Abstract
Background
Survival following human immunodeficiency virus (HIV) infection has improved significantly following the advent of highly active antiretroviral therapy. A large percentage of HIV-infected patients consume and abuse alcohol. Erosion of lean body mass is an important contributing factor to patient morbidity and mortality, and is a common feature of both chronic alcohol (ALC) consumption and acquired immunodeficiency syndrome (AIDS). We hypothesized that alcohol-induced loss in lean body mass is likely to exacerbate the AIDS wasting syndrome, particularly at the terminal stage of AIDS (SAIDS).
Methods
This study examined the impact of chronic, intra-gastric ALC (5 h/d × 4 d/wk; blood alcohol levels = 55 mM to 60 mM) administration on body composition and muscle mass in simian immunodeficiency virus (SIV)-infected male Rhesus macaques in contrast to SIV-infected isocaloric (22 kcal/kg/d) sucrose (SUC)-infused control animals at the terminal stage of SIV infection.
Results
At terminal stage, ALC/SIV+ animals had significantly lower body weight, body mass index, and limb muscle area than SUC/SIV+ animals. Both ALC/SIV+ and SUC/SIV+ animals had suppressed expression of insulin-like growth factor-I and increased expression of the ubiquitin ligase muscle-specific RING finger-1 mRNA. ALC increased mRNA expression of atrogin- 1 (pre-SIV and at SAIDS) and tumor necrosis factor (TNF)-α (SAIDS). These changes were not associated with significant differences in fractional rates of muscle protein synthesis or in overall survival rate. These data show that chronic ALC exacerbated the loss of muscle mass at terminal SAIDS.
Conclusion
Our findings suggest the involvement of TNF-α and increased muscle proteolysis via atrogin-1 for the greater erosion of lean body mass at terminal SAIDS in ALC-treated Rhesus macaques.
Keywords: Rhesus, Alcohol, Simian Immunodeficiency Virus, Muscle
According to the Center for Disease Control, more than 400,000 people were living with acquired immunodeficiency syndrome (AIDS) in the US at the end of 2003 (CDC, 2004). The substantial reduction in human immunodeficiency virus (HIV) associated morbidity and mortality, resulting from the use of highly active antiretroviral therapy (HAART), has made HIV infection a chronic disease during which individuals are likely to engage in alcohol and drug abuse at rates comparable to the noninfected population (Lefevre et al., 1995). Chronic alcohol consumption remains the most common and costly form of drug abuse with approximately 14 million Americans (about 7 percent of the adult population) fulfilling the diagnostic criteria for alcohol abuse and/or alcoholism (SAMHSA, 2005). Indeed, alcohol abuse and HIV infection frequently co-exist (Lee et al., 2001).
Muscle wasting remains an important determinant of the increased morbidity and mortality observed in AIDS (Grunfeld and Feingold, 1992; Tang et al., 2002; Van Loan et al., 1999; Wanke et al., 2000; Grinspoon and Mulligan, 2003). Chronic alcohol abuse is also associated with skeletal muscle myopathy (Preedy et al., 1994) resulting from decreased muscle protein synthesis (Lang et al., 1999a; Pacy et al., 1991; Reilly et al., 1997) and possibly accelerated muscle proteolysis (Teschner et al., 1988). In addition, indirect effects of alcohol such as alterations in nutritional state, micronutrient availability and growth factor expression have also been implicated in the etiology of the alcohol-induced muscle wasting (Molina et al., 1996; Bergheim et al., 2003; Yeomans et al., 2003). Because the loss of lean body mass (LBM) has a deleterious impact on overall survival of HIV-infected individuals, factors that accelerate this process, such as chronic alcohol consumption, are likely to accelerate the progression of disease and the development of AIDS-associated wasting (Coodley et al., 1994; Suttmann et al., 1995; Thiebaut et al., 2000;Wheeler et al., 1998).
The controlled study of the interaction of alcohol on the pathophysiological mechanisms underlying the progression of disease in HIV patients is confounded by the concomitant use of prescribed and recreational drugs, the stage of infection, comorbid conditions and intercurrent infections (Forrester et al., 2000). Experimental Simian Immunodeficiency Virus (SIV) infection of rhesus macaque results in a disease process that is remarkably similar to human AIDS (Arthur et al., 1986; Baskin et al., 1991, 1992). The disease is characterized by an initial asymptomatic period ultimately progressing to a clinical AIDS stage, making this an optimal animal model to examine the interactions of alcohol and SIV disease progression in a controlled environment. The present study was designed to address the hypothesis that chronic alcohol consumption exacerbates the severity of muscle wasting associated with terminal stage acquired immune deficiency syndrome (SAIDS) in SIV-infection of Rhesus macaques.
MATERIALS AND METHODS
All experiments were approved by the Institutional Animal Care and Use Committee at both the Tulane National Primate Research Center (TNPRC) in Covington, Louisiana, and Louisiana State University Health Sciences Center in New Orleans and adhered to the National Institutes of Health guidelines for the use of experimental animals. Four to six year old male Rhesus monkeys (Macaca mulatta) obtained from the breeding colonies at the TNPRC were studied. Age- and body weight-matched animals were randomized to either: isocaloric sucrose-fed SIV infection (SUC/SIV+) or chronic alcohol-fed with SIV infection (ALC)/SIV+. In all, 27 animals were included in the study, 13 in the SUC/SIV+ group and 14 in the ALC/SIV+ group. Animals were individually housed in a Biosafety Level-2 containment building. Animals were infused intra-gastrically with alcohol daily for 4 consecutive days each week for 3 months prior to intravenous SIV inoculation as previously described (Bagby et al., 2003). Time-matched control animals were infused with isocaloric sucrose. Alcohol and sucrose infusions were continued following SIV inoculation using the same protocol of administration (detailed below).
Animal Selection Criteria
Excellent health of the animals was determined by a) a complete physical exam by a veterinarian, b) a complete blood count (CBC) and serum chemistries, and c) negative serological status for simian retrovirus [confirmed by Genetic amplification of DNA (nested PCR), enzyme immunoassay and Western immunoblot] and simian T-lymphotropic virus-1 (EIA and Western immunoblot) based on assays performed by the Pathogen Detection Laboratory (California National Primate Research Center, Davis, CA). To reduce and refine the use of nonhuman primates in our research project, animals identified as “slow progressors” were selected to provide a more homogeneous cohort of animals. This approach has been used to decrease variability between the experimental groups, and increase the ability to evaluate the impact of alcohol as a co-factor of disease progression in our sample size. Selection was based on low capacity of in vitro infection by SIVDeltaB670 of peripheral blood mononuclear lymphocytes (PBML) (Seman et al., 2000). Monkeys infected with this SIV isolate show variable rates of disease progression to SAIDS. The rate of in vivo disease progression has been correlated to the ability of PBMCs to express reverse transcriptase activity following in vitro infection. Slow progressors were animals that had rates of viral replication by cultured PBML in the lower half of tested animals.
Surgical Procedures, Alcohol Administration, and SIV Infection
Gastric catheter placement for alcohol delivery, the alcohol delivery protocol, and route of SIV infection, have been previously described in detail (Bagby et al., 2003). A primed (2.5 g/kg) constant (160 mg/kg/h) ethanol (30% w/v water) infusion was administered intragastrically over a 5-h period on 4 consecutive days per week. We selected controlled delivery over voluntary alcohol consumption to reduce experimental variability and ensure maximal control of alcohol exposure throughout the duration of SIV infection. This protocol of alcohol administration resulted in sustained intoxicating blood alcohol concentrations between 50 mM and 60 mM throughout the 5-h infusion period. Infusion rates (g ethanol/Kg body weight) were not altered throughout the experimental period, suggesting no significant alteration in rate of alcohol metabolism. Time-matched control monkeys were subjected to the same surgical procedures, but received an isocaloric sucrose infusion. Total calories provided by alcohol and sucrose averaged 15%.
Three months after initiating chronic alcohol administration, animals were inoculated intravenously with 10,000 times the ID50 (50% infective dose) of either SIVΔB670 (n = 12; 7 ALC and 5 SUC) or SIVmac251 (n = 15; 7 ALC and 8 SUC) provided by Drs. LouisMartin and Preston Marx. Intravenous (saphenous) inoculation was performed at the conclusion of an ethanol or sucrose session on the second day of a 4-consecutive day alcohol infusion when blood alcohol levels were elevated to simulate infection during an alcohol-binge. The progression of SIV disease was monitored throughout the study period through clinical and biochemical parameters including CBC, lymphocyte subsets, serum chemistries [total protein, albumin, globulin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase and plasma SIV gagRNA]. Serum chemistries were determined using a CobasMira Chemistry Analyzer (Roche, Rotkreutz, Switzerland) at the Clinical Chemistry Laboratory at the TRPRC. Serum globulins were calculated as the difference between total serum protein and albumin concentrations. Clinical variables monitored included: body weight and rectal temperature, general physical condition, presence of lymphadenopathy, splenomegaly, presence of rashes or skin abnormalities, condition of oral and conjunctival mucosa, diarrhea and signs of opportunistic infection. All parameters were assessed on a nonalcohol day following an overnight fast.
Diet
Animals were provided ad libitum with Monkey chow (Lab Fiber Plus Primate diet-DT, PMI Nutrition International, St. Louis, MO) and supplemented with fruits, vitamins and Noyes’ treats (Research Diets, New Brunswick, NJ).
Experimental Protocol
Animals were studied during the basal period, after an initial 3-month period of alcohol (or isocaloric sucrose) administration prior to SIV infection, and at end-stage SAIDS. End-stage SAIDS was defined according to presence of three or more of the following criteria: weight loss greater than 15%, hypoalbuminemia (< 3 mg/dl) in the presence of edema, anemia and thrombocytopenia, 3 days of complete anorexia, major organ failure or medical conditions unresponsive to treatment (including respiratory distress, intractable diarrhea, or persistent vomiting, secondary infections that required antibiotic treatment), neurological manifestations of disease, severe diarrhea and dehydration. At the time that animals were identified to fit the criteria for end-stage SAIDS they were studied under anesthesia prior to euthanasia and necropsy.
The following anthropometric measures were obtained to the nearest millimeter while animals were anesthetized: crown-rump length, triceps, subscapular, abdominal and thigh skin-fold thickness, waist, hip and thigh circumferences by two independent investigators blinded to treatment protocol. These values were used to calculate body mass index (BMI) as adapted for the use in monkeys (weight, kg/crown-rump length cm2) (Colman et al., 1999; Jayo et al., 1993). In addition, the obtained measures were used to calculate corrected arm and thigh girth (abbreviated CAG and CTG, respectively) as well as an estimate of skeletal muscle mass using arm muscle area based on established formulas used in humans (Heymsfield et al., 1982). As follows:
CAG: Upper arm circumference – π Triceps skin fold thickness
CTG: Thigh circumference – π Thigh skin fold thickness
Arm muscle area: CAG/4π
These calculations are estimates based on the assumptions that 1) mid-arm (and mid-thigh) circumference is circular and includes bone area, 2) the triceps (and thigh) skin fold thickness is twice the average fat rim diameter, and 3) muscle mass of the upper extremity is an overall reflection of total body muscle mass.
In Vivo Measure of Muscle Protein Synthesis
The rate of muscle protein synthesis was assessed using the “flooding-dose” technique as previously described (Garlick et al., 1989). None of the animals were studied while intoxicated to avoid possible confounding acute effects of alcohol (Lang et al., 1999a). Animals were sedated with Ketamine and lightly anesthetized with Isoflurane (1.5%), and intravenous catheters were placed in the saphenous and antecubital veins for blood sampling and for isotope infusion, respectively. Monkeys were injected (over a 10-min period) with L-[2H5] phenylalanine combined with unlabeled amino acid (180 mg/kg; Ajinomoto, Tokyo, Japan) so that enrichment was ~15%. Blood samples for precursor enrichment determination were collected at 0, 5, 15, 45 and 90 minutes. Muscle biopsies (3 to 4) were obtained from the right quadriceps using a Max Core® (BARD, Murray Hill, NJ) biopsy needle at the 90 minutes time point and immediately frozen in liquid nitrogen and stored at −80°C until analysis.
The determination of L-[2H5]phenylalanine enrichment in both plasma samples and in hydrolyzed tissue (muscle) protein was made by gas chromatography-mass spectrometry (GC/MS) under electron impact ionization and selective ion recording (EI-SIR) as previously described (Hashiguchi et al., 1997). Fractional rates of protein synthesis were calculated by: kS = [(P × 100) / (F × t)], where kS is the % of the tissue protein pool (mixed muscle protein) renewed daily by synthesis (% per day), P is the enrichment of phenylalanine in tissue protein (atom % excess), F is the plasma phenylalanine enrichment (atom % excess) over the time interval (0 minutes to 90 minutes) after phenylalanine injection, and t is the period of incorporation (days) (Garlick et al., 1989).
Hematology and Lymphocyte Subset Analysis
Complete and differential blood counts were performed in the Clinical Laboratory at the TRPRC using a Coulter counter for total leukocyte counts and Wright-Giemsa staining of blood smears for leukocyte differentials. Blood lymphocyte subsets were determined as previously described (Stoltz et al., 2000) using fluorochrome (fluorescein and phycoerythrin) conjugated monoclonal antibodies against phenotypic cell surface antigens followed by flow cytometric analysis, as previously described (Molina et al., 2006).
Real Time Polymerase Chain Reaction Assay for Simian Immunodeficiency Virus-RNA
Plasma SIV copy number was measured by a real-time RTPCR assay using gag primers and probe as previously described (Suryanarayana et al., 1998) and adopted by our core laboratories (Bagby et al., 2003). Viral RNA was isolated from 500 µl of plasma using the Purescript kit according to manufacturer’s instructions in a final volume of 80 µl (Gentra Systems, Minneapolis, MN). RT-PCR reaction was performed in triplicate with 15 µl of the isolated RNA in 30 µl and then 25 µl of cDNA product used as template in the PCR reaction. The gag probe was double-labeled with a reporter fluorochrome, 6-carboxy-fluorescein, and a quencher fluorochrome, 6-carboxy-tetramethyl rhodamine. Fluorescence was measured with a PE Applied Biosystems GeneAmp 5700 (Applied Biosystems, Foster City, CA) in each of 55 cycles. The normalized fluorescence parameter, ΔRn, was calculated by integrated software. ΔRn was plotted as a function of amplification cycle, and the cycle at which fluorescence is greater than background, or threshold cycle (CT), was determined. A ΔB670 gag clone was generated by RT-PCR of SIVΔB670 using primers 5′gcgcgagaaactccgtcttgt and 5′gcactatttgttttgcttcctcag, and used as standard. The standard RT-PCR was run for 40 cycles in triplicate at 10-fold dilutions from a copy number of 10 to 107. Plasma samples were measured in triplicate and copy number was determined from the standard curve. Flow cytometry analysis and SIV mRNA quantifications were performed by the LSUHSC Alcohol Research Center Core Laboratory.
Muscle Cytokine, Insulin-Like Growth Factor-I, Myostatin, Muscle-Specific RING Finger-1 and Atrogin-1/MAFbx Expression
Quadriceps muscle samples were used for analysis of gene expression of myostatin (a negative regulator of muscle growth), ubiquitin ligases muscle-specific RING finger-1 (MuRF-1) and atrogin-1, proinflammatory cytokines [tumor necrosis factor (TNF) and interleukin 6 (IL-6)], and insulin-like growth factor-I (IGF-I). Muscle samples were frozen in liquid nitrogen and stored at −80°C until analyzed. Frozen muscle biopsies were homogenized and total RNAs were isolated using RNeasy Mini Kits from Qiagen (Valencia, CA). The quantity and purity of RNA were determined by OD260 and OD280 measurements.
Real Time qRT-PCR
Total RNAs were reverse transcribed with a Superscipt™ First Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA) using random hexamers and 1.5 µg of each RNA. No-template and no-amplification controls were included. Quantitative PCR was performed for 40 cycles using Quantitect™ SYBR Green PCR Kits (Qiagen) with final PCR primer concentrations of 300 nm in a reaction volume of 50 µl. Primers were designed to span exon/intron junctions or to lie on separate exons (Giegerich et al., 1996). Areas of known variation were avoided. Reactions were done in triplicate. PCR analyses were performed using an ABI Prism 7700 Sequence Detection System (Applied Biosystems) by the Functional Genomics Core Facility at Penn State College of Medicine. Amplification annealing temperatures were 58°C for TNF-α, 59°C for myostatin, and 60°C for all other genes. Melting curve analyses were performed to assess specificity of the PCR products.
After PCR was completed, baseline and threshold levels were adjusted using the ABI Prism 7700 software and CT values were determined. Care was taken to ensure that threshold levels were clearly set within the log-linear range of all amplification plots. Ribosomal 18S RNA content was quantified as an endogenous control against which each gene was then normalized. Due to varying amplification efficiencies between PCR primer sets, individual standard curves were generated for each gene as well as 18S. The relative standard curve method was then used to analyze the data, with relative amounts of unknown samples being calculated using linear regression analysis. The relative values for each gene were then divided by the 18S values and the control group was set as the calibrator, a value of 1.0.
Data Analysis
All data are presented as mean ± SEM for n=14 to 16 in each experimental group. Treatment (SIV and alcohol) and time (pre-SIV and SAIDS) effects were established using two-way repeated measures analysis of variance followed by pair-wise multiple comparison procedures (Holm-Sidak method) when indicated. Survival data were analyzed using the LIFETEST procedure. Statistical significance was set at p < 0.05.
RESULTS
Viral Load, CD4+/CD8+, and Survival
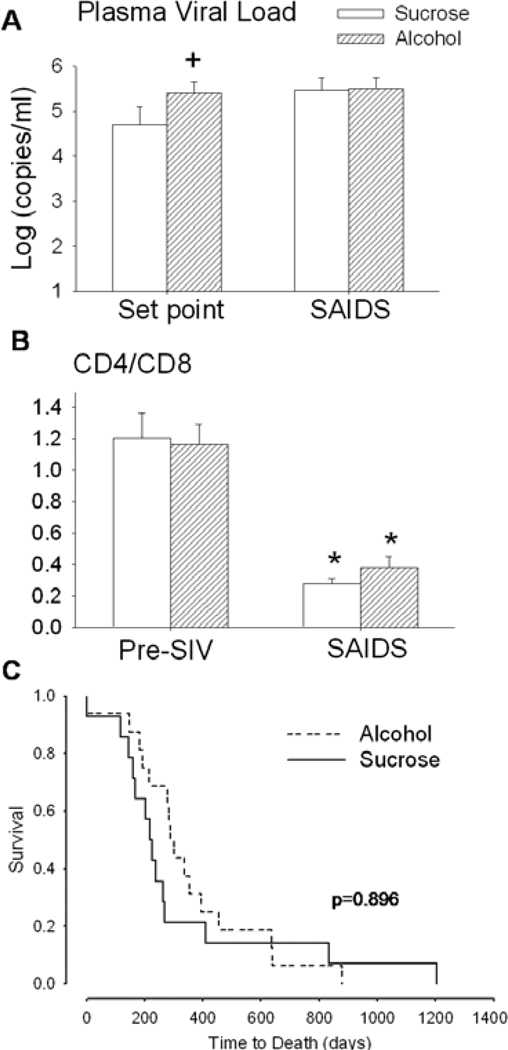
Viral load at set point, defined as the viral load established during the 13 to 15-wk period following the initial acute infection, was significantly higher in alcohol-treated compared to sucrose-treated SIV-infected animals Fig. 1A). However, at end-stage, no significant difference in viral load was detected between the two groups. The ratio of CD4+ to CD8+ lymphocytes was similar in sucrose- and alcohol-treated animals pre-SIV infection. Both sucrose-and alcohol-treated animals showed a significant decline in the CD4+ to CD8+ lymphocyte ratio at end-stage, which was comparable in magnitude (Fig. 1B). No significant difference was observed in time to death between sucroseand alcohol-treated animals.
Fig. 1.
(A) Plasma viral load at set point and at end stage disease (SAIDS) in sucrose/simian immunodeficiency virus (SIV) positive (open bars) and alcohol/SIV positive (hatched bars) animals. (B) CD4+/CD8+ lymphocyte ratio in sucrose- (open bars) and alcohol- (hatched bars) treated rhesus macaques prior to SIV infection (pre-SIV) and at SAIDS. (C) Time to death (days) from day of SIV infection in sucrose (solid lines) and alcohol (dotted lines) treated rhesus macaques. Values are means ± SEM, n = 13 to 14 in each group. *p < 0.05 versus basal values, +p < 0.05 versus time-matched sucrose/SIV positive.
Serum Chemistries
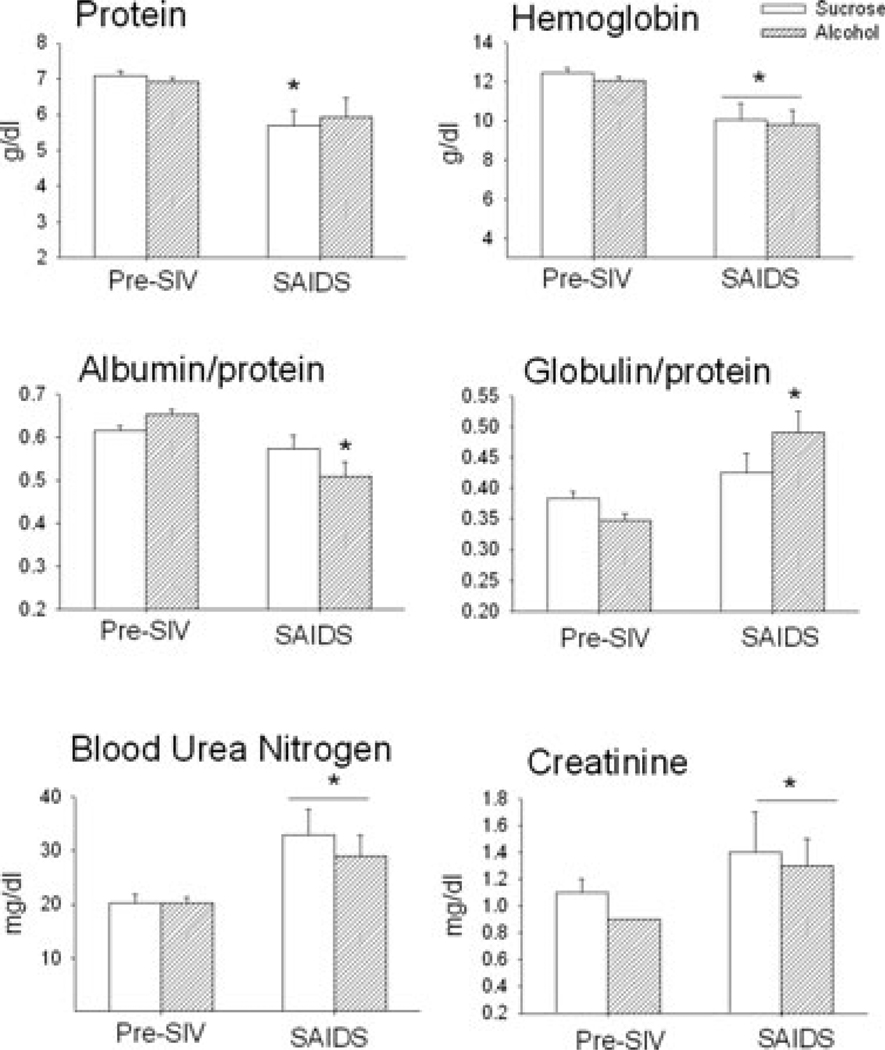
Basal values for protein (7.1 ± 0.1 g/dl), albumin (4.4 ± 0.1 g/dl), globulin (2.7 ± 0.1 g/dl), hemoglobin (12.4 ± 0.4 g/dl), blood urea nitrogen (BUN; 20 ± 2 mg/dl), and creatinine (1.1 ± 0.1 mg/dl) were similar in sucrose- and alcohol-treated animals during the baseline pre- SIV period (Fig. 2). Three months of alcohol administration alone did not alter these variables. At end-stage SAIDS, sucrose-treated SIV-infected animals had significantly decreased total plasma protein, albumin, and hemoglobin and a marked increase in BUN and creatinine levels. Similarly, alcohol-treated SIV-infected animals, had significantly lower protein, albumin (data not shown), and hemoglobin levels from those measured pre-SIV infection, as well as elevated BUN and creatinine levels at end-stage. No differences in total plasma protein were detected in alcohol-treated SIV infected animals. However, the albumin to protein ratio was significantly decreased in alcohol-treated SIV infected animals at end-stage SAIDS, while that of sucrose-treated animals was not significantly altered from baseline values (Fig. 2). Furthermore, alcohol-treated animals had an increased globulin to protein ratio which was not observed in sucrose-treated SIV-infected animals.
Fig. 2.
Serum values for protein (g/dl), hemoglobin (g/dl), albumin to protein and globulin to protein ratios, blood urea nitrogen (mg/dl) and creatinine (mg/dl) in sucrose (open bars) and alcohol (hatched bars) treated rhesus macaques during baseline pre-SIV period and at end-stage disease (SAIDS). Values are means ± SEM, n = 13 to 14 in each group. *p < 0.05 versus basal values, +p < 0.05 versus time-matched sucrose/simian immunodeficiency virus (SIV) positive.
The mean basal circulating concentrations of markers of liver function, ALT (30 ± 3 U/l) and AST (41 ± 4 U/l) were not significantly different between sucrose- and alcohol-treated animals at basal nor were they altered at end-stage SAIDS in either sucrose-treated or alcohol-treated SIV infected animals (data not shown).
Anthropometric Measurements
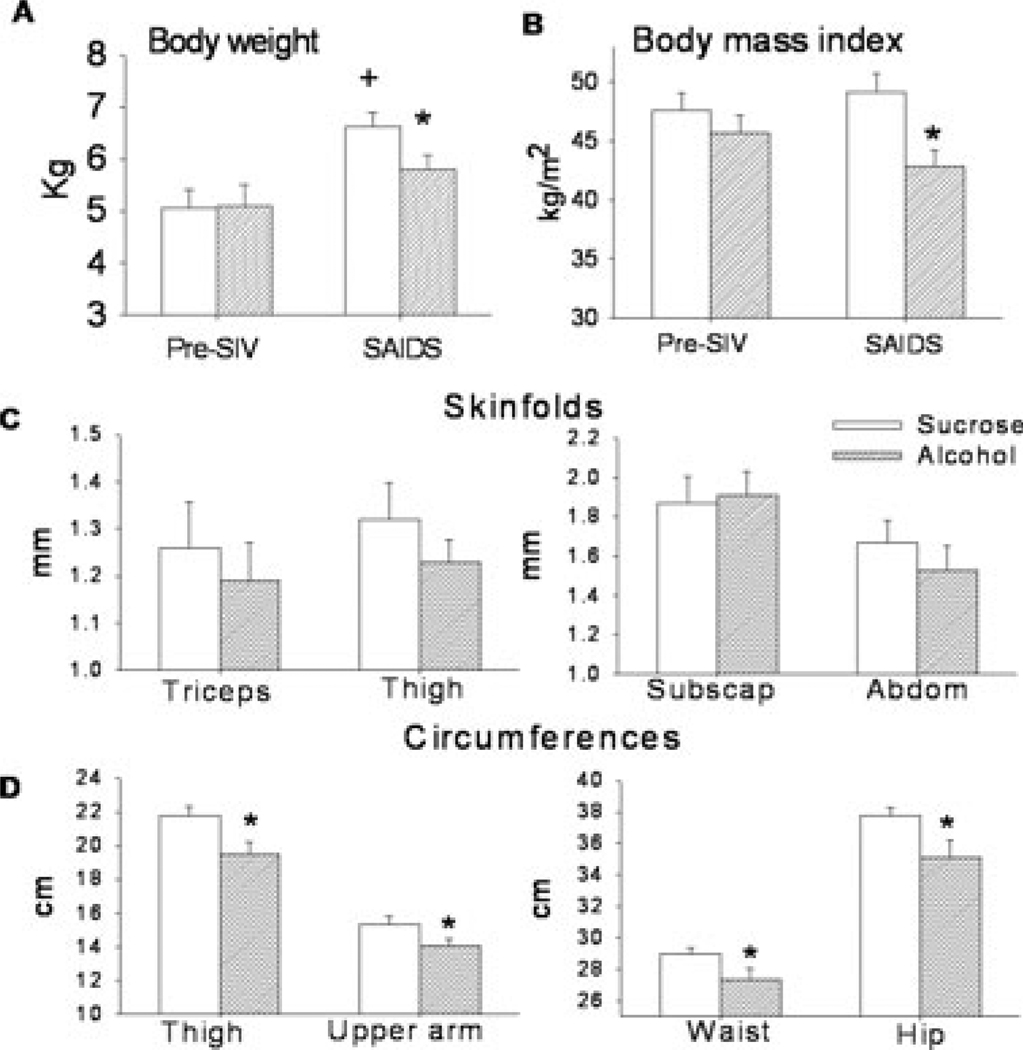
There were no significant differences in the anthropometric measures of sucrose- and alcohol-treated animals determined during the basal pre-SIV period (Table 1). When animals reached end-stage SAIDS, body weight had increased an average of 1.6 ± 0.3 kg from pre-SIV in sucrose-fed animals (Fig. 3A). In contrast, alcohol-treated SIV infected animals demonstrated 70% less weight gain (0.52 ± 0.25 kg) during the course of disease. Body weight of alcohol-treated SIV-infected animals averaged 5.6 ± 0.3 kg at end-stage and this was significantly lower (p < 0.05) than that of sucrose-treated SIV infected animals (6.6 ± 0.3 kg). Body mass index (BMI) was not significantly different at end stage SAIDS (49.1 ± 1.6 kg/m2) from that determined pre-SIV in sucrose-treated animals. In contrast, BMI of alcohol-treated animals was significantly lower than that of sucrose-treated SIV-infected animals (42.8 ± 1.4 kg/m2) at end-stage SAIDS (Fig. 3B). No differences in skin-fold thickness determined in the triceps, subscapular, abdominal and thigh regions were noted in the sucrose- and alcohol-treated SIV-infected animals (Fig. 3C). However, significantly lower circumferences were determined in waist, hip, thigh and upper arm in the alcohol-treated animals when compared to sucrose-treated SIV-infected animals at end-stage SAIDS (Fig. 3D). No differences in waist to hip ratio measured in sucrose-(0.77 ± 0.01) and alcohol-treated (0.77 ± 0.01) animals were noted at the end-stage SAIDS (data not shown).
Table 1.
Anthropometric Measures Obtained During the Basal Pre-simian Immunodeficiency Virus (SIV) Infection Period in Sucrose- and Alcohol-Treated Animals
| Skinfold (mm) | Circumference (cm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Tric. | Sub-SC | Abdom | Thigh | Waist | Hip | Thigh | Arm | |
| Sucrose | 1.1 ± 0.1 | 2.2 ± 0.1 | 1.8 ± 0.1 | 1.3 ± 0.1 | 27.1 ± 0.9 | 32.9 ± 1.1 | 20.3 ± 0.88 | 14.1 ± 0.5 |
| Alcohol | 1.2 ± 0.1 | 2.1 ± 0.1 | 1.7 ± 0.1 | 1.3 ± 0.1 | 26.7 ± 0.9 | 32.5 ± 1.3 | 20.4 ± 0.8 | 14.2 ± 0.6 |
Values are average ± SEM, n = 16 in each group expressed as millimeters for skinfolds and centimeters for circumferences. Tric., triceps; sub-sc, subscapular; abdom, abdominal.
Fig. 3.
(A) Body weight (Kg), (B) estimates of body mass index (kg/m2), (C) skinfold thickness (triceps, thigh, subscapular and abdominal) expressed in millimeters, (D) circumferences (thigh, upper arm, waist and hip) expressed in centimeters, obtained pre-SIV infection and at end stage (SAIDS) in sucrose-SIV infected (open bars) and alcohol-treated SIV infected rhesus macaques (hatched bars). Values are means ± SEM, n = 13 to 14 animals/group. *p < 0.05 versus end-stage sucrose-treated SIV infected animals. +p < 0.05 versus alcohol-treated SAIDS. Pre-SIV values were not different between groups and are presented in Table 1.
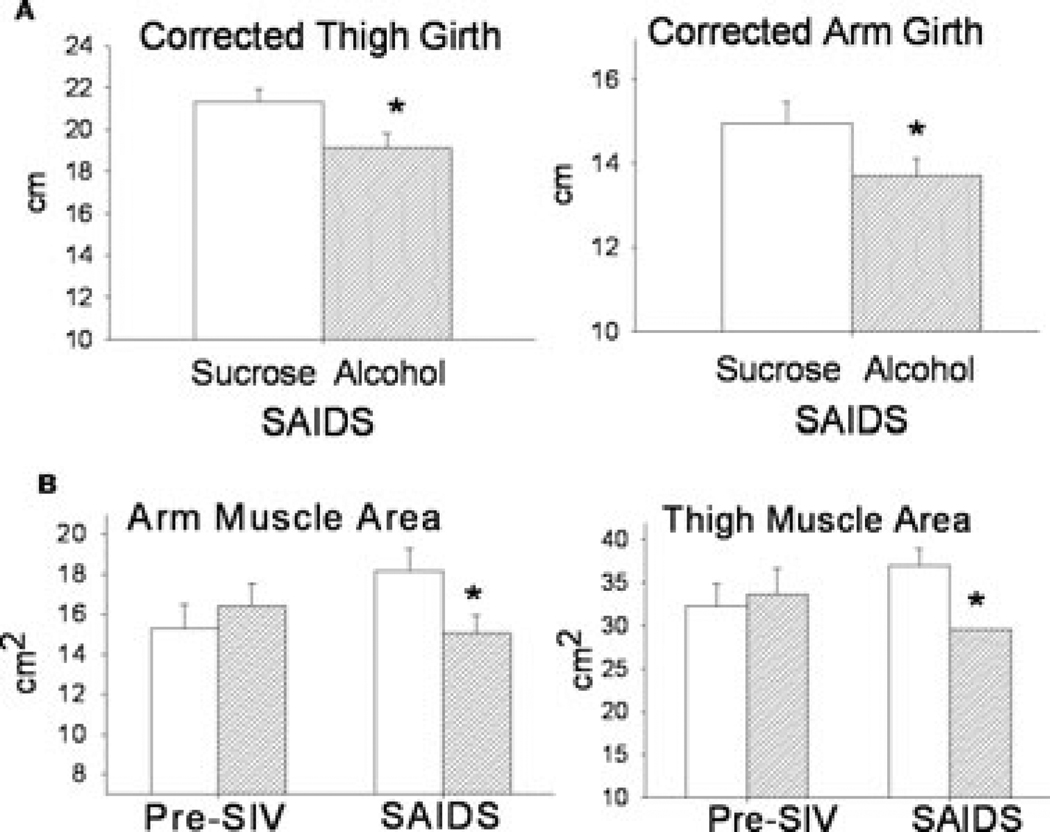
Anthropometric measures have been used to estimate muscle mass in clinical studies. In particular, limb circumferences corrected for subcutaneous adipose tissue thickness are valid predictors of total body skeletal muscle mass. Using the anthropometric measures obtained, we calculated the CAG and CTG of animals at the basal pre-SIV infection period and at end-stage SAIDS (Fig. 3). No differences were detected between the sucrose- and alcohol-treated animals arm (13.7 ± 0.5 cm and 14.2 ± 0.6 cm) or thigh (19.9 ± 0.8 cm and 20.3 ± 0.8 cm) girth prior to SIV infection. However, corrected thigh and arm girth were significantly lower in alcohol-treated (19.1 ± 0.7 cm and 13.7 ± 0.4 cm; respectively) than in sucrose-treated (21.3 ± 0.6 cm and 14.9 ± 0.5 cm; respectively) SIV infected animals at SAIDS (Fig. 4A). In addition, the estimated arm and thigh muscle areas were significantly lower in alcohol-treated SIV-infected animals than in sucrose SIV-infected (Fig. 4B).
Fig. 4.
(A) Corrected thigh and arm girth (cm) obtained at end stage of AIDS (SAIDS) in sucrose- and alcohol-treated SIV-infected rhesus, (B) arm and thigh muscle area (cm2), obtained pre-SIV infection and at end stage SAIDS in sucrose (open bars) and alcohol (hatched bars) treated rhesus macaques. Values are means ± SEM, n=13 to 14 animals/group. *p < 0.05 versus end-stage sucrose-treated SIV-infected animals.
Muscle Cytokine, Ubiquitin Ligase, and Growth Factor Expression
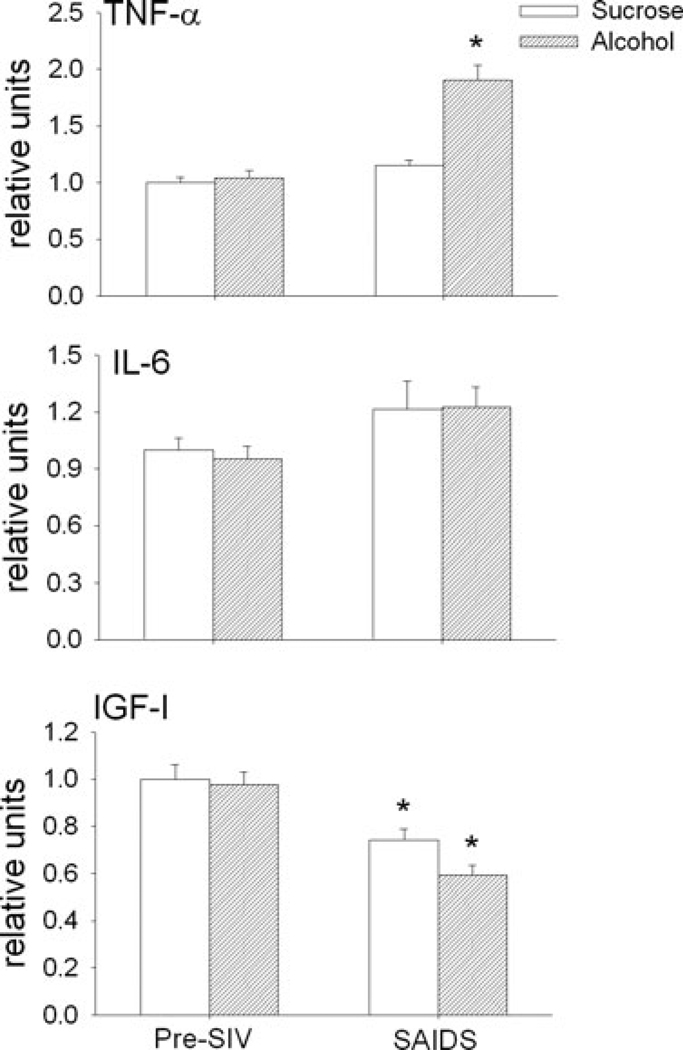
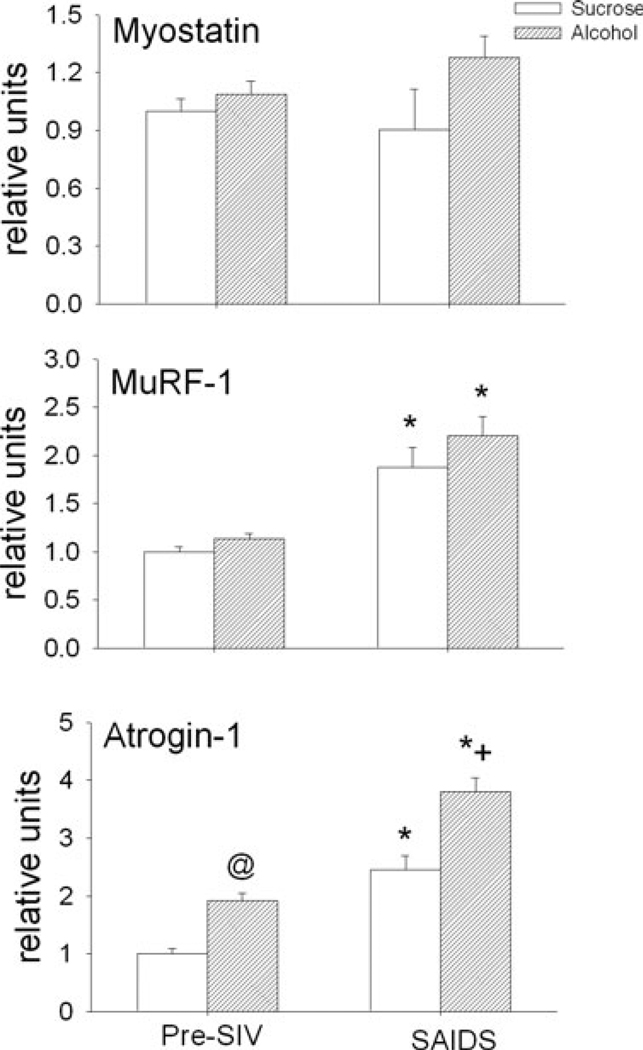
Skeletal muscle mRNA expression of TNF-α, IL-6, IGF-I, myostatin and MuRF-1 was not different between sucrose-and alcohol-treated animals during the basal pre-SIV infection period (Figs 5 and 6). However, there was a 2-fold increase in atrogin-1 expression following 3 months of alcohol administration. This was the only difference observed following alcohol administration alone prior to SIV infection, compared to values from the time-matched sucrose-fed animals. End-stage SAIDS was characterized by a significant decrease in skeletal muscle expression of IGF-I, increased expression of MuRF-1, and atrogin, and no change in that of TNF-α, IL-6 or myostatin in sucrose-treated SIV-infected animals. In alcohol-treated animals, the SIV-induced changes in skeletal muscle expression of IGF-I and MuRF-1 were similar to those observed at end-stage in sucrose-treated animals. However, alcohol-treated SIV-infected animals had significantly higher skeletal muscle expression of TNF-α and atrogin-1 at end-stage compared to that of sucrose-treated SIV-infected animals.
Fig. 5.
Skeletal muscle (quadriceps) expression (relative units) of tumor necrosis factor (TNF)-α, interleukin 6 (IL-6), and insulin-like growth factor-I (IGF-I) in sucrose- (open bars) and alcohol- (hatched bars) treated rhesus macaques prior to SIV infection (pre-SIV) and at end stage (SAIDS) infection. Values are means ± SEM, n = 13 to 14 in each group. *p < 0.05 versus pre-SIV.
Fig. 6.
Skeletal muscle (quadriceps) expression (relative units) of myostatin, muscle-specific RING finger-1 (MuRF-1), and atrogin-1 in sucrose-(open bars) and alcohol- (hatched bars) treated rhesus macaques prior to SIV infection (pre-SIV) and at end stage of AIDS (SAIDS) infection. Values are means ± SEM, n = 13 to 14 in each group. *p < 0.05 versus pre-SIV. @p < 0.05 versus pre-SIV sucrose-treated animals. +p < 0.05 versus sucrose-treated SAIDS.
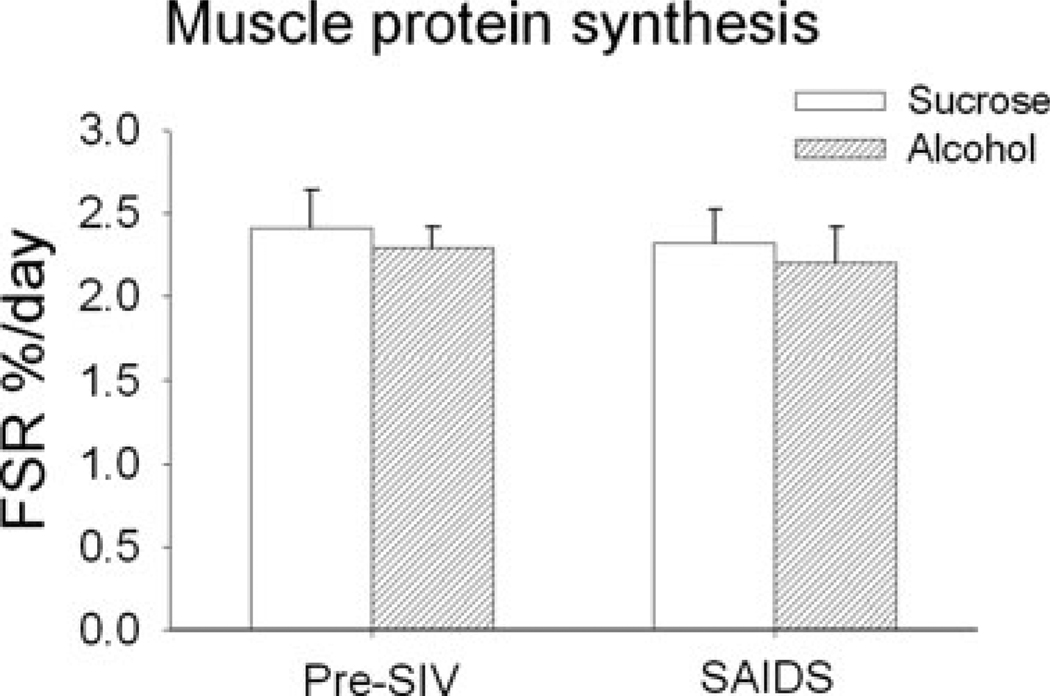
Muscle Protein Synthesis
Rates of skeletal muscle protein synthesis determined at basal pre-SIV infection averaged 2.4 ± 0.2%/ d and 2.3 ± 0.1%/d in sucrose- and alcohol-treated animals. Rates determined at the end stage SAIDS were not different between the two experimental groups nor where they different from rates determined pre-SIV infection (Fig. 7).
Fig. 7.
Fractional synthetic rates of skeletal muscle protein synthesis (%/d) in sucrose- (open bars) and alcohol- (hatched bars) treated rhesus macaques prior to SIV infection (pre-SIV) and at end stage of AIDS (SAIDS) infection. Values are means ± SEM, n = 13 to 14 in each group. No differences were detected between groups.
DISCUSSION
The present study examined the impact of chronic alcohol intake on organ pathology, with a focus on body composition and potential regulatory mechanisms of skeletal muscle mass, during the terminal SAIDS stage in rhesus macaques. Chronic alcohol administration increased viral load at set point and had a profound effect on BMI resulting primarily from decreased skeletal muscle mass. These changes were associated with a significantly augmented SIV-induced increase in skeletal muscle TNF-α and atrogin-1 expression. These findings suggest that the ability of alcohol to amplify the pro-inflammatory and catabolic responses to SIV infection is an important mechanism involved in the loss of muscle mass. Furthermore, they confirm our previous hypothesis that the early alcohol-induced nutritional and metabolic alterations during the asymptomatic phase of SIV infection lead to disruption of the host adaptive response during the symptomatic phase of disease aggravating end-stage disease (Molina et al., 2006).
Previous studies from our laboratory, as well as those of others, have found that chronic alcohol consumption increased viral load during the initial period of SIV infection (Bagby et al., 2003; Kumar et al., 2005; Molina et al., 2006; Poonia et al., 2005), but not during the end-stage of disease (Bagby et al., 2006). The findings from the present study confirm these previous reports and, in addition, show similar patterns of increased set point in alcohol-treated animals regardless of the virus used. In the present study, approximately 50% of the animals were infected with MAC251 while others were infected with B670, a virus previously utilized by our laboratory. Previously, Bagby et al. (2003) reported decreased survival time in alcohol-treated animals when compared to that of sucrose-treated animals. Our results did not show a significant difference in time to death between the two experimental groups. When analyzed according to virus strain used to infect the animals, it became apparent that time to death in SIVMAC251 infected animals was more rapid compared to animals infected with SIVB670. This difference in rate of disease progression between animals infected with MAC251 and B670 may explain the differing observations between Bagby et al. and the present study. Nevertheless, the results from this study show that alcohol exacerbates losses of skeletal muscle mass and alterations in body composition during progression to end stage disease and increases the incidence of organ system pathology.
Previously we showed that chronic alcohol ingestion reduces dietary intake and alters nutrient selection leading to growth retardation when compared to macaques receiving isocaloric sucrose (Molina et al., 2006). Accurate quantification of caloric intake during the end stage disease was not possible due to the large variability as animals approached end-stage. However, the results from the present study do show that total weight gain from the time of infection to end-stage disease in sucrose-treated SIV infected animals is similar to that observed 10 months post-SIV infection when animals were still asymptomatic (1.5 ± 0.4 kg). This suggests negligible cumulative weight gain in SIV-infected animals once they enter the symptomatic phase of infection. In contrast, weight gain in alcohol-treated SIV infected animals was significantly reduced during the asymptomatic phase of infection and lower than that of sucrose-treated animals, suggesting that these animals not only failed to gain weight but had significant weight loss during the symptomatic phase of SIV infection. Moreover, while we have previously reported that BMI of alcohol-treated SIV-infected animals is still preserved despite a significant decrease in caloric intake during the asymptomatic phase of the infection, the current study provides clear evidence for the debilitating effect of alcohol. Sucrose-fed animals did not show significant alterations in BMI resulting from SIV infection; while BMI in alcohol-treated SIV-infected animals was 20% lower at end-stage when compared to that of sucrose-treated SIV infected animals. Weight loss, particularly loss of metabolically active lean tissue, has been associated with accelerated disease progression, loss of muscle protein mass, and impairment of strength and functional status in HIV-infected individuals (Mangili et al., 2006).
Anthropometric measures obtained in this study revealed that limb, abdominal and hip circumferences were all 5 to 10% smaller in alcohol-treated SIV infected animals. The lack of differences in skinfold measurement suggests no change in subcutaneous fat. Therefore, the changes in circumference are most likely due to changes in muscle mass. Furthermore, the similar waist to hip ratio between groups suggests there was no overt alteration in fat redistribution in either the sucrose-or alcohol-treated animals as a result of SIV infection or of chronic alcohol administration. Whether rhesus macaques develop similar lipodystrophy as that described in humans following HAART is not known and is the subject of further investigation.
The results from the present study suggest that loss of LBM may be accelerated or accentuated by life-style factors such as chronic alcohol intake. Our results showed similar increases in BUN and creatinine levels at end stage disease, suggestive of increased protein catabolism and loss of renal function in both experimental groups. We did not observe significant changes in the rates of skeletal muscle protein synthesis in the present study. Previous studies from our laboratory have shown that during the asymptomatic phase of the disease as well, there is no evident alteration in the rate of skeletal muscle protein synthesis (Molina et al., 2006). These findings are in contrast to previous reports of alcohol-induced suppression of muscle protein synthesis, in human and animal models (Lang et al., 1999a; Preedy and Peters, 1988). We speculate that different protocols of alcohol administration, time from alcohol administration to measurement of rates of protein synthesis as well as circulating alcohol levels at the time of study could account for the different response (Reilly et al., 2000; Vary et al., 2004). Whether responses to anabolic agents are preserved in these animals is not known. In this regard, because protein synthesis was determined in the postabsorptive state, it is possible that we missed differences in protein synthesis in response to feeding. Several strategies have been proposed to improve nitrogen balance and prevent muscle wasting (Kotler, 2000), including amino acid supplementation (Selberg et al., 1995), anabolic hormone administration (Hengge et al., 2003; Mulligan et al., 1993; Storer et al., 2005) and resistance exercise (Roubenoff et al., 1999). The results from the present study suggest that the effectiveness of these therapies is likely to be further compromised in chronic alcohol-consuming HIV-infected patients. The greater TNF-α response in skeletal muscle of alcohol treated animals would be expected to not only decrease local production of anabolic hormones, but in addition to impair tissue responsiveness to hormonal or nutrient stimulation of protein synthesis (Gelato et al., 2002; McNurlan et al., 1997). Moreover, one would predict that throughout the course of infection, skeletal muscle protein synthesis undergoes rapid adjustments, particularly following periods of acute intercurrent infections. One could speculate that alcohol impairs the ability of the host to undergo those periods of enhanced synthetic rates, accounting for the overall lower LBM despite similar rates of muscle protein synthesis measured in the basal post-absorption state.
Previous studies in patients with advanced disease (AIDS wasting) have provided evidence for the involvement of the ubiquitin-ATP-dependent proteolytic system in loss of skeletal muscle mass and it has been suggested that this pathway is activated by pro-inflammatory cytokines, such as TNF and glucocorticoids, leading to AIDS-associated wasting (Llovera et al., 1998). Two genes which have been identified as markers of the skeletal atrophy process are MuRF-1 and atrogin-1, both of which encode muscle-specific E3 ubiquitin ligases (Glass, 2003). Previously we have observed that during the asymptomatic phase of the disease MuRF-1 mRNA was decreased in alcohol-treated SIV infected animals (Molina et al., 2006). This suppressed expression appears to be a transient change, possibly controlled as a compensatory mechanism to maintain muscle mass during the asymptomatic phase of the disease. Both MuRF-1 and atrogin-1 mRNA expression was increased at end-stage in sucrose-fed SIV+ animals. However, atrogin-1 mRNA expression showed a marked increase in alcohol-treated animals prior to infection, of similar magnitude to that observed in SIV-infected control animals at end stage SAIDS. At terminal SAIDS, the magnitude of the difference between control and alcohol-treated animals remained similar to that which was observed prior to SIV infection. These findings indicate an underlying alcohol-mediated up-regulation of atrogin-1 mRNA expression which was further accentuated by SIV infection. The increased atrogin-1 expression observed at end-stage would be expected to accentuate the overall catabolic balance in skeletal muscle, given that IGF-I mRNA was significantly suppressed in both groups. The suppression of IGF-I was not previously observed during the asymptomatic phase of the disease (Molina et al., 2006). Consistent with our previous findings, myostatin mRNA was not altered in either experimental group at end-stage. Taken together, these findings suggest that the decrease in muscle mass at end-stage is likely the result of a pro-inflammatory/anabolic imbalance favoring overall catabolic processes leading to loss of muscle mass associated with impaired muscle growth. Clinical studies indicate that inflammation impacts the responsiveness of muscle tissue to an anabolic stimulus (Gelato et al., 2002). Moreover, similar up-regulation in muscle TNF-α expression has been demonstrated following an inflammatory challenge (Lang et al., 2003) and these muscle responses are generally associated with altered protein metabolism (Frost and Lang, 2005).
The findings from our study show that chronic alcohol consumption affects muscle-specific mechanisms, underlying the pathophysiology of alcohol-mediated effects on body composition. Taken together, our results from this and our previous study suggest that higher viral set-point, alterations in feeding behavior, and accentuated localized pro-inflammatory state associated with diminished anabolic signals (decreased tissue IGF-I) contribute to a decrease in LBM during the end-stage of the disease in chronic alcohol-fed animals. We speculate this imbalance between increased inflammatory and attenuated anabolic signals leads to skeletal muscle catabolism and dysfunction. The higher viral set point as well as the enhanced TNF-α expression in skeletal muscle observed in alcohol-treated SIV-infected animals indicates chronic alcohol consumption can further compromise the integrity of the infected host immune function. The pro-inflammatory effect of chronic alcohol observed in muscle raises the possibility that alcohol-induced accentuation of inflammatory cytokines, particularly TNF-α, in other tissue compartments may enhance viral replication (Connolly et al., 2005; Decrion et al., 2005). Future studies will examine whether this increased pro-inflammatory cytokine expression, particularly of TNF-α, is also observed in compartments that would be primary sites for viral replication including spleen, lymph nodes and mucosa. In summary, the results from this study confirm our hypothesis that chronic binge-like alcohol intake during SIV infection produces detrimental effects on the host immune, nutritional and metabolic function accentuating the loss of LBM at end-stage disease.
ACKNOWLEDGMENTS
The authors would like to acknowledge the technical expertise of Connie Porretta, Jean Carnal, Rhonda Martinez, Jane Schexneider, Howard Blakesly, Curtis Vande Stouwe and Jay Nystrom as well as the excellent veterinary care provided by Dr. Jason Dufour and contributions of Dr. Kirsten Zambell. Supported by AA-07577, AA-09803, AA-11290, DK072909.
REFERENCES
- Arthur LO, Raymond VG, Marx P, Gardner MB. Simian acquired immunodeficiency syndrome. Prog Allergy. 1986;37:332–352. doi: 10.1159/000318452. [DOI] [PubMed] [Google Scholar]
- Bagby GJ, Stoltz DA, Zhang P, Kolls JK, Brown J, Bohm RP, Jr, Rockar R, Purcell J, Murphey-Corb M, Nelson S. The effect of chronic binge ethanol consumption on the primary stage of SIV infection in rhesus macaques. Alcohol Clin Exp Res. 2003;27:495–502. doi: 10.1097/01.ALC.0000057947.57330.BE. [DOI] [PubMed] [Google Scholar]
- Bagby GJ, Zhang P, Purcell JE, Didier PJ, Nelson S. Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease. Alcohol Clin Exp Res. 2006;30:1781–1790. doi: 10.1111/j.1530-0277.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- Baskin GB, Murphey-Corb M, Martin LN, Soike KF, Hu FS, Kuebler D. Lentivirus-induced pulmonary lesions in rhesus monkeys (Macaca mulatta) infected with simian immunodeficiency virus. Vet Pathol. 1991;28:506–513. doi: 10.1177/030098589102800607. [DOI] [PubMed] [Google Scholar]
- Baskin GB, Murphey-Corb M, Roberts ED, Didier PJ, Martin LN. Correlates of SIV encephalitis in rhesus monkeys. J Med Primatol. 1992;21:59–63. [PubMed] [Google Scholar]
- Bergheim I, Parlesak A, Dierks C, Bode CJ, Bode C. Nutritional deficiencies in German middle-class male alcohol consumers: relation to dietary intake and severity of liver disease. Eur J Clin Nutr. 2003;57:431–438. doi: 10.1038/sj.ejcn.1601557. [DOI] [PubMed] [Google Scholar]
- CDC. HIV / AIDS Surveillance Report, 2003 (Vol. 15) Atlanta, GA: US Department of Health and Human Services, CDC; 2004. pp. 1–46. [Google Scholar]
- Colman RJ, Hudson JC, Barden HS, Kemnitz JW. A comparison of dual-energy X-ray absorptiometry and somatometrics for determining body fat in rhesus macaques. Obes Res. 1999;1:90–96. doi: 10.1002/j.1550-8528.1999.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Connolly NC, Riddler SA, Rinaldo CR. Proinflammatory cytokines in HIV disease-a review and rationale for new therapeutic approaches. AIDS Rev. 2005;7:168–180. [PubMed] [Google Scholar]
- Coodley GO, Loveless MO, Merrill TM. The HIV wasting syndrome: a review. J Acquir Immune Defic Syndr. 1994;7:681–694. [PubMed] [Google Scholar]
- Decrion AZ, Dichamp I, Varin A, Herbein G. HIV and inflammation. Curr HIV Res. 2005;3:243–259. doi: 10.2174/1570162054368057. [DOI] [PubMed] [Google Scholar]
- Forrester JE, Woods MN, Knox TA, Spiegelman D, Skinner SC, Gorbach SL. Body composition and dietary intake in relation to drug abuse in a cohort of HIV-positive persons. J Acquir Immune Defic Syndr. 2000;1(25):S43–S48. doi: 10.1097/00042560-200010001-00007. [DOI] [PubMed] [Google Scholar]
- Frost RA, Lang CH. Skeletal muscle cytokines: regulation by pathogenassociated molecules and catabolic hormones. Curr Opin Clin Nutr Metab Care. 2005;8:255–263. doi: 10.1097/01.mco.0000165003.16578.2d. [DOI] [PubMed] [Google Scholar]
- Garlick PJ, Wernerman J, McNurlan MA, Essen P, Lobley GE, Milne E, Calder GA, Vinnars E. Measurement of the rate of protein synthesis in muscle of postabsorptive young men by injection of a ‘flooding dose’ of [1–13C]leucine. Clin Sci (Lond) 1989;77:329–336. doi: 10.1042/cs0770329. [DOI] [PubMed] [Google Scholar]
- Gelato MC, Mynarcik DC, McNurlan MA. Soluble tumor necrosis factor alpha receptor 2, a serum marker of resistance to the anabolic actions of growth hormone in subjects with HIV disease. Clin Sci. 2002;102:85–90. [PubMed] [Google Scholar]
- Giegerich R, Meyer F, Scheiermacher C. GeneFisher - software support for the detection of postulated genes. Proc Int Conf Intell Syst Mol Biol. 1996;4:68–77. [PubMed] [Google Scholar]
- Glass DJ. Molecular mechanisms modulating muscle mass. Trends Mol Med. 2003;9:344–350. doi: 10.1016/s1471-4914(03)00138-2. [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Mulligan K. Department of Health and Human Services Working Group on the Prevention and Treatment of Wasting and Weight Loss. Weight loss and wasting in patients infected with human immunodeficiency virus. Clin Infect Dis. 2003;36 Suppl. 2:S69–S78. doi: 10.1086/367561. [DOI] [PubMed] [Google Scholar]
- Grunfeld C, Feingold KR. Metabolic disturbances and wasting in the acquired immunodeficiency syndrome. New Engl J Med. 1992;327:329–337. doi: 10.1056/NEJM199207303270506. [DOI] [PubMed] [Google Scholar]
- Hashiguchi Y, Molina PE, Dorton S, McNurlan MA, Garlick PJ, Reddy D, Abumrad NN. Central opiate l-receptor mediated suppression of tissue protein synthesis. Am J Physiol. 1997;273:R920–R927. doi: 10.1152/ajpregu.1997.273.3.R920. [DOI] [PubMed] [Google Scholar]
- Hengge UR, Stocks K, Faulkner S, Wiehler H, Lorenz C, Jentzen W, Hengge D, Ringham G. Oxymetholone for the treatment of HIV-wasting: a double-blind, randomized, placebo-controlled phase III trial in eugonadal men and women. HIV Clin Trials. 2003;4:150–163. doi: 10.1310/hct.2003.4.3.002. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, McManus C, Smith J, Stevens V, Nixon DW. Anthropometric measurement of muscle mass: revised equations for calculating bone-free arm muscle area. Am J Clin Nutr. 1982;36:680–690. doi: 10.1093/ajcn/36.4.680. [DOI] [PubMed] [Google Scholar]
- Jayo JM, Shively CA, Kaplan JR, Manuck SB. Effects of exercise and stress on body fat distribution in male cymolgous monkeys. Int J Obesity. 1993;17:597–604. [PubMed] [Google Scholar]
- Kotler DP. Nutritional alterations associated with HIV infection. J Acquir Immune Defic Syndr. 2000;25 Suppl. 1:S81–S87. doi: 10.1097/00042560-200010001-00013. [DOI] [PubMed] [Google Scholar]
- Kumar R, Perez-Casanova A, Tirado G, Noel RJ, Torres C, Rodriguez I, Martinez M, Staprans S, Kraiselburd E, Yamamura Y, Higley JD, Kumar A. Increased viral replication in simian immunodeficiency virus / simian-HIV-infected macaques with self-administering model of chronic alcohol consumption. J Acquir Immune Defic Syndr. 2005;39:386–390. doi: 10.1097/01.qai.0000164517.01293.84. [DOI] [PubMed] [Google Scholar]
- Lang CH, Silvis C, Deshpande N, Nystrom G, Frost RA. Endotoxin stimulates in vivo expression of inflammatory cytokines tumor necrosis factor alpha, interleukin-1beta, −6, and high-mobility-group protein-1 in skeletal muscle. Shock. 2003;19:538–546. doi: 10.1097/01.shk.0000055237.25446.80. [DOI] [PubMed] [Google Scholar]
- Lang CH, Wu D, Frost RA, Jefferson LS, Kimball SR, Vary TC. Inhibition of muscle protein synthesis by alcohol is associated with modulation of eIF2B and eIF4E. Am J Physiol Endocrinol Metab. 1999a;277:E268–E276. doi: 10.1152/ajpendo.1999.277.2.E268. [DOI] [PubMed] [Google Scholar]
- Lee LM, Karon JM, Selik R, Neal JJ, Fleming PL. Survival after AIDS diagnosis in adolescents and adults during the treatment era, United States, 1984–1997. JAMA. 2001;285:1308–1315. doi: 10.1001/jama.285.10.1308. [DOI] [PubMed] [Google Scholar]
- Lefevre F, O’Leary B, Moran M, Mossar M, Yarnold PR, Martin GJ, Glassroth J. Alcohol consumption among HIV-infected patients. J Gen Intern Med. 1995;10:458–460. doi: 10.1007/BF02599920. [DOI] [PubMed] [Google Scholar]
- Llovera M, Garcia-Martinez C, Agell N, Lopez-Soriano FJ, Authier FJ, Gherardi RK, Argiles JM. Ubiquitin and proteasome gene expression is increased in skeletal muscle of slim AIDS patients. Int J Mol Med. 1998;2:69–73. [PubMed] [Google Scholar]
- Mangili A, Murman DH, Zampini AM, Wanke CA. Nutrition and HIV infection: review of weight loss and wasting in the era of highly active antiretroviral therapy from the nutrition for healthy living cohort. Clin Infect Dis. 2006;42:836–842. doi: 10.1086/500398. [DOI] [PubMed] [Google Scholar]
- McNurlan MA, Garlick PJ, Steigbigel RT, DeCristofaro KA, Frost RA, Lang CH, Johnson RW, Santasier AM, Cabahug CJ, Fuhrer J, Gelato MC. Responsiveness of muscle protein synthesis to growth hormone administration in HIV-infected individuals declines with severity of disease. J Clin Inves. 1997;100:2125–2132. doi: 10.1172/JCI119747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE, Fan J, Gelato M, Lang CH, Abumrad NN. Modulation of insulin-like growth factor-I: a specific role for vitamin B1 (thiamine) Nutr Biochem. 1996;7:207–213. [Google Scholar]
- Molina PE, McNurlan M, Rathmacher J, Lang CH, Zambell KL, Purcell J, Bohm RP, Zhang P, Bagby GJ, Nelson S. Chronic alcohol accentuates nutritional, metabolic, and immune alterations during asymptomatic simian immunodeficiency virus infection. Alcohol Clin Exp Res. 2006;12:2065–2078. doi: 10.1111/j.1530-0277.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- Mulligan K, Grunfeld C, Hellerstein MK, Neese RA, Schambelan M. Anabolic effects of recombinant human growth hormone in patients with wasting associated with human immunodeficiency virus infection. J Clin Endocrinol Metab. 1993;77:956–962. doi: 10.1210/jcem.77.4.8408471. [DOI] [PubMed] [Google Scholar]
- Pacy PJ, Preedy VR, Peters TJ, Read M, Halliday D. The effect of chronic alcohol ingestion on whole body and muscle protein synthesis – a stable isotope study. Alcohol Alcohol. 1991;26:505–513. doi: 10.1093/oxfordjournals.alcalc.a045152. [DOI] [PubMed] [Google Scholar]
- Poonia B, Nelson S, Bagby GJ, Zhang P, Quniton L, Veazey RS. Chronic alcohol consumption results in higher simian immunodeficiency virus replication in mucosally inoculated rhesus macaques. AIDS Res Hum Retroviruses. 2005;21:863–868. doi: 10.1089/aid.2005.21.863. [DOI] [PubMed] [Google Scholar]
- Preedy VR, Peters TJ. Acute effects of ethanol on protein synthesis in different muscles and muscle protein fractions of the rat. Clin Sci (Lond) 1988;74:461–466. doi: 10.1042/cs0740461. [DOI] [PubMed] [Google Scholar]
- Preedy VR, Salisbury JR, Peters TJ. Alcoholic muscle disease: features and mechanisms. J Pathol. 1994;173:309–315. doi: 10.1002/path.1711730405. [DOI] [PubMed] [Google Scholar]
- Reilly ME, Mantle D, Richardson PJ, Salisbury J, Jones J, Peters TJ, Preedy VR. Studies on the time-course of ethanol’s acute effects on skeletal muscle protein synthesis: comparison with acute changes in proteolytic activity. Alcohol Clin Exp Res. 1997;21:792–798. [PubMed] [Google Scholar]
- Reilly ME, Mantle D, Salisbury J, Peters TJ, Preedy VR. Comparative effects of acute ethanol dosage on liver and muscle protein metabolism. Biochem Pharmacol. 2000;60:1773–1785. doi: 10.1016/s0006-2952(00)00504-9. [DOI] [PubMed] [Google Scholar]
- Roubenoff R, McDermott A, Weiss L, Suri J, Wood M, Bloch R, Gorbach S. Short-term progressive resistance training increases strength and lean body mass in adults infected with human immunodeficiency virus. AIDS. 1999;13:231–239. doi: 10.1097/00002030-199902040-00011. [DOI] [PubMed] [Google Scholar]
- Selberg O, Suttmann U, Melzer A, Deicher H, Muller MJ, Henkel E, McMillan DC. Effect of increased protein intake and nutritional status on whole-body protein metabolism of AIDS patients with weight loss. Metabolism. 1995;44:1159–1165. doi: 10.1016/0026-0495(95)90009-8. [DOI] [PubMed] [Google Scholar]
- Seman AL, Pewen WF, Fresh LF, Martin LN, Murphey-Corb M. The replicative capacity of rhesus macaque peripheral blood mononuclear cells for simian immunodeficiency virus in vitro is predictive of the rate of progression to AIDS in vivo. J Gen Virol. 2000;81:2441–2449. doi: 10.1099/0022-1317-81-10-2441. [DOI] [PubMed] [Google Scholar]
- Stoltz DA, Nelson S, Kolls JK, Zhang P, Bohm RP, Murphey-Corb M, Bagby GJ. In vitro ethanol suppresses alveolar macrophage TNF-α during simian immunodeficiency virus infection. Am J Respir Crit Care Med. 2000;161:135–140. doi: 10.1164/ajrccm.161.1.9905016. [DOI] [PubMed] [Google Scholar]
- Storer TW, Woodhouse LJ, Sattler F, Singh AB, Schroeder ET, Beck K, Padero MC, Mac P, Yarasheski KE, Geurts P, Willemsen A, Harms MK, Bhasin SA. Randomized, placebo-controlled trial of nandrolone decanoate in human immunodeficiency virus-infected men with mild to moderate weight loss with recombinant human growth hormone as active reference treatment. J Clin EndoMetab. 2005;90:4474–4482. doi: 10.1210/jc.2005-0275. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Overview of Findings from the 2004 National Survey on Drug Use and Health ((Office of Applied Studies, NSDUH Series H-27, DHHS Publication No. SMA 05-4061) Rockville, MD: 2005. [Google Scholar]
- Suryanarayana K, Wiltrout T, Vasquez G, Hirsch V, Lifson J. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res Hum Retroviruses. 1998;14:183–189. doi: 10.1089/aid.1998.14.183. [DOI] [PubMed] [Google Scholar]
- Suttmann U, Ockenga J, Selberg O, Hoogestraat L, Deicher H, Muller MJ. Incidence and prognostic value of malnutrition and wasting in human immunodeficiency virus infected outpatients. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:239–246. doi: 10.1097/00042560-199503010-00004. [DOI] [PubMed] [Google Scholar]
- Tang AM, Forrester J, Spiegelman D, Knox TA, Tchetgen E, Gorbach SL. Weight loss and survival in HIV-positive patients in the era of highly active antiretroviral therapy. Acquir Immune Defic Syndr. 2002;31:230–236. doi: 10.1097/00126334-200210010-00014. [DOI] [PubMed] [Google Scholar]
- Teschner M, Schaefer RM, Weissinger F, Kulzer P, Duelk MJ, Peter G, Heidland A. Chronic ethanol ingestion enhances catabolism and muscle protease activity in acutely uremic rats. Nephron. 1988;50:338–344. doi: 10.1159/000185199. [DOI] [PubMed] [Google Scholar]
- Thiebaut R, Malvy D, Marimoutou C, Davis F. Anthropometric indices as predictors of survival in AIDS adults. Aquitaine Cohort, France, 1985–1997. Groupe d-Epidemiologie Clinique du Sida en Aquitaine (GE-CSA) Eur J Epidemiol. 2000;16:633–639. doi: 10.1023/a:1007696530440. [DOI] [PubMed] [Google Scholar]
- Van Loan MD, Strawford A, Jacob M, Hellerstein M. Monitoring changes in fat-free mass in HIV-positive men with hypotestosteronemia and AIDS wasting syndrome treated with gonadal hormone replacement therapy. AIDS. 1999;13:241–248. doi: 10.1097/00002030-199902040-00012. [DOI] [PubMed] [Google Scholar]
- Vary TC, Nairn AC, Lang CH. Restoration of protein synthesis in heart and skeletal muscle after withdrawal of alcohol. Alcohol Clin Exp Res. 2004;28:517–525. doi: 10.1097/01.alc.0000121653.80502.54. [DOI] [PubMed] [Google Scholar]
- Wanke CA, Silva M, Knox TA, Forrester J, Speigelman D, Gorbach SL. Weight loss and wasting remain common complications in individuals infected with human immunodeficiency virus in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;31:803–805. doi: 10.1086/314027. [DOI] [PubMed] [Google Scholar]
- Wheeler DA, Gilbert CL, Launer CA, Muurahainen N, Elion RA, Abrams DI, Bartsch GE. Weight loss as a predictor of survival and disease progression in HIV infection. Terry Beirn Community Programs for Clinical Research on AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:80–85. doi: 10.1097/00042560-199805010-00012. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Caton S, Hetherington MM. Alcohol and food intake; nutrition and physiological function. Curr Opin Clin Nutr Metab Care. 2003;6:639–644. doi: 10.1097/00075197-200311000-00006. [DOI] [PubMed] [Google Scholar]