Abstract
Purpose The aim of this study is to investigate the short-term influence of a period of dynamic exercise on axial length (AXL) and intraocular pressure (IOP) in young adult subjects.
Patients and methods In all, 20 young adult subjects (10 myopes and 10 emmetropes) participated. Baseline measures of ocular biometrics, IOP and ocular pulse amplitude (OPA) were taken following a 20-min rest period. Subjects then performed 10 min of moderate intensity, low impact dynamic exercise (bicycle ergometry). Measures of ocular biometrics, IOP and OPA were repeated immediately after, and then 5 and 10 min after this exercise task. Systemic blood pressure and pulse rate were also monitored. A repeated measures analysis of variance was used to investigate the changes in the measured parameters.
Results Exercise resulted in significant changes in a range of ocular parameters. A small but significant decrease in AXL was observed following exercise (P<0.0001). The largest change in AXL was noted immediately following exercise (mean decrease −17±10 μm). IOP and OPA also decreased significantly following exercise (P<0.0001). A moderate but significant positive association was found between the changes in AXL and the changes in IOP (r2=0.36, P<0.0001). There were no significant differences found between the myopic and emmetropic subjects in the magnitude of changes observed in ocular parameters following exercise.
Conclusion The physiological effects of dynamic exercise lead to changes in a range of ocular parameters, including significant reductions in IOP, OPA and decreases in AXL.
Keywords: exercise, axial length, intraocular pressure, myopia, refractive error
Introduction
Axial length (AXL) is an important biometric determinant of the refractive state of an eye. For an eye to remain emmetropic, a precise matching of the AXL of an eye to its refractive power must occur. The development of precise optical techniques for the measurement of AXL has shown that a range of different physiological factors can lead to short-term changes in this biometric parameter. Changes in accommodation,1, 2, 3 imposed defocus,4 alterations in IOP5, 6 and water loading7 have all been found to be associated with short term changes in human AXL. Significant diurnal variations in AXL have also been documented.8, 9, 10 The exact cause underlying these diurnal variations has not been determined, although changes in choroidal thickness (ChT)11 and IOP10 have been implicated.
Performing physical activity (ie, dynamic exercise) has been demonstrated to lead to changes in a range of ocular parameters. The influence of exercise on IOP has been well studied, with the majority of studies finding periods of dynamic exercise to be associated with reductions in IOP.12, 13, 14, 15, 16 The magnitude of reduction in IOP has been found to be related to the intensity of exercise.13, 15 Dynamic exercise has also been reported to lead to changes in ocular blood flow. Price et al17 and Lovasik and Kergoat18 reported increases in pulsatile ocular blood flow in subjects following a short period of dynamic exercise. Similarly, Lovasik et al19 and Okuno et al20 reported small increases in choroidal blood flow with dynamic exercise. These changes in choroidal blood flow have been found to be substantially smaller than the concomitant increases in ocular perfusion pressure (OPP) that accompanied the exercise, suggesting a degree of regulation of ocular blood flow in response to increased perfusion pressure.19 Changes in retinal blood flow with dynamic exercise have also been studied, with reported changes typically being of small magnitude, suggesting a stronger auto-regulatory capacity in the retinal vasculature.20, 21
Physical activity has also been found to cause changes in other ocular parameters such as tonic accommodation,22 pupil size,23 anterior chamber angle24 and retinal activity;25 however, the influence of exercise on ocular biometrics such as AXL is not known. Given that exercise is known to alter IOP, and that changes in IOP have been found to be associated with variations in AXL, we were interested to investigate the influence of a period of dynamic exercise on AXL (and a range of other ocular biometrics) and IOP in a population of young adult subjects.
Subjects and methods
In all, 20 young adult subjects (mean age 25±4 years) participated in this study. A total of 12 of the 20 subjects were male. The subjects were from a range of ethnic backgrounds with 13 being of Caucasian, 5 of Indian and 2 of East Asian ethnic origin. All subjects reported normal ocular health (with no history of ocular disease, injury or surgery), and no history of any systemic health problems or cardiovascular disease. As previous studies26 have indicated that choroidal blood flow regulation may be altered in chronic smokers, no regular cigarette smokers were included in the study. All subjects were recruited from the staff and students of our university, and all gave written informed consent to participate. Approval from the university human research ethics committee was obtained before the commencement of the study. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
All subjects underwent an ophthalmic examination before their participation in the study to determine their refractive status and confirm normal ocular health. Following this examination, subjects were classified on the basis of their subjective spherical equivalent refraction (SER) as being either myopic (SER ⩾−1.00 D, n=10) or emmetropic (SER from +0.50 to −0.50 D, n=10). The mean SER of the myopes was −2.53±1.43 D and the emmetropes was −0.03±0.26 D. No subject exhibited cylindrical refraction of >1.00 DC or anisometropia of >1.00 DS. All subjects exhibited best-corrected visual acuity of logMAR 0.00 or better. The myopic and emmetropic populations of subjects were evenly matched for gender (both populations consisted of 60% male subjects), age (mean age of 23±3 years and 26±4 years for the myopes and emmetropes, respectively) and ethnicity (myopes consisted of seven Caucasian, two Indian and one East Asian, and emmetropes six Caucasian, three Indian and one East Asian subject).
Following this preliminary examination, the influence of a period of moderate intensity dynamic exercise on ocular biometrics and IOP was investigated. As both IOP and AXL are known to vary diurnally,10 all measures were carried out between 1300 and 1600 hours to ensure diurnal variations did not confound the results. Subjects were also instructed to refrain from ingesting food or liquid in the 30 min before the experiment, as water loading has previously been found to influence AXL and IOP.7 All ocular measures were conducted on the right eye.
Ocular biometric measures were collected using the Lenstar LS 900 optical biometer (Haag Streit AG, Koeniz, Switzerland), an instrument based on the principle of optical low coherence reflectometry, that has previously been shown to be highly precise and accurate.27 Measurements were collected according to manufacturer specifications with five repeated measures collected from each subject at each measurement session. IOP was measured with the dynamic contour tonometer (DCT, Ziemer Ophthalmic Systems, Port, Switzerland), a contact tonometer based on the principle of contour matching.28 This instrument has been found to provide reliable measures of IOP that correlate closely with measures from the Goldmann applanation tonometer29 along with reliable measures of ocular pulse amplitude (OPA, the difference between the diastolic and systolic IOP).30 Three valid measures with the DCT (ie, those exhibiting a quality score of 1–3) were collected for each subject at each measurement session following the instillation of a drop of local anesthetic (0.4% oxybuprocaine hydrochloride). Brachial arterial blood pressure (BP) was also measured before and after exercise using an electronic sphygmomanometer. This instrument also provided a measurement of pulse rate. In addition, pulse rate was also monitored before and during the exercise task using a wireless chest strap electronic heart rate monitor. The pulse rate derived from these two instruments was found to correlate closely (r2=0.72).
Baseline measurements of ocular biometrics, IOP, BP and pulse rate were collected following a 20-min rest period, in which subjects were seated viewing a television at a distance of 6 m. Each subject then performed a 10-min period of low impact, moderate intensity exercise (riding a bicycle ergometer) while they continued to maintain distance viewing. To standardize the intensity of the exercise between subjects, heart rate was monitored throughout the exercise task and subjects were instructed to cycle to an intensity in order to maintain their heart rate in a target range between 50 and 70% of their ‘heart rate reserve' above their resting heart rate. Heart rate reserve was defined as maximum heart rate−resting heart rate,31 with the resting heart rate derived from the pulse readings after the 20 min of rest and the maximum heart rate defined as 208−(0.7 × age).32 Immediately following exercise, ocular biometrics, IOP and BP were measured. Measurements were then repeated at 5 min and then 10 min after completion of the exercise task. All measurements were collected with subjects seated, and the order of measurement at each session was standardized with ocular biometrics measured first followed by IOP and BP measures. During the rest and exercise periods, all subjects wore their habitual distance spherocylindrical spectacle correction.
Data analysis
Following data collection, the IOP, OPA and ocular biometric measures from each subject were averaged. The biometric measures automatically derived from the Lenstar instrument include central corneal thickness (CCT, distance anterior to posterior cornea), anterior chamber depth (ACD, distance from posterior cornea to anterior lens), lens thickness (LT, distance from anterior to posterior lens) and AXL (distance from anterior cornea to retinal pigment epithelium). It has previously been shown that additional manual analysis of the Lenstar A-scan data originating from the posterior eye can provide measurements of retinal thickness (RT, the distance from the inner limiting membrane to the retinal pigment epithelium) and ChT (the distance from the retinal pigment epithelium to posterior choroid).4, 7 This manual analysis in this study was performed by two independent masked observers. The estimates of ChT and RT from the two observers correlated closely (r2=0.94 and 0.99 for ChT and RT, respectively). The systemic BP readings were also analyzed to calculate the mean arterial BP (MAP; MAP=diastolic BP+(1/3 (systolic BP−diastolic BP)), which was subsequently used to calculate the mean OPP for each subject at each measurement session (OPP=2/3 MAP–IOP).33 To investigate the influence of exercise on each of the measured variables, a repeated measures analysis of variance (ANOVA) was carried out with one within-subjects factor (time of measurement) and one between-subjects factor (refractive error group). An analysis of covariance (ANCOVA)34 was also carried out to investigate any association between AXL and the other measured variables.
Results
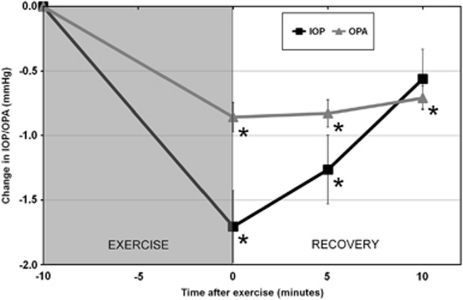
In this population of young adult subjects, 10 min of dynamic exercise was found to lead to significant changes in AXL, IOP and OPA. Repeated measures ANOVA revealed significant reductions in both IOP and OPA following exercise (P<0.0001; Figure 1). The mean IOP at baseline was 16.52±2.21 mm Hg, which reduced on average by −1.71±1.24 mm Hg immediately after exercise, −1.26±1.19 mm Hg at 5 min and −0.56±1.03 mm Hg at 10 min after exercise. Bonferroni corrected pair-wise comparisons revealed the changes in IOP were significantly different to baseline immediately after and 5 min after exercise (P<0.01); however, at 10 min after exercise IOP was not significantly different to baseline (P=0.16). At baseline the mean OPA was 2.33±0.74 mm Hg, and this reduced on average by −0.86±0.51 mm Hg, −0.83±0.47 mm Hg and −0.71±0.41 mm Hg immediately after, and 5 and 10 min after exercise, respectively. The changes in OPA were significantly different to baseline (P<0.0001) at all post-exercise measures. The IOP and OPA data exhibited no significant between-subjects effect of refractive error or any significant time by refractive error interaction, indicating similar mean levels and pattern of change between the myopic and emmetropic subjects.
Figure 1.
The mean change in IOP and OPA following exercise. ANOVA for repeated measures revealed the changes in both IOP and OPA were statistically significant following exercise (P<0.0001). Asterisk indicates a significant difference from baseline (P<0.05). Error bars represent the standard error of the mean.
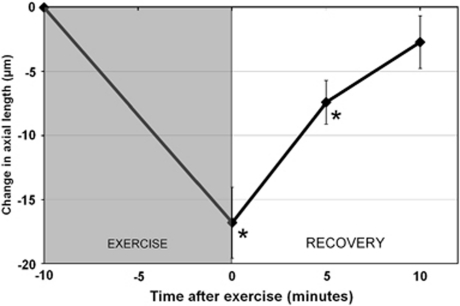
The mean ocular biometric measures and their changes following exercise are displayed in Table 1 . AXL exhibited a significant reduction following exercise (P<0.0001; Figure 2). Pair-wise comparisons revealed the decrease in AXL immediately after (mean change −17±12 μm), and 5 min after exercise (mean change −7±8 μm) were significantly different to baseline (P<0.01), but 10 min after the exercise task, AXL had returned to baseline (mean change −3±9 μm). A significant between-subjects effect of refractive error (P<0.001) was found, indicative of longer AXL in the myopes (mean AXL at baseline was 24.84±0.71 and 23.63±0.61 μm in the myopes and emmetropes, respectively). Although the reduction in AXL immediately following exercise was of slightly greater magnitude in the myopes (mean change −19±14 μm) compared with the emmetropes (mean change −15±12 μm), the difference in AXL changes between the refractive error groups following exercise was not significant (P>0.05).
Table 1. Mean ocular biometric measures at baseline and their changes immediately, and 5 and 10 min after a period of moderate intensity exercise.
| Mean±SD at baseline |
Mean±SD change after exercise |
P-value | |||
|---|---|---|---|---|---|
| 0 min | 5 min | 10 min | |||
| CCT (μm) (n=20) | 538±29 | −0.1±2.6 | 0.0±3.2 | −0.8±3.0 | 0.3 |
| ACD (mm) (n=20) | 3.16±0.35 | 0.001±0.03 | 0.01±0.02 | 0.02±0.03* | 0.01 |
| LT (mm) (n=17)* | 3.56±0.28 | −0.01± 0.03 | −0.01±0.04 | −0.02±0.04 | 0.2 |
| RT (mm) (n=17) | 0.20±0.02 | −0.002±0.003 | −0.001± 0.005 | 0.001±0.005 | 0.1 |
| ChT (mm) (n=16) | 0.28±0.06 | 0.008±0.01 | 0.006±0.01 | 0.005±0.01 | 0.1 |
| AXL (mm) (n=20) | 24.23±0.90 | −0.017±0.01* | −0.007±0.01* | −0.003±0.01 | <0.0001 |
Abbreviations: ACD, anterior chamber depth; AXL, axial length; CCT, central corneal thickness; ChT, choroidal thickness; LT, lens thickness; RT, retinal thickness.
P-value represents the within-subject effect of time from the repeated measures analysis of variance.
Asterisk denotes Bonferroni adjusted pair-wise comparisons, indicating a significant change from baseline (P<0.05).
Figure 2.
The mean change in axial length following exercise. ANOVA for repeated measures revealed the changes in axial length following exercise were statistically significant (P<0.0001). Asterisk indicates a significant change from baseline (P<0.05). Error bars represent the standard error of the mean.
The mean ChT at baseline was 275±62 μm. On average, there was a small increase in ChT observed after exercise with the largest increase (8±11 μm) observed at the immediate post-exercise measurement. However, the changes in ChT following exercise just failed to reach statistical significance (P=0.1). Changes in RT following exercise were also not statistically significant (P=0.1) with the average magnitude of change being small (∼1–2 μm). Neither ChT nor RT exhibited a significant between-subjects effect of refraction, or time by refractive error interaction.
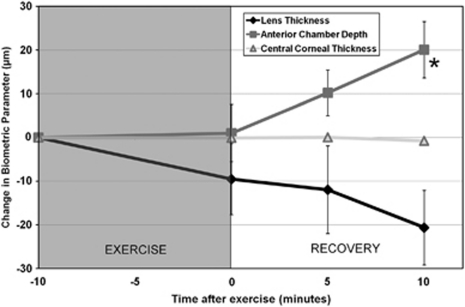
Figure 3 illustrates the change in anterior eye biometrics following exercise. There were no significant changes in CCT following exercise (P=0.33). ACD exhibited a significant increase following exercise (P=0.01). Pair-wise comparisons revealed that only the increase in ACD at the 10 min post-exercise measurement was significantly different to baseline (mean increase of 20±29 μm, P=0.04). Although LT exhibited similar magnitude but opposite direction of change at 10 min after exercise (ie, a decrease in LT of −21±35 μm), the changes in LT were not statistically significant (P=0.2). ACD and LT both exhibited a significant between-subjects effect of refractive error (P<0.05), indicating that on average the myopic subjects exhibited slightly deeper anterior chambers (mean ACD at baseline was 3.34±0.32 mm in the myopes and 3.00±0.30 mm in the emmetropes) and slightly thinner crystalline lenses (mean LT at baseline was 3.40±0.27 mm in the myopes and 3.70±0.24 mm in the emmetropes). Neither parameter exhibited a significant time by refractive error interaction.
Figure 3.
Mean change in anterior eye ocular biometrics following exercise. ANOVA for repeated measures revealed the changes in anterior chamber depth (ACD) were statistically significant (P<0.05). The changes in central corneal thickness (CCT) and lens thickness (LT) were not statistically significant. Asterisk indicates a significant difference from baseline. Error bars represent the standard error of the mean.
Analysis of systemic hemodynamic parameters revealed a significant increase in mean arterial pressure (MAP) following exercise (P<0.001). The largest mean increase of 5.4±5.0 mm Hg in MAP was noted at the first post-exercise measurement. The changes in BP coupled with the reduction in IOP lead to significant increases in OPP following exercise (P<0.0001). The largest increase in OPP of 5.3±3.6 mm Hg was found at the first post-exercise measurement with increases of 3.0±3.6 and 2.6±3.6 mm Hg found at 5 and 10 min after exercise, respectively. The mean resting pulse rate was 72±10 BPM, which increased on average by a magnitude of 66±6 BPM during exercise. At the first post-exercise measurement, an increase in pulse rate of 21±11 BPM was found with increases of 16±10 and 15±8 BPM found at the 5 and 10 min post-exercise measures, respectively. There were no significant between-subjects effects of refractive error or refractive error by time interactions for any of the measured systemic hemodynamic parameters (P>0.05).
ANCOVA revealed that the change in AXL had a significant positive association with the change in IOP (r2=0.36, P<0.0001) and OPA (r2=0.22, P<0.01), and a significant negative association with the change in pulse rate (r2=0.31, P<0.0001). In addition, the change in OPA had a significant positive association with the change in IOP (r2=0.50, P<0.0001) and a significant negative association with the MAP (r2=0.18, P<0.01) and pulse rate (r2=0.61, P<0.0001).
Discussion
This study demonstrates in a population of young adult subjects that a period of dynamic exercise leads to significant changes in ocular parameters including IOP, OPA and AXL. Although a number of previous studies have noted changes in a range of ocular measures following dynamic exercise, including IOP,12, 13, 14, 15, 16 OPA17, 18 and choroidal blood flow;19, 20 this is the first study to demonstrate that AXL also changes following exercise. The short-term changes in AXL are small and unlikely to be of clinical significance, however, they demonstrate that the physiological changes occurring as a consequence of a short period of dynamic exercise, lead to transient changes in ocular biometrics.
Although the exact mechanism underlying the changes in AXL following exercise is not known, a significant association was found between the changes in AXL and IOP. It is therefore possible that the reductions observed in IOP may have contributed to the exercise induced AXL changes. Previous studies in humans have shown that reductions in IOP (either mechanically or pharmacologically induced) are associated with short-term decreases in AXL, suggesting a mechanical contraction/expansion of the globe with changes in IOP.5, 6 Our findings of a reduction in AXL accompanying the reduced IOP following a short period of dynamic exercise are consistent with these previous findings.
Dynamic exercise has also previously been found to lead to small increases in ocular blood flow.17, 18, 19, 20 Lovasik et al19 reported a significant increase of choroidal blood flow of 5–6% during a period of dynamic exercise. Although choroidal blood flow was not measured in this study, a small magnitude increase in this parameter would be expected to lead to expansion of the choroid, and an accompanying reduction in AXL. We found a significant reduction in AXL and observed a trend for a small increase in ChT (mean change of ∼3%) following exercise, however, the changes in ChT did not reach statistical significance. Further research using a technique capable of more precise determination of ChT is required to confirm whether significant changes in choroidal biometrics occur following exercise.
We also found significant changes in ACD occur after exercise, indicating a deepening of the anterior chamber following exercise. Although the observed decreases in LT were not statistically significant, they were of similar magnitude to the increases in the ACD, which suggests that these changes may be occurring because of the alterations in the accommodative tone after exercise, which could potentially reflect changes in the autonomic nervous system associated with dynamic exercise.35 This is consistent with previous research that indicates a relationship between cardiovascular function, systemic autonomic tone and the ocular accommodation system.36 Changes in aqueous fluid dynamics in the eye could also potentially be involved in the anterior chamber variations observed, particularly considering that previous studies have also found reductions in IOP to be accompanied by increases in ACD.5
Our findings may have implications for longer-term refractive error development. A number of recent studies have noted a link between outdoor activities and refractive error development with the majority of these studies suggesting that greater amounts of time spent on outdoor activities are associated with reduced chances of developing myopia.37, 38, 39, 40, 41, 42 Cross-sectional studies of children examining the relationship between refractive error and both subjective reporting of sports and outdoor activities37, 42 and objectively measured physical activity levels41 have noted a lower prevalence of myopia in children performing greater amounts of physical and outdoor activities. These associations seem to be independent of near work activities.40, 42 Similarly, longitudinal studies of both children38 and young adults39 have found that subjects performing greater amounts of physical and outdoor activities were less likely to subsequently develop myopia. The exact mechanism underlying this apparent protective effect of outdoor activity is unclear, although it has been hypothesized that the biochemical effects of exercise39, 42 or the high intensity of lighting experienced outdoors40 may have an inhibitory effect on eye growth.
It has also been suggested that an imbalance of the autonomic nervous system (eg, a deficit in ocular sympathetic innervations to the accommodation system) may be a factor associated with myopia development.43 The acute effects of exercise noted in this study and the known association between cardiovascular function and ocular accommodation36 suggest that exercise induced changes in autonomic balance44 could potentially influence ocular accommodation and could have implications for refractive error development. However, it should be noted that there is limited evidence to support a direct link between autonomic imbalance and refractive error.45
Our findings tend to support the potential role of physical activity in the protective effects of outdoor activity in myopia development, particularly given that previous studies with experimental animals have shown that short term increases in choroidal blood flow46 and ChT47 are associated with an inhibition of myopic eye growth. However, it is also possible that other factors (such as light intensity) do also have a role. Recent evidence from animal studies supports the potential role of light intensity in the inhibition of myopic eye growth.48
Although there is some evidence to suggest that exercise training may reduce resting levels of IOP,49, 50 the influence of physical fitness on refractive error development remains to be determined. However, our findings coupled with recent epidemiological research41, 42 suggest this may be an area worthy of future research. It should also be noted that our exercise task was performed indoors under standard laboratory lighting conditions. Previous findings of increases in choroidal blood flow associated with higher ambient lighting levels51, 52 suggest that the magnitude of exercise induced ocular change could be larger as a result of exercise performed outdoors in sunlight. Further research is required to clarify the relative importance of lighting and physical activity on both short-term and long-term ocular change and their association with refractive error development.
The decrease in IOP and OPA, that we have observed in our population of young adult subjects using the DCT instrument, largely confirm previous studies investigating the influence of dynamic exercise on IOP and OPA using a pneumotonometer.17, 18 The reduction in OPA was found to be moderately associated with the changes in pulse rate after exercise, indicating that as the pulse rate increases, the OPA decreases. This implies that exercise induced increases in pulse rate are associated with a decrease in pulse volume (as the change in IOP with each pulse decreased as pulse rate increased), as has been previously documented.18
Conclusions
A short period of dynamic exercise leads to significant changes in a range of ocular parameters of young adult subjects. These findings illustrate that significant biometric changes occur in the human eye following exercise. The significant reduction in AXL may be related to exercise induced changes in IOP and blood flow, and may have implications for refractive error development in human subjects.
Acknowledgments
We thank Beata Sander and Stephen Witt for their assistance with analysis procedures in this study. We acknowledge the support of the QUT Myopia Research Fellowship.
The authors declare no conflict of interest.
References
- Drexler W, Findl O, Schmetterer L, Hitzenberger CK, Fercher AF. Eye elongation during accommodation in humans: differences between emmetropes and myopes. Invest Ophthalmol Vis Sci. 1998;39:2140–2147. [PubMed] [Google Scholar]
- Mallen EA, Kashyap P, Hampson KM. Transient axial length change during the accommodation response in young adults. Invest Ophthalmol Vis Sci. 2006;47:1251–1254. doi: 10.1167/iovs.05-1086. [DOI] [PubMed] [Google Scholar]
- Read SA, Collins MJ, Woodman EW, Cheong S-H. Axial length changes during accommodation in myopes and emmetropes. Optom Vis Sci. 2010;87:656–662. doi: 10.1097/OPX.0b013e3181e87dd3. [DOI] [PubMed] [Google Scholar]
- Read SA, Collins MJ, Sander BP. Human optical axial length changes in response to defocus. Invest Ophthalmol Vis Sci. 2010;51 (12:6262–6269. doi: 10.1167/iovs.10-5457. [DOI] [PubMed] [Google Scholar]
- Leydolt C, Findl O, Drexler W. Effects of change in intraocular pressure on axial eye length and lens position. Eye. 2008;22:657–661. doi: 10.1038/sj.eye.6702709. [DOI] [PubMed] [Google Scholar]
- Ebneter A, Wagels B, Zinkernagel MS. Non-invasive biometric assessment of ocular rigidity in glaucoma patients and controls. Eye. 2009;23:606–611. doi: 10.1038/eye.2008.47. [DOI] [PubMed] [Google Scholar]
- Read SA, Collins MJ. Water drinking influences eye length and IOP in young healthy subjects. Exp Eye Res. 2010;91:180–185. doi: 10.1016/j.exer.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Stone RA, Quinn GE, Francis EL, Ying G-S, Flitcrift DI, Parekh P, et al. Diurnal axial length fluctuations in human eyes. Invest Ophthalmol Vis Sci. 2004;45:63–70. doi: 10.1167/iovs.03-0294. [DOI] [PubMed] [Google Scholar]
- Wilson LB, Quinn GE, Ying G-S Francis EL, Schmid G, Lam A, et al. The relation of axial length and intraocular pressure fluctuations in human eyes. Invest Ophthalmol Vis Sci. 2006;47:1778–1784. doi: 10.1167/iovs.05-0869. [DOI] [PubMed] [Google Scholar]
- Read SA, Collins MJ, Iskander DR. Diurnal variation of axial length, intraocular pressure, and anterior eye biometrics. Invest Ophthalmol Vis Sci. 2008;49:2911–2918. doi: 10.1167/iovs.08-1833. [DOI] [PubMed] [Google Scholar]
- Brown JS, Flitcroft DI, Ying G-S, Francis EL, Schmid GF, Quinn GE, et al. In vivo human choroidal thickness measurements: evidence for diurnal fluctuations. Invest Ophthalmol Vis Sci. 2009;50:5–12. doi: 10.1167/iovs.08-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton DA, Phillips CI. Effect of moderate exercise on the ocular tension. Br J Ophthalmol. 1970;54:599–605. doi: 10.1136/bjo.54.9.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teraslinna KP, Rowe DG, Jackson J. Standardized aerobic and anaerobic exercise: differential effects on intraocular tension, blood pH and lactate. Invest Ophthalmol Vis Sci. 1975;14:782–785. [PubMed] [Google Scholar]
- Ashkenazi I, Melamed S, Blumenthal M. The effect of continuous strenuous exercise on intraocular pressure. Invest Ophthalmol Vis Sci. 1992;33:2874–2877. [PubMed] [Google Scholar]
- Harris A, Malinovsky V, Martin B. Correlates of acute exercise-induced ocular hypotension. Invest Ophthalmol Vis Sci. 1994;35:3852–3857. [PubMed] [Google Scholar]
- Martin B, Harris A, Hammel T, Malinovsky V. Mechanism of exercise-induced ocular hypotension. Invest Ophthalmol Vis Sci. 1999;40:1011–1015. [PubMed] [Google Scholar]
- Price EL, Gray LS, Humphries L, Zweig C, Button NF. Effect of exercise on intraocular pressure and pulsatile ocular blood flow in a young normal population. Optom Vis Sci. 2003;80:460–466. doi: 10.1097/00006324-200306000-00013. [DOI] [PubMed] [Google Scholar]
- Lovasik JV, Kergoat H. Consequences of an increase in the ocular perfusion pressure on the pulsatile ocular blood flow. Optom Vis Sci. 2004;81:692–698. doi: 10.1097/01.opx.0000144748.65471.e5. [DOI] [PubMed] [Google Scholar]
- Lovasik JV, Kergoat H, Riva CE, Petric BL, Geiser M. Choroidal blood flow during exercise-induced changes in the ocular perfusion pressure. Invest Ophthalmol Vis Sci. 2003;44:2126–2132. doi: 10.1167/iovs.02-0825. [DOI] [PubMed] [Google Scholar]
- Okuno T, Sugiyama T, Kohyama M, Kojima S, Oku H, Ikeda T. Ocular blood flow changes after dynamic exercise in humans. Eye. 2006;20:796–800. doi: 10.1038/sj.eye.6702004. [DOI] [PubMed] [Google Scholar]
- Iester M, Torre PG, Bricola G, Bagnis A, Calabria G. Retinal blood flow autoregulation after dynamic exercise in healthy young subjects. Ophthalmologica. 2007;221:180–185. doi: 10.1159/000099298. [DOI] [PubMed] [Google Scholar]
- Ritter AD, Huhn-Beck H. Dark focus of accommodation and nervous system activity. Optom Vis Sci. 1993;70:532–534. [PubMed] [Google Scholar]
- Hayashi N, Someya N, Fukuba Y. Effect of intensity of dynamic exercise on pupil diameter in humans. J Physiol Anthropol. 2010;29:119–122. doi: 10.2114/jpa2.29.119. [DOI] [PubMed] [Google Scholar]
- Haargaard B, Jensen PK, Kessing SV, Nissen OI. Exercise and iris concavity in healthy eyes. Acta Ophthalmol. 2001;79:277–282. doi: 10.1034/j.1600-0420.2001.790313.x. [DOI] [PubMed] [Google Scholar]
- Kergoat H, Forcier P. Correlation of an exercise-induced increase in systemic circulation with neural retinal function in humans. Doc Ophthalmol. 1996;92:145–147. doi: 10.1007/BF02583286. [DOI] [PubMed] [Google Scholar]
- Wimpissinger B, Resch H, Berisha F, Weigert G, Polak K, Schmetterer L. Effects of isometric exercise on subfoveal choroidal blood flow in smokers and nonsmokers. Invest Ophthalmol Vis Sci. 2003;44:4859–4863. doi: 10.1167/iovs.03-0391. [DOI] [PubMed] [Google Scholar]
- Buckhurst PJ, Wolffsohn JS, Shah S, Naroo SA, Davies N, Berrow EJ. A new optical coherence reflectometry device for ocular biometry in cataract patients. Br J Ophthalmol. 2009;93:949–953. doi: 10.1136/bjo.2008.156554. [DOI] [PubMed] [Google Scholar]
- Kanngiesser HE, Kniestedt C, Robert YCA. Dynamic contour tonometry. Presentation of a new tonometer. J Glaucoma. 2005;14:344–350. doi: 10.1097/01.ijg.0000176936.16015.4e. [DOI] [PubMed] [Google Scholar]
- Kaufmann C, Bachmann LM, Thiel MA. Comparison of dynamic contour tonometry with goldmann applanation tonometry. Invest Ophthalmol Vis Sci. 2004;45:3118–3121. doi: 10.1167/iovs.04-0018. [DOI] [PubMed] [Google Scholar]
- Kaufmann C, Bachman LM, Robert YC, Thiel MA. Ocular pulse amplitude in healthy subjects as measured by dynamic contour tonometry. Arch Ophthalmol. 2006;124:1104–1108. doi: 10.1001/archopht.124.8.1104. [DOI] [PubMed] [Google Scholar]
- Fletcher GF. How to implement physical activity in primary and secondary prevention. A statement for healthcare professionals from the task force on risk reduction, American Heart Association. Circulation. 1997;96:355–357. doi: 10.1161/01.cir.96.1.355. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- Riva CE, Sinclair SH, Grunwald JE. Autoregulation of retinal circulation in response to decrease of perfusion pressure. Invest Ophthalmol Vis Sci. 1981;21:34–38. [PubMed] [Google Scholar]
- Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: part 1 - correlation within subjects. BMJ. 1995;310:446. doi: 10.1136/bmj.310.6977.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwinkel ET, Bloomfield DM, Arwady MA, Goldsmith RL. Exercise and autonomic function in health and cardiovascular disease. Cardiol Clin. 2001;19:369–387. doi: 10.1016/s0733-8651(05)70223-x. [DOI] [PubMed] [Google Scholar]
- Davies LN, Wolffsohn JS, Gilmartin B. Autonomic correlates of ocular accommodation and cardiovascular function. Ophthal Physiol Opt. 2009;29:427–435. doi: 10.1111/j.1475-1313.2009.00635.x. [DOI] [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Moeschberger ML, Jones LA, Zadnik K. Parental myopia, near work, school achievment and children's refractive error. Invest Ophthalmol Vis Sci. 2002;43:3633–3640. [PubMed] [Google Scholar]
- Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007;48:3524–3532. doi: 10.1167/iovs.06-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen N, Jensen H, Goldschmidt E. Does the level of physical activity in university students influence development and progression of myopia? A 2 year prospective cohort study. Invest Ophthalmol Vis Sci. 2008;49:1322–1327. doi: 10.1167/iovs.07-1144. [DOI] [PubMed] [Google Scholar]
- Rose KA, Morgan IG, Ip J, Kifley A, Huynh S, Smith W, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279–1285. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Deere K, William C, Leary S, Mattocks C, Ness A, Blair SN, et al. Myopia and later physical activity in adolescence: a prospective study. Br J Sports Med. 2009;43:542–544. doi: 10.1136/bjsm.2008.049288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirani M, Tong L, Gazzard G, Zhang X, Chia A, Young TL, et al. Outdoor activity and myopia in Singapore teenage children. Br J Ophthalmol. 2009;93:997–1000. doi: 10.1136/bjo.2008.150979. [DOI] [PubMed] [Google Scholar]
- Chen JC, Schmid KL, Brown B. The autonomic control of accommodation and implications for human myopia development: a review. Ophthal Physiol Opt. 2003;23:401–422. doi: 10.1046/j.1475-1313.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- Goldsmith RT, Bigger JT, Steinman RC, Fleiss JL. Comparison of 24-h parasympathetic activity in endurance trained and untrained young men. J Am Coll Cardiol. 1992;20:552–558. doi: 10.1016/0735-1097(92)90007-a. [DOI] [PubMed] [Google Scholar]
- Mallen EAH, Gilmartin B, Wolffsohn JS. Sympathetic innervation of ciliary muscle and oculomotor function in emmetropic and myopic yound adults. Vision Res. 2005;45:1641–1651. doi: 10.1016/j.visres.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MEC, Wildsoet CF, Reiner A. Temporal relationship of choroidal blood flow and thickness changes during recovery from form deprivation myopia in chicks. Exp Eye Res. 2002;74:561–570. doi: 10.1006/exer.2002.1142. [DOI] [PubMed] [Google Scholar]
- Nickla DL. Transient increases in choroidal thickness are consistently associated with brief daily visual stimuli that inhibit ocular growth in chicks. Exp Eye Res. 2007;84:951–959. doi: 10.1016/j.exer.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Ashby R, Ohlendorf A, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2009;50:5348–5354. doi: 10.1167/iovs.09-3419. [DOI] [PubMed] [Google Scholar]
- Sargent RG, Blair SN, Magun JC, Krejci RC, Sacoco C, Langley TD, et al. Physical fitness and intraocular pressure. Am J Optom Physiol Opt. 1981;58:460–466. doi: 10.1097/00006324-198106000-00005. [DOI] [PubMed] [Google Scholar]
- Passo MS, Goldberg L, Elliot DL, Van Buskirk M. Exercise training reduces intraocular pressure among subjects suspected of having glaucoma. Arch Ophthalmol. 1991;109:1096–1098. doi: 10.1001/archopht.1991.01080080056027. [DOI] [PubMed] [Google Scholar]
- Longo A, Geiser M, Riva CE. Subfoveal choroidal blood flow in response to light-dark exposure. Invest Ophthalmol Vis Sci. 2000;41:2678–2683. [PubMed] [Google Scholar]
- Fuchsjäger-Mayrl G, Polska E, Malec M, Schmetterer L. Unilateral light-dark transitions affect choroidal blood flow in both eyes. Vision Res. 2001;41:2919–2924. doi: 10.1016/s0042-6989(01)00171-7. [DOI] [PubMed] [Google Scholar]