tissue recombination study with ERα null mice determined definitively that DES blocks the expression of p63 via ERα within the MDE (Kurita et al., 2004). In the study, DES blocked p63 expression in wild-type but not ERα null MDE. DES actions via ERα in the mesenchymal cells did not affect the expression of p63 in the epithelial cells. Our recent study indicates that DES via ERα blocks p63 expression in MDE cells autonomously (Fig. 3). In the experiment, uterine epithelial cells from P1 ERα null and wild-type mice were mixed and combined with vaginal mesenchyme, which was then grafted under the subrenal capsule of nude mice (Fig. 3). In intact hosts, uterine epithelium was induced to express p63 in both ERα positive and negative epithelial cells (Fig. 3A–C). In contrast, when hosts were supplemented with DES, p63 expression was detected only in the ERα negative cells (Fig. 3D – F). These experiments clearly demonstrate that DES does not block expression of p63 in the MDE through the down-regulation of mesenchymal factors (red arrows in Fig. 2A′ and B′). Cell-autonomous inhibition of p63 expression by DES via epithelial ERα also refutes the involvement of autocrine factors produced by the MDE in the pathogenesis of DES-induced adenosis.
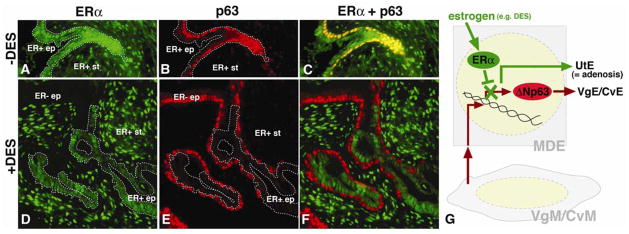
Figure 3. Cell-autonomous inhibition of p63 expression by DES/ERα in MDE.
ERα; estrogen receptor α, UtE; uterine epithelium, VgE; vaginal epithelium, CvE; cervical epithelium, VgM; vaginal mesenchyme, CvM; cervical mesenchyme, ep; epithelium, st; stroma, ER+; ERα positive ER−; ERα negative. Dotted line indicates ER+ epithelial cells.
The uterine epithelial cells from P1 ERα null and wild-type mice were mixed, combined with P1 rat VgM and grafted under the subrenal capsule of female host nude mice with/without subcutaneous implantation of a 25μg DES pellet (Kurita et al., 2004). In the absence of DES, p63 was induced in the entire epithelium, which contained both ERα positive and negative epithelial cells. The panels A –C show the area with double positive epithelial cells for ERα and p63. In contrast, in the presence of DES, p63 was induced only in the ERα negative cells. The panels D– F show exclusive expression of ERα and p63 in the epithelium in the DES-treated host. These data confirm the conclusion of our previous study that DES inhibits expression of p63 through ERα in the epithelial cells. ERα in the VgM/CvM does not inhibit induction of p63 in the MDE. Furthermore, co-localization of ER+/p63-negative and ER−/p63-positive epithelial cells indicates that the inhibitory effect of DES/ERα on p63 expression is cell-autonomous, as illustrated in G.
(b). Clear Cell Adenocarcinoma
The relative risk of CCAC in DES Daughters has been estimated to be greater than 40 times compared to the general population (Hatch et al., 1998). Therefore, it has been considered that in utero exposure to DES is the cause of CCACs of the cervix and vagina. However, the incidence of CCAC has been estimated to be 1 to 1.5 per 1,000 DES daughters (Melnick et al., 1987; Herbst, 2000), whereas the prevalence of cervical/vaginal adenosis in DES daughters was as high as ~90% (Johnson et al., 1979; Sherman et al., 1974; Robboy et al., 1984). The discrepancy in the incidences of DES-induced cervical/vaginal adenosis versus CCACs indicates that oncogenesis of cervical/vaginal CCACs requires factors other than the developmental loss of p63 expression. Moreover, the prevalence of adenosis in the general population has been estimated to be 1 to 10 % (Herbst et al., 1975; Chattopadhyay et al., 2001), which is 5 – 90 times lower than that of DES daughters. Thus, the > 40-fold increase in CCAC incidence in DES daughters appears to be proportional to the increased rate of adenosis lesions. These numbers strongly suggest that the in utero DES exposure increased the risk of cervical/vaginal CCACs by increasing the prevalence of cervical/vaginal adenosis lesions; however the chance of the malignant transformation of adenosis to adenocarcinoma may be identical between DES-daughters and general population. Progression of adenosis to CCACs may be independent of DES-exposure.
The molecular mechanism underlying the transformation of the adenosis lesion to CCAC is not known. For example, a screening for common mutations to cancers, such as Ras, p53 and WT1, failed to detect mutations in all 16 cervical/vaginal CCAC cases (Boyd et al., 1996). While infection of high-risk types of the human papillomavirus (HPV) is the major cause of both cervical squamous cell carcinomas (SCC) and non-clear cell adenocarcinomas (Clifford et al., 2003; Castellsague et al., 2006), there is no association between this infection and CCACs (Pirog et al., 2000; Goto et al., 2005; Stewart et al., 2006; Liebrich et al., 2009). Even in a case of synchronous SCC and CCAC of the cervix (co-existing cervical SCC and CCAC in the same patient), HPV was found only in SCC but not in CCAC, suggesting different etiologies in these two types of cervical cancers (Goto et al., 2005). The low prevalence of this cancer makes research difficult. Although the etiology of cervical/vaginal CCAC remains unknown, it should be noted that in a study, loss of function mutations in PTEN (phosphatase and tensin homolog deleted on chromosome 10) were detected in 36% (4/11) of HPV-negative cervical adenocarcinomas (Minaguchi et al., 2004), suggesting a link between alterations in the phosphatidylinositol 3,4,5-trisphosphate (PIP3) signaling pathway and CCACs of the cervix.
Cervical/vaginal adenosis and CCAC in post-DES era
Although DES is no longer prescribed to pregnant women, cervical/vaginal adenosis and CCACs are still relevant to current women’s health. There have been cases of cervical/vaginal adenoses/CCACs reported in women who have no history of DES exposure (Kurman and Scully, 1974; Robboy et al., 1986; Accetta et al., 2001; Herbst et al., 1975; Chattopadhyay et al., 2001). The persisting incidence of cervical/vaginal adenosis in the general population even after the ban of DES usage suggests the existence of other causal factors in our environment. In utero exposure to environmental chemicals may be the cause of adenosis in the cervix and vagina. In this regard, there are a number of environmental chemicals that disrupt the endocrine systems of animals and humans by mimicking or antagonizing the biological activities of natural hormones, thus known as endocrine disruptors (Colborn, 1995; Diamanti-Kandarakis et al., 2009). Some environmental chemicals such as bisphenol A (BPA) have been reported to elicit estrogenic activities, which may explain the underlying cause of non DES-associated adenosis (Newbold et al., 2009).
Uterine Squamous Metaplasia
In addition to cervical/vaginal adenosis, perinatal DES exposure of mice also induced uterine squamous metaplasia, development of squamous cells in normally columnar uterine epithelium (McLachlan et al., 1980). When pregnant mice were treated with 100 μg DES/kg/day from gestational day 14 to day 17, a small number of p63 positive cells were observed in the uterine epithelium at P7 (Fig. 4A), which were considered to be the seed of squamous metaplasia (Kurita et al., 2004). The metaplastic squamous epithelial cells in the adult uterus expressed vaginal epithelial markers such as K14, and PR was also expressed in the vaginal pattern (Kurita et al., 2004). These observations indicate that uterine squamous metaplasia is the formation of cervical/vaginal epithelium in the uterus. The uterus of p63 null mice was resistant to DES-induced squamous metaplasia, confirming the essential role of p63 in cervical/vaginal epithelial differentiation (Kurita et al., 2004). While cervical and vaginal adenoses are congenital, squamous metaplasia can develop in the adult uterus. For example, uterine squamous metaplasia is a classic histological change caused by chronic estrogen exposure (McEuen, 1936; Gitlin, 1954; Selye et al., 1935) or vitamin A deficiency (VAD) (Wolbach and Howe, 1925; Moll et al., 1983; Darwiche et al., 1993) in mature animals. When CD-1 mice were fed a vitamin A deficient diet from weaning, expression of p63 was detected in the uterine epithelium at four months old (Fig. 4E). This observation is in agreement with the tissue recombination study that demonstrated the presence of developmental plastic cells in adult uterine epithelium (Fig. 3C).
Canonical Wnt signaling pathway maintains uterine epithelial identity
As described above, epithelial differentiation in MDE was first “induced” and then “stabilized” during development. The Wnt (wingless-type MMTV integration site family member) pathway plays an important role in the “stabilization” of the epithelial cell fate in the uterus. In the Wnt7a null mutant mice, uterine epithelial cells are columnar with proper differentiation markers by puberty (Parr and McMahon, 1998; Carta and Sassoon, 2004; Kurita and Nakamura, 2008), thus Wnt7a is dispensable for the cell fate determination of MDE in the neonatal uterus and cervix/vagina (Fig. 5A). However, Wnt7a is essential for the “stabilization” of the uterine epithelial cell fate, and thus uterine epithelium of Wnt7a null mice gradually transforms into a squamous epithelium. The uterine squamous metaplasia becomes prominent in fully mature Wnt7a null mice (Miller and Sassoon, 1998), and the post-pubertal onset of uterine squamous metaplasia in Wnt7a null mice suggests the involvement of ovarian steroid hormones in its pathogenesis. Indeed, our study demonstrated the essential role of estrogen signaling in the formation of uterine squamous metaplasia in the postaxial hemimelia mice (Kurita and Nakamura, 2008), a spontaneous Wnt7a null mutant strain (Parr et al., 1998). When Wnt7a null mice were ovariectomized at P35, the uterine epithelium remained columnar, negative for p63 (Fig. 5B) and positive for PR in the absence of estrogen at P60 (Kurita and Nakamura, 2008) (Fig. 5B′). However, 17β-estradiol treatment at P60 induced p63 expression and squamous transformation in the uterine epithelium of ovariectomized Wnt7a null mice (Fig. 5C) (Kurita and Nakamura, 2008).
Figure 5.
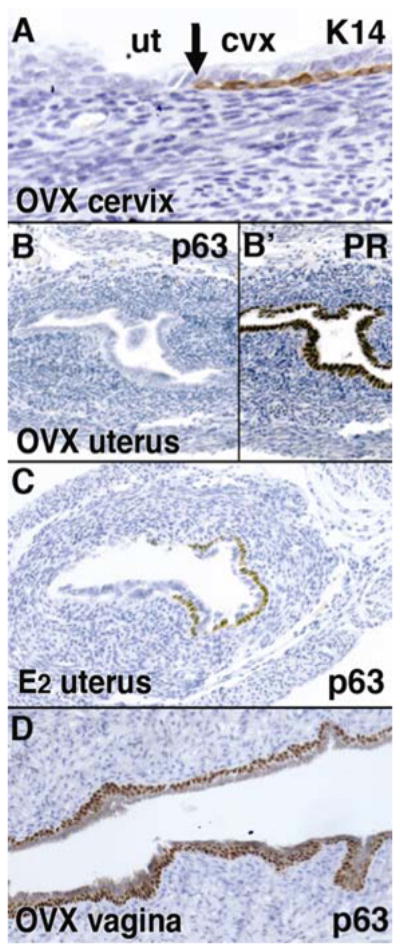
Uterine and vaginal epithelial differentiation phenotypes of Wnt7a null mutant, postaxial hemimelia (px) mice. Female px mice were ovariectomized (OVX) at P35 and the expressions of K14 (A), PR (B′) and p63 (B, C and D) were analyzed at P60. The SCJ was normally formed at cervix (A). UtE was columnar, positive for PR (B′) and negative for p63 and K14 (B), whereas CvE/VgE were stratified squamous with the expression of p63 (D). However, IP injection of 125ng 17β-estradiol (E2)/day for 3 days from P60 induced p63 expression in the uterus (C). Therefore, Wnt7a is required for stabilization of the epithelial cell fate in the uterus, but is dispensable for induction of the uterine/vaginal epithelial cell fate.
The Canonical Wnt pathway is initiated by the binding of the Wnt protein to the membrane receptors, followed by downstream cytoplasmic events that lead to stabilization and nuclear transportation of β-catenin. In the nucleus, β-catenin binds to the T-cell factor/lymphocyte enhancer factor (Tcf/Lef) transcription factor, resulting in the activation of Wnt target genes (Cadigan and Peifer, 2009). The conditional inactivation of β-catenin in the uterus induced expression of p63 in the uterine epithelium of adult mice (Jeong et al., 2009), confirming the importance of the canonical Wnt/β-catenin signaling pathway in the maintenance of the uterine epithelial identity. The regulation of p63 by Wnts in the uterine epithelium, however, appears to involve more than just Wnt7a and β-catenin. In the uterus, transcripts for Wnts 4, 5a, 7a, 7b, 10b 11 and 16 have been detected (Wang and Shackleford, 1996; Hayashi et al., 2009; Miller et al., 1998), thus the loss of β-catenin in the uterus can affect the downstream signaling of many Wnt family members. Indeed, the conditional inactivation of Wnt4 in the uterus also induced p63 expression in the adult uterine epithelium (Franco et al., 2010). Since Wnt4 was also down-regulated in the uterus of Wnt7a null mutant mice (Miller and Sassoon, 1998), it is possible that the development of uterine squamous metaplasia in the Wnt7a null mutant mice was mediated by the down-regulation of Wnt4. In addition, the loss of Wnt7a affects expression of Wnt5a, Hoxa10 and Hoxa11 in the adult uterus (Miller and Sassoon, 1998). Therefore, in the adult uterus, epithelial differentiation is maintained by a complex signaling network in which canonical Wnt signaling pathway plays the pivotal role. Wnt/β-catenin signaling pathway controls maintenance and differentiation cell fate of epithelial stem cells in the epidermis and gastrointestinal tract (DasGupta and Fuchs, 1999; Huelsken et al., 2001; Korinek et al., 1998; Grigoryan et al., 2008). The same signaling pathway may also control the number and cell fate of stem cells in the FRT, and uterine squamous metaplasia may be a result of the dysregulation of the stem cell maintenance in the adult uterine epithelium.
Acknowledgments
The author thanks Dr. Gerald R. Cunha for the invitation to write this review,, Stacy Ann Druschitz and So-youn Kim for editing. The work is supported by NIH/NCI R01 CA154358.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accetta SG, Rivoire WA, Monego HI, Vettori DV, De Oliveira Freitas DM, Edelweiss MI, Capp E. Vaginal adenosis in a non-diethylstilbestrol-exposed 6-year-old patient. Gynecologic and obstetric investigation. 2001;51:271–273. doi: 10.1159/000058063. [DOI] [PubMed] [Google Scholar]
- Acién P. Embryological observations on the female genital tract. Human reproduction (Oxford, England) 1992;7:437–445. doi: 10.1093/oxfordjournals.humrep.a137666. [DOI] [PubMed] [Google Scholar]
- Arey LB. Developmental anatomy: a textbook and laboratory manual of embryology. W.B. Saunders; Philadelphia: 1954. [Google Scholar]
- Bloomfield A, Frazer JE. The Development of the Lower End of the Vagina. Journal of anatomy. 1927;62:9–32. [PMC free article] [PubMed] [Google Scholar]
- Boutin EL, Battle E, Cunha GR. The response of female urogenital tract epithelia to mesenchymal inductors is restricted by the germ layer origin of the epithelium: prostatic inductions. Differentiation; research in biological diversity. 1991a;48:99–105. doi: 10.1111/j.1432-0436.1991.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Boutin EL, Battle E, Cunha GR. The germ layer origin of mouse vaginal epithelium restricts its responsiveness to mesenchymal inductors: uterine induction. Differentiation; research in biological diversity. 1992;49:101–107. doi: 10.1111/j.1432-0436.1992.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Boutin EL, Sanderson RD, Bernfield M, Cunha GR. Epithelial-mesenchymal interactions in uterus and vagina alter the expression of the cell surface proteoglycan, syndecan. Developmental biology. 1991b;148:63–74. doi: 10.1016/0012-1606(91)90317-v. [DOI] [PubMed] [Google Scholar]
- Boyd J, Takahashi H, Waggoner SE, Jones LA, Hajek RA, Wharton JT, Liu FS, Fujino T, Barrett JC, McLachlan JA. Molecular genetic analysis of clear cell adenocarcinomas of the vagina and cervix associated and unassociated with diethylstilbestrol exposure in utero. Cancer. 1996;77:507–513. doi: 10.1002/(SICI)1097-0142(19960201)77:3<507::AID-CNCR12>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Brody JR, Cunha GR. Histologic, morphometric, and immunocytochemical analysis of myometrial development in rats and mice: I. Normal development. The American journal of anatomy. 1989;186:1–20. doi: 10.1002/aja.1001860102. [DOI] [PubMed] [Google Scholar]
- Bulmer D. The development of the human vagina. Journal of anatomy. 1957;91:490–509. [PMC free article] [PubMed] [Google Scholar]
- Bulmer D. The epithelium of the urogenital sinus in female human foetuses. Journal of anatomy. 1959;93:491–498. [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Peifer M. Wnt signaling from development to disease: insights from model systems. Cold Spring Harbor perspectives in biology. 2009;1:a002881. doi: 10.1101/cshperspect.a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y. Revisiting old vaginal topics: conversion of the Mullerian vagina and origin of the “sinus” vagina. The International journal of developmental biology. 2009;53:925–934. doi: 10.1387/ijdb.082846yc. [DOI] [PubMed] [Google Scholar]
- Carlson BM. Human Embryology and Developmental Biology. Mosby; St. Louis: 1999. [Google Scholar]
- Carta L, Sassoon D. Wnt7a is a suppressor of cell death in the female reproductive tract and is required for postnatal and estrogen-mediated growth. Biology of reproduction. 2004;71:444–454. doi: 10.1095/biolreprod.103.026534. [DOI] [PubMed] [Google Scholar]
- Castellsague X, Diaz M, de Sanjose S, Munoz N, Herrero R, Franceschi S, Peeling RW, Ashley R, Smith JS, Snijders PJ, Meijer CJ, Bosch FX. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. Journal of the National Cancer Institute. 2006;98:303–315. doi: 10.1093/jnci/djj067. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay I, Cruickshan DJ, Packer M. Non diethylstilbesterol induced vaginal adenosis--a case series and review of literature. European journal of gynaecological oncology. 2001;22:260–262. [PubMed] [Google Scholar]
- Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. British journal of cancer. 2003;88:63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T. Pesticides--how research has succeeded and failed to translate science into policy: endocrinological effects on wildlife. Environ Health Perspect. 1995;103(Suppl 6):81–85. doi: 10.1289/ehp.95103s681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR. The dual origin of vaginal epithelium. The American journal of anatomy. 1975;143:387–392. doi: 10.1002/aja.1001430309. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Stromal induction and specification of morphogenesis and cytodifferentiation of the epithelia of the Müllerian ducts and urogenital sinus during development of the uterus and vagina in mice. The Journal of experimental zoology. 1976;196:361–370. doi: 10.1002/jez.1401960310. [DOI] [PubMed] [Google Scholar]
- Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH, Oren M, Jetten AM. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 2004;287:C171–181. doi: 10.1152/ajpcell.00226.2003. [DOI] [PubMed] [Google Scholar]
- Darwiche N, Celli G, Sly L, Lancillotti F, De Luca LM. Retinoid status controls the appearance of reserve cells and keratin expression in mouse cervical epithelium. Cancer Res. 1993;53:2287–2299. [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development (Cambridge, England) 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Del Vecchio FR. Development of the caudal portions of the Mullerian ducts in the rat (Rattus norvegicus) Acta Anat (Basel) 1982;113:235–245. [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews U. Helper function of the Wolffian ducts and role of androgens in the development of the vagina. Sex Dev. 2007;1:100–110. doi: 10.1159/000100031. [DOI] [PubMed] [Google Scholar]
- Drews U, Sulak O, Schenck PA. Androgens and the development of the vagina. Biology of reproduction. 2002;67:1353–1359. doi: 10.1095/biolreprod67.4.1353. [DOI] [PubMed] [Google Scholar]
- Ferris DG. Anatomy and Histology of Normal Female Lower Genital Tract. In: Ferris DG, Cox JT, O’Connor DM, Wright VC, Foerster J, editors. Modern Colposcopy: Textbook and Atlas. Kendall Hunt; Dubuque: 2004. pp. 13–40. [Google Scholar]
- Fliegner JRH. Vaginal Agenesis and the Embryology of Vaginal Epithelium. The Australian & New Zealand journal of obstetrics & gynaecology. 1994;34:562–566. doi: 10.1111/j.1479-828x.1994.tb01273.x. [DOI] [PubMed] [Google Scholar]
- Forsberg JG. The institute of Anatomy. LUND; 1963. Derivation and differentiation of the vaginal epithelium; p. 197. [Google Scholar]
- Forsberg JG. An experimental approach to the problem of the derivation of the vaginal epithelium. Journal of embryology and experimental morphology. 1965;14:213–222. [PubMed] [Google Scholar]
- Forsberg JG. Cervicovaginal epithelium: its origin and development. American journal of obstetrics and gynecology. 1973;115:1025–1043. doi: 10.1016/0002-9378(73)90687-x. [DOI] [PubMed] [Google Scholar]
- Forsberg JG. Animal model of human disease: adenosis and clear-cell carcinomas of vagina and cervix. The American journal of pathology. 1976;84:669–672. [PMC free article] [PubMed] [Google Scholar]
- Forsberg JG. Development of the human vaginal epithelium. In: Hafez ESE, Evans TN, editors. The Human Vagina. North-Holland; New York: 1978. pp. 3–19. [Google Scholar]
- Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D, Jeong JW, Lydon JP, Bagchi IC, Bagchi MK, Demayo FJ. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010 doi: 10.1096/fj.10-175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutiger P. On the early development of the ductus paramesonephricus and the Mullerian capsule in man. Acta anatomica. 1969;72:233–245. [PubMed] [Google Scholar]
- Gilbert SF. Developmental Biology. Sinauer Associates; Sunderland: 2003. [Google Scholar]
- Gitlin G. On the mode of development of stratified squamous epithelium in the rat’s uterus following prolonged estrogen administration. The Anatomical record. 1954;120:637–661. doi: 10.1002/ar.1091200308. [DOI] [PubMed] [Google Scholar]
- Giusti RM, Iwamoto K, Hatch EE. Diethylstilbestrol revisited: a review of the long-term health effects. Annals of internal medicine. 1995;122:778–788. doi: 10.7326/0003-4819-122-10-199505150-00008. [DOI] [PubMed] [Google Scholar]
- Goto K, Takeuchi Y, Yakihara A, Kotsuji F. Synchronous invasive squamous cell carcinoma and clear cell adenocarcinoma of the uterine cervix: a different human papillomavirus status. Gynecologic oncology. 2005;97:976–979. doi: 10.1016/j.ygyno.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes & development. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünwald P. The relation of the growing tip of the Müllerian duct to the Wolffian duct and its importance for the genesis of malformations. The Anatomical record. 1941;81:1–19. [Google Scholar]
- Guioli S, Sekido R, Lovell-Badge R. The origin of the Müllerian duct in chick and mouse. Developmental biology. 2007;302:389–398. doi: 10.1016/j.ydbio.2006.09.046. [DOI] [PubMed] [Google Scholar]
- Hart DB. Preliminary Note on the Development of the Clitoris, Vagina, and Hymen. J Anat Physiol. 1896;31:18–28. 11. [PMC free article] [PubMed] [Google Scholar]
- Hart DB. Morphology of the Human Urinogenital Tract. J Anat Physiol. 1901;35:330–375. [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R. Development of the human Müllerian duct in the sexually undifferentiated stage. Anat Rec A Discov Mol Cell Evol Biol. 2003;272:514–519. doi: 10.1002/ar.a.10061. [DOI] [PubMed] [Google Scholar]
- Hatch EE, Palmer JR, Titus-Ernstoff L, Noller KL, Kaufman RH, Mittendorf R, Robboy SJ, Hyer M, Cowan CM, Adam E, Colton T, Hartge P, Hoover RN. Cancer risk in women exposed to diethylstilbestrol in utero. JAMA: the journal of the American Medical Association. 1998;280:630–634. doi: 10.1001/jama.280.7.630. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Erikson DW, Tilford SA, Bany BM, Maclean JA, 2nd, Rucker EB, 3rd, Johnson GA, Spencer TE. Wnt genes in the mouse uterus: potential regulation of implantation. Biology of reproduction. 2009;80:989–1000. doi: 10.1095/biolreprod.108.075416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst AL. Behavior of estrogen-associated female genital tract cancer and its relation to neoplasia following intrauterine exposure to diethylstilbestrol (DES) Gynecologic oncology. 2000;76:147–156. doi: 10.1006/gyno.1999.5471. [DOI] [PubMed] [Google Scholar]
- Herbst AL, Poskanzer DC, Robboy SJ, Friedlander L, Scully RE. Prenatal exposure to stilbestrol. A prospective comparison of exposed female offspring with unexposed controls. The New England journal of medicine. 1975;292:334–339. doi: 10.1056/NEJM197502132920704. [DOI] [PubMed] [Google Scholar]
- Herbst AL, Scully RE. Adenocarcinoma of the vagina in adolescence. A report of 7 cases including 6 clear-cell carcinomas (so-called mesonephromas) Cancer. 1970;25:745–757. doi: 10.1002/1097-0142(197004)25:4<745::aid-cncr2820250402>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. The New England journal of medicine. 1971;284:878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee HS, Franco HL, Broaddus RR, Taketo MM, Tsai SY, Lydon JP, DeMayo FJ. beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene. 2009;28:31–40. doi: 10.1038/onc.2008.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LD, Driscoll SG, Hertig AT, Cole PT, Nickerson RJ. Vaginal adenosis in stillborns and neonates exposed to diethylstilbestrol and steroidal estrogens and progestins. Obstetrics and gynecology. 1979;53:671–679. [PubMed] [Google Scholar]
- Josso N, di Clemente N, Gouedard L. Anti-Mullerian hormone and its receptors. Molecular and cellular endocrinology. 2001;179:25–32. doi: 10.1016/s0303-7207(01)00467-1. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet. 2003;4:969–980. doi: 10.1038/nrg1225. [DOI] [PubMed] [Google Scholar]
- Koff AK. Development of the vagina in the human fetus. Contrib Embryol. 1933;24:59–91. [PubMed] [Google Scholar]
- Komatsu M, Fujita H. Electron-microscopic studies on the development and aging of the epithelium of mice. Anat Embryol (Berl) 1978;152:243–259. doi: 10.1007/BF00350523. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nature genetics. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- Kurita T. Developmental origin of vaginal epithelium. Differentiation; research in biological diversity. 2010;80:99–105. doi: 10.1016/j.diff.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (Müllerian) epithelial differentiation. Developmental biology. 2001;240:194–211. doi: 10.1006/dbio.2001.0458. [DOI] [PubMed] [Google Scholar]
- Kurita T, Cunha GR. Roles of p63 in differentiation of Müllerian duct epithelial cells. Annals of the New York Academy of Sciences. 2001;948:9–12. doi: 10.1111/j.1749-6632.2001.tb03982.x. [DOI] [PubMed] [Google Scholar]
- Kurita T, Cunha GR, Robboy SJ, Mills AA, Medina RT. Differential expression of p63 isoforms in female reproductive organs. Mechanisms of development. 2005;122:1043–1055. doi: 10.1016/j.mod.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee K, Cooke PS, Taylor JA, Lubahn DB, Cunha GR. Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biology of reproduction. 2000;62:821–830. doi: 10.1093/biolreprod/62.4.821. [DOI] [PubMed] [Google Scholar]
- Kurita T, Mills AA, Cunha GR. Roles of p63 in the diethylstilbestrol-induced cervicovaginal adenosis. Development (Cambridge, England) 2004;131:1639–1649. doi: 10.1242/dev.01038. [DOI] [PubMed] [Google Scholar]
- Kurita T, Nakamura H. Embryology of the uterus. In: Aplin JD, Fazleabas AT, Glasser SR, Giudice LC, editors. Endometrium. Informa UK Ltd; London, UK: 2008. pp. 1–18. [Google Scholar]
- Kurman RJ, Scully RE. The incidence and histogenesis of vaginal adenosis. An autopsy study. Human pathology. 1974;5:265–276. doi: 10.1016/s0046-8177(74)80111-5. [DOI] [PubMed] [Google Scholar]
- Liebrich C, Brummer O, Von Wasielewski R, Wegener G, Meijer C, Iftner T, Petry KU. Primary cervical cancer truly negative for high-risk human papillomavirus is a rare but distinct entity that can affect virgins and young adolescents. European journal of gynaecological oncology. 2009;30:45–48. [PubMed] [Google Scholar]
- Mauch RB, Thiedemann KU, Drews U. The vagina is formed by downgrowth of Wolffian and Müllerian ducts. Graphical reconstructions from normal and Tfm mouse embryos. Anat Embryol (Berl) 1985;172:75–87. doi: 10.1007/BF00318946. [DOI] [PubMed] [Google Scholar]
- McEuen CS. Metaplasia of uterine epithelium produced in rats by prolonged administration of oestrin. Am J Cancer. 1936;27:91–94. doi: 10.1158/ajc.1936.91. [DOI] [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, Bullock BC. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 1980;40:3988–3999. [PubMed] [Google Scholar]
- Melnick S, Cole P, Anderson D, Herbst A. Rates and risks of diethylstilbestrol-related clear-cell adenocarcinoma of the vagina and cervix. An update. The New England journal of medicine. 1987;316:514–516. doi: 10.1056/NEJM198702263160905. [DOI] [PubMed] [Google Scholar]
- Miller C, Pavlova A, Sassoon DA. Differential expression patterns of Wnt genes in the murine female reproductive tract during development and the estrous cycle. Mechanisms of development. 1998;76:91–99. doi: 10.1016/s0925-4773(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Miller C, Sassoon DA. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development (Cambridge, England) 1998;125:3201–3211. doi: 10.1242/dev.125.16.3201. [DOI] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Minaguchi T, Yoshikawa H, Nakagawa S, Yasugi T, Yano T, Iwase H, Mizutani K, Shiromizu K, Ohmi K, Watanabe Y, Noda K, Nishiu M, Nakamura Y, Taketani Y. Association of PTEN mutation with HPV-negative adenocarcinoma of the uterine cervix. Cancer letters. 2004;210:57–62. doi: 10.1016/j.canlet.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Moll R, Levy R, Czernobilsky B, Hohlweg-Majert P, Dallenbach-Hellweg G, Franke WW. Cytokeratins of normal epithelia and some neoplasms of the female genital tract. Laboratory investigation; a journal of technical methods and pathology. 1983;49:599–610. [PubMed] [Google Scholar]
- Moore KL, Persaud TVN. The Urogenital System. In: Moore KL, Persaud TVN, editors. The Developing Human: Clinically Oriented Embryology. W B Saunders Co; Philadelphia: 2002. pp. 287–328. [Google Scholar]
- Morris JM. The syndrome of testicular feminization in male pseudohermaphrodites. American journal of obstetrics and gynecology. 1953;65:1192–1211. doi: 10.1016/0002-9378(53)90359-7. [DOI] [PubMed] [Google Scholar]
- Nakamuta N, Kobayashi S. Developmental expression of p63 in the mouse testis. J Vet Med Sci. 2004a;66:681–687. doi: 10.1292/jvms.66.681. [DOI] [PubMed] [Google Scholar]
- Nakamuta N, Kobayashi S. Expression of p63 in the mouse primordial germ cells. J Vet Med Sci. 2004b;66:1365–1370. doi: 10.1292/jvms.66.1365. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Jefferson WN, Padilla-Banks E. Prenatal exposure to bisphenol a at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ Health Perspect. 2009;117:879–885. doi: 10.1289/ehp.0800045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR, McLachlan JA. Vaginal adenosis and adenocarcinoma in mice exposed prenatally or neonatally to diethylstilbestrol. Cancer Res. 1982;42:2003–2011. [PubMed] [Google Scholar]
- O’Rahilly R. The embryology and anatomy of the uterus. In: Wynn RM, editor. The Uterus. The Williams & Wilkins Co; Baltimore: 1973. pp. 17–39. [Google Scholar]
- Orvis GD, Behringer RR. Cellular mechanisms of Müllerian duct formation in the mouse. Developmental biology. 2007;306:493–504. doi: 10.1016/j.ydbio.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr BA, Avery EJ, Cygan JA, McMahon AP. The classical mouse mutant postaxial hemimelia results from a mutation in the Wnt 7a gene. Developmental biology. 1998;202:228–234. doi: 10.1006/dbio.1998.9007. [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature. 1998;395:707–710. doi: 10.1038/27221. [DOI] [PubMed] [Google Scholar]
- Petre-Lazar B, Livera G, Moreno SG, Trautmann E, Duquenne C, Hanoux V, Habert R, Coffigny H. The role of p63 in germ cell apoptosis in the developing testis. Journal of cellular physiology. 2007;210:87–98. doi: 10.1002/jcp.20829. [DOI] [PubMed] [Google Scholar]
- Petre-Lazar B, Moreno SG, Livera G, Duquenne C, Habert R, Coffigny H. p63 expression pattern in foetal and neonatal gonocytes after irradiation and role in the resulting apoptosis by using p63 knockout mice. Int J Radiat Biol. 2006;82:771–780. doi: 10.1080/09553000600960019. [DOI] [PubMed] [Google Scholar]
- Pirog EC, Kleter B, Olgac S, Bobkiewicz P, Lindeman J, Quint WG, Richart RM, Isacson C. Prevalence of human papillomavirus DNA in different histological subtypes of cervical adenocarcinoma. The American journal of pathology. 2000;157:1055–1062. doi: 10.1016/S0002-9440(10)64619-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plapinger L, Bern HA. Adenosis-like lesions and other cervicovaginal abnormalities in mice treated perinatally with estrogen. Journal of the National Cancer Institute. 1979;63:507–518. [PubMed] [Google Scholar]
- Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev. 1995;16:271–321. doi: 10.1210/edrv-16-3-271. [DOI] [PubMed] [Google Scholar]
- Robboy SJ, Hill EC, Sandberg EC, Czernobilsky B. Vaginal adenosis in women born prior to the diethylstilbestrol era. Human pathology. 1986;17:488–492. doi: 10.1016/s0046-8177(86)80039-9. [DOI] [PubMed] [Google Scholar]
- Robboy SJ, Young RH, Welch WR, Truslow GY, Prat J, Herbst AL, Scully RE. Atypical vaginal adenosis and cervical ectropion. Association with clear cell adenocarcinoma in diethylstilbestrol-exposed offspring. Cancer. 1984;54:869–875. doi: 10.1002/1097-0142(19840901)54:5<869::aid-cncr2820540519>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Russell DL. Embryology. In: Kaufman RH, Friedrich EGJ, Gardner HL, editors. Benign Diseases of the Vulva and Vagina. Year Book Medical Publishers; Chicago: 1989. pp. 16–25. [Google Scholar]
- Sadler TW. Urogenital System. Langman’s Medical Embryology. 2004:321–362. [Google Scholar]
- Saito K, Kawakami S, Okada Y, Takazawa R, Koga F, Kageyama Y, Kihara K. Spatial and isoform specific p63 expression in the male human urogenital tract. The Journal of urology. 2006;176:2268–2273. doi: 10.1016/j.juro.2006.07.057. [DOI] [PubMed] [Google Scholar]
- Sajjad Y. Development of the genital ducts and external genitalia in the early human embryo. J Obstet Gynaecol Res. 2010;36:929–937. doi: 10.1111/j.1447-0756.2010.01272.x. [DOI] [PubMed] [Google Scholar]
- Sánchez-Ferrer ML, Acien MI, Sanchez del Campo F, Mayol-Belda MJ, Acien P. Experimental contributions to the study of the embryology of the vagina. Human reproduction (Oxford, England) 2006;21:1623–1628. doi: 10.1093/humrep/del031. [DOI] [PubMed] [Google Scholar]
- Selye H, Thomson DL, Collip JB. Metaplasia of Uterine Epithelium Produced by Chronic Œstrin Administration. Nature. 1935;135:65–66. [Google Scholar]
- Shapiro E, Huang HY, Wu XR. Uroplakin and androgen receptor expression in the human fetal genital tract: insights into the development of the vagina. The Journal of urology. 2000;164:1048–1051. doi: 10.1097/00005392-200009020-00031. [DOI] [PubMed] [Google Scholar]
- Sherman AI, Goldrath M, Berlin A, Vakhariya V, Banooni F, Michaels W, Goodman P, Brown S. Cervical-vaginal adenosis after in utero exposure to synthetic estrogens. Obstetrics and gynecology. 1974;44:531–545. [PubMed] [Google Scholar]
- Signoretti S, Waltregny D, Dilks J, Isaac B, Lin D, Garraway L, Yang A, Montironi R, McKeon F, Loda M. p63 is a prostate basal cell marker and is required for prostate development. The American journal of pathology. 2000;157:1769–1775. doi: 10.1016/S0002-9440(10)64814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, 3rd, Bevans-Wilkins K, Ye C, Kurtycz DF. Clear-cell endocervical adenocarcinoma in a 19-year-old woman. Diagn Cytopathol. 2006;34:839–842. doi: 10.1002/dc.20569. [DOI] [PubMed] [Google Scholar]
- Teixeira J, Maheswaran S, Donahoe PK. Müllerian inhibiting substance: an instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr Rev. 2001;22:657–674. doi: 10.1210/edrv.22.5.0445. [DOI] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development (Cambridge, England) 1995;121:4057–4065. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- Ulfelder H, Robboy SJ. The embryologic development of the human vagina. American journal of obstetrics and gynecology. 1976;126:769–776. doi: 10.1016/0002-9378(76)90666-9. [DOI] [PubMed] [Google Scholar]
- Verloop J, van Leeuwen FE, Helmerhorst TJ, van Boven HH, Rookus MA. Cancer risk in DES daughters. Cancer causes & control: CCC. 2010;21:999–1007. doi: 10.1007/s10552-010-9526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shackleford GM. Murine Wnt10a and Wnt10b: cloning and expression in developing limbs, face and skin of embryos and in adults. Oncogene. 1996;13:1537–1544. [PubMed] [Google Scholar]
- Witschi E. Development and differentiation of the uterus. In: Mack HC, editor. Prenatal Life. Wayne State University Press; Detroit: 1970. pp. 11–34. [Google Scholar]
- Wolbach SB, Howe PR. Tissue Changes Following Deprivation of Fat-Soluble a Vitamin. J Exp Med. 1925;42:753–777. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, McKeon F. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- Yin Y, Ma L. Development of the mammalian female reproductive tract. J Biochem (Tokyo) 2005;137:677–683. doi: 10.1093/jb/mvi087. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Fujino A, MacLaughlin DT, Manganaro TF, Szotek PP, Arango NA, Teixeira J, Donahoe PK. Müllerian inhibiting substance regulates its receptor/SMAD signaling and causes mesenchymal transition of the coelomic epithelial cells early in Müllerian duct regression. Development (Cambridge, England) 2006;133:2359–2369. doi: 10.1242/dev.02383. [DOI] [PubMed] [Google Scholar]