Abstract
Purpose
To determine whether the incidence rate and severity of dry eye after hematopoietic stem cell transplantation varies with donor vsrecipient gender.
Methods
We limited this study to patients received bone marrow transplantation (BMT). In all, 172 patients received BMT at Keio University School of Medicine between January 2000 and May 2007. Of them, 136 recipients who survived at least 70 days were studied prospectively. We classified the 136 patients according to the gender of the donor and the recipient (group I: female to female; group II: male to male; group III: male to female; group IV: female to male). The incidence and severity of chronic graft-vs-host disease-associated dry eye were determined for each group. The donor gender was masked when we assessed dry eye and calculate the incidence.
Results
The incidence of dry eye was 47.4% for group I, 37.5% for group II, 58.6% for group III, and 42.9% for group IV. The percentage of patients with severe dry eye was 44.4, 50.0, 35.3, and 77.8% respectively. There was a significant difference between the percent severe dry eye/total dry eye incidences in groups III and IV (P=0.0375) (odds ratio, 7.6; 95% confidence interval, 1.00–101.01).
Conclusions
Close attention must be paid to the development of dry eye in cases of female to male BMTs, because the ratio of severe/total dry eye is more common in cases of female to male BMTs than in other gender combination.
Keywords: dry eye, allogeneic hematopoietic stem cell transplantation, chronic graft-vs-host disease, gender-mismatched transplantation, minor histocompatibility antigens
Introduction
Graft-vs-host disease (GVHD) is a major complication subsequent to hematopoietic stem cell transplantation (HSCT). Many HSCT recipients become long-term survivors of their disease, and chronic GVHD (cGVHD) occurs in 30–70% of allogeneic HSCT recipients.1, 2 As a result of this, assuring patient quality of life (QOL) and addressing late complications after HSCT have become increasingly important. The eye, mouth, liver, lung, skin, and intestine are preferential targets of cGVHD.3 Dry eye has emerged as a major complication of systemic cGVHD as well as ocular cGVHD that strongly affects patient QOL.4 So far, neither radical treatment nor prophylaxis has been established for cGVHD-related dry eye. This study was intended to learn which patients are at the higher risk for developing progressive dry eye.
It is generally known that a donor–recipient gender mismatch in HCST often leads to severe GVHD.5 In particular, GVHD in male recipients of female donors tends to be especially severe.6, 7, 8, 9 Given the current knowledge about gender mismatch and GVHD, it seems likely that HSCT with a female donor and male recipient could increase the patient's risk of developing cGVHD-related severe dry eye. However, no report on donor–recipient gender mismatch affecting ocular GVHD has been published in the ophthalmologic literature.
In this retrospective study, we focused on donor–recipient gender only in cases treated by bone marrow transplantation (BMT), and investigated the incidence and severity of dry eye associated with matched and mismatched gender in donor–recipient pairs.
Patients and methods
Study design
This was a retrospective survey evaluating the late effects of BMT. All research and measurements followed the tenets of the Declaration of Helsinki. The study was approved by the ethics committee of the Keio University School of Medicine. All patients underwent standardized clinical and ophthalmological evaluation, as described below, before BMT and 3, 6, 9, 12, 18, 24, and 30 months after BMT, as well as on additional occasions as indicated.
Between January 2000 and May 2007, 172 patients underwent BMT at Keio University School of Medicine. We selected patients who survived for at least 70 days after BMT for this study. This criterion selected patients who survived beyond the time of acute GVHD (aGVHD) development and who could potentially develop cGVHD. We excluded patients under the age of 15 and those who underwent re-transplantation. In total, 36 of the 172 recipients were excluded from this study, and the remaining 136 patients were studied prospectively. When we examined BMT patients, the donor genders were masked. We classified the 136 patients according to the genders of the donor and recipient, and determined the incidence rate and severity of dry eye among these groups ((donor to recipient) group I: female to female; group II: male to male; group III: male to female; group IV: female to male).
Clinical evaluation
Ocular surface vital staining
The fluorescein and rose bengal stain scores for the ocular surface were obtained using the double-vital staining method.10, 11, 12, 13 Two microliter of a preservative-free solution of 1% rose bengal and 1% fluorescein was instilled into the conjunctival sac by a micropipette.10 The van Bijsterveld scoring system was used for the rose bengal staining.14 For the rose bengal score, the ocular surface was divided into the three zones: nasal conjunctival, corneal, and temporal. A score of 0–3 points was used for each zone, with a minimum possible score of 0 and a maximum possible score of 9. Scarce punctuate staining was given 1 point. Denser staining not covering the entire zone was given 2 points. Denser staining over the entire zone was given 3 points. For fluorescein staining, the cornea was divided three equal upper, middle, and lower zone. Each zone has a staining score ranging from 0 to 3 points, as with the rose bengal stain, and a minimum and maximum score was 0 and 9, respectively. The presence of scarce staining in a zone was scored 1; frequent puncta not covering the entire zone was scored as 2 points; and puntate staining covering the entire zone was scored as 3 points.15
Tear function test
Tear film break-up time (TBUT) was measured three times at the time of double staining, and the median value was calculated.11 The Schirmer test was performed using standard strips (Alcon, Fort Worth, TX, USA) placed in the lower conjunctival sac for 5 min without anesthesia. For the Schirmer test with nasal stimulation, the standard strips were placed in the conjunctival sac for 5 min, while a cotton–wool swab was inserted into the nose until the tip reached the nasal membrane of the ethmoid sinus. The middle turbinate was touched with the cotton–wool swab, which was kept in place for 5 min. We then measured the length of the moistened part of the standard strip from the conjunctival sac.16
Diagnostic criteria
Dry eye was diagnosed as a disorder of tear film caused by tear deficiency and/or excessive tear evaporation, which cause the damage of ocular surface with or without symptom.12 Dry eye was diagnosed when the tear film of patients showed disturbance of tear dynamics (TBUT ≤5 s, Schirmer test ≤5 mm) and the ocular surface was abnormal (rose bengal score ≥3, fluorescein score ≥1).13 Severe dry eye was defined as reduced reflex tearing (Schirmer test with nasal stimulation ≤10 mm) and abnormality of the ocular surface (rose bengal score ≥3 and/or fluorescein score ≥3)14 and/or a grade of 3 or 4 according to the dry eye workshop (DEWS) report 2007.17 Briefly, DEWS proposed the classification of dry eye severity level, which is classified according to the grade of symptom, ocular surface findings, and tear dynamics. The severity level 3 or 4 was regarded as the sign of severe frequent or constant and/or disabling symptom, severe ocular surface damage accompanied by marked injection, filamentary keratitis, mucous clumping, tear debris, and ulceration. When trichiasis, keratinization, and symblepharon along with sign of symptom of dry eye were present, dry eye was also regarded as grade 3 and 4. In addition, TBUT and Schirmer scores are ≤5 s and ≤5 mm for grade 3 and immediate and ≤2 mm for grade 4, respectively.
Mild dry eye was defined as abnormality of the ocular surface (rose bengal score ≥3, fluorescein score ≥1) without reduced reflex tearing (Schirmer test with nasal stimulation >10 mm). According to the grading system of the severity level of dry eye based on DEWS report, grade 1 and 2 were regarded as mild to moderate stress, and none to mild, or variable conjuntival injection, conjunctival or corneal staining or corneal/tear signs. In addition, TBUT and Schirmer scores are variable for grade 1, and ≤10 s and ≤10 mm for grade 2, respectively. Patients who had dry eye before BMT were considered to have a sustained a dry eye incident only when the severity of the dry eye worsened after the transplantation.
Statistical analysis
Fisher's direct method was used to evaluate differences among the groups. Statistical analyses were performed using R statistical software (Free Software Foundation, Boston, MA, USA). R is available freely on web site. This is an open source software for statistical analysis.18, 19
In this article, version-2.9.1 was available. Significant difference was defined as P≤0.05.
Results
The 136 subjects survived for at least 70 days after BMT and were evaluable for the presence and severity of dry eye. The median age of the patients was 44 years (range 18–61 years). The median age of the donors was 35 years (range 18–80 years). Clinical characteristics of the 136 patients are shown in Table 1. There were no significant differences between patient and donor age in any group. The percentage of unrelated donors was higher than that of related donors in all groups. There was no statistical significant when we carried out a statistical analysis concerning related/unrelated parameters of donor, that is, the genetic influence in all four groups (Table 1).
Table 1. Patient characteristics in the four donor–recipient gender groups.
| I (F → F) | II (M → M) | III (M → F) | IV (F → M) | |
|---|---|---|---|---|
| n=38 | n=48 | n=29 | n=21 | |
| Patient age (range) | 39.5 (20–60) | 44.5 (18–59) | 43 (25–61) | 47 (18–59) |
| Donor age (range) | 36 (33–63) | 33 (17–58) | 38 (21–80) | 36 (23–52) |
| Donor relation | ||||
| Unrelated (%) | 27 (71) | 36 (75) | 23 (79.3) | 17 (81) |
| Related (%) | 11 (29) | 12 (25) | 6 (20.7) | 4 (19) |
Abbreviations: F, female; M, male.
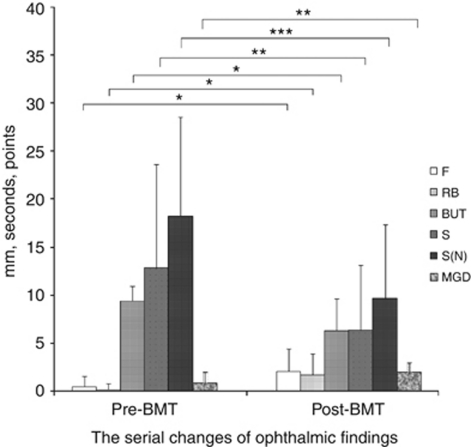
We showed the baseline profile of pre-BMT and the serial change of ophthalmic findings at pre- and post-BMT (Tables 2 and Table 3, Figure 1). There were significant differences of the clinical variables of ocular surface findings and tear dynamics between at the pre- and post-BMT (Figure 1).
Table 2. Baseline profile ocular surface findings and tear dynamics of pre-BMT.
| Pre-F | RB | TBUT | S | S(N) | MGD |
|---|---|---|---|---|---|
| 0.49±1.09 (n=136) | 0.19±1.07 (n=135) | 9.36±1.05 (n=117) | 12.95±1.72 (n=129) | 19.02±10.31 (n=84) | 0.86±1.18 (n=40) |
Abbreviations: F, fluorescein score; MGD, meibomian gland dysfunction; RB, rose bengal score; S, value of Schirmer test; S (N), value of Schirmer test with nasal stimulation; TBUT, tear film break-up time.
Table 3. Summary of clinical evaluation at pre- and post-BMT.
| F | RB | TBUT | S | S(N) | MGD | |
|---|---|---|---|---|---|---|
| Pre | ||||||
| I (n=38) | 0.5±0.9(38) | 0.1±0.5 (37) | 9.0±2.1 (34) | 14±11.8 (35) | 18.0±10.4 (20) | 0.8±1.1 (8) |
| II (n=48) | 0.5±1.2 (48) | 0.1±0.5 (48) | 9.7±1.1 (38) | 11.9±10.5 (48) | 16.1±9.0 (34) | 0.9±1.3 (15) |
| III (n=29) | 0.3±0.7 (29) | 0.1±0.6 (29) | 9.6±1.1 (27) | 11.8±8.5 (29) | 20.4±11.2 (20) | 0.8±0.7 (12) |
| IV (n=21) | 0.8±1.5 (21) | 0.4±0.9 (21) | 9.2±1.6 (18) | 14.2±12.0 (20) | 21.6±11.3 (10) | 1.0±1.3 (5) |
| Post | ||||||
| I (n=38) | 2.1±2.6 (38) | 1.7±2.2 (35) | 5.7±3.5 (34) | 5.6±7.9 (17) | 6.4±5.6 (9) | 1.9±1.0 (12) |
| II (n=48) | 2.1±2.4 (47) | 1.6±2.3 (44) | 7.0±3.1 (46) | 6.8±5.2 (18) | 12.1±8.6 (15) | 1.7±0.9 (9) |
| III (n=29) | 2.1±2.1 (28) | 1.5±2.1 (27) | 6.2±3.3 (27) | 6.1±6.0 (12) | 8.9±7.6 (7) | 2.3±0.7 (7) |
| IV (n=21) | 2.0±2.1 (21) | 2.0±2.2 (20) | 5.9±3.2 (21) | 6.9±7.5 (11) | 9.4±6.5 (10) | 3.0±0.0 (3) |
Abbreviations: F, fluorescein score; MGD, meibomian gland dysfunction; Pre, pre-BMT; Post, post-BMT; RB, rose bengal score; S, value of Schirmer test; S (N), value of Schirmer test with nasal stimulation; TBUT, tear film break-up time.
Figure 1.
Clinical variables for dry eye parameters at pre- and post-BMT. There were significant differences in ophthalmic findings of pre- and post-BMT. Ocular surface findings and tear dynamics were deteriorated after BMT. F, fluorescein score; RB, rose bengal score; TBUT, tear film break-up time; S, value of Schirmer test; S(N), value of Schirmer test with nasal stimulation; MGD, meibomian gland dysfunction. *P<0.001, **P<0.01, ***P<0.05.
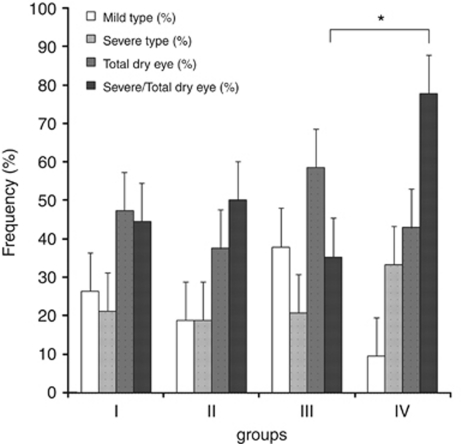
The incidence rate of mild dry eye, severe dry eye, and total dry eye was not significantly different in any of the four groups (Table 4). However, the percentage of patients with dry eye that became severe was greatest in group IV (77.8%), and the incidence was significantly higher than in group III (35.3%) (P=0.0375). Odds ratio (OR) was 7.6 (95% confidence interval: 1.00–101.01). The severity of dry eye was more prevalent in group IV more than group III (Table 4, Figure 2). We double checked the severity of dry eye using diagnostic criteria 2007, but the severity determinations were the same for our usual criteria12 and the DEWS criteria.17
Table 4. Incidence rate of dry eye, mild dry eye, severe dry eye, and severe/total dry eye in the four groups.
| I | II | III | IV | |
|---|---|---|---|---|
| Mild type (%) | 26.3 | 18.8 | 37.9 | 9.5 |
| Severe type (%) | 21.1 | 18.8 | 20.7 | 33.3 |
| Total dry eye (%) | 47.4 | 37.5 | 58.6 | 42.9 |
| Severe/total dry eye (%) | 44.4 | 50 | 35.3 | 77.8* |
*P=0.0375.
Figure 2.
Comparison of the frequency of various type dry eye among four groups. The patients with dry eye that became severe was greatest in group IV (77.8%), and the incidence was significantly higher than in group III (35.3%) (*P=0.0375). OR was 7.6 (95% confidence interval: 1.00–101.01). The severity of dry eye was more prevalent in group IV more than group III.
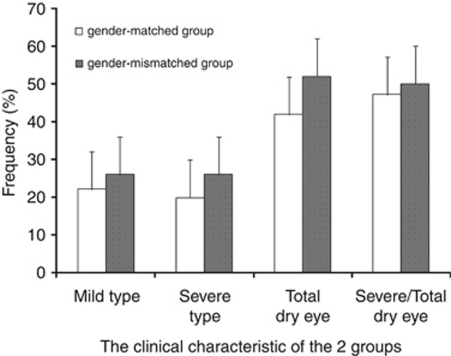
We then evaluated the results by pooling the two gender-matched groups and the two gender-mismatched ones. The incidence rate for total, mild, and severe dry eye was slightly higher in the gender-mismatched group, although significant differences were not observed between these two groups (Figure 3).
Figure 3.
Comparison of the frequency of various type dry eye between gender-matched and gender-mismatched groups. There were no significant differences between gender-matched and gender-mismatched group. However the incidence rate of total, mild, and severe dry eye was slightly higher in gender-mismatch group.
Discussion
Here, we investigated the incidence rate and severity of dry eye associated with matched and mismatched genders in donors and recipients after HSCT, in patient groups with few differences in patient characteristics (Table 1).
The efficacy of HSCT is improved by the graft-vs-leukemia (GVL) effect, a result of moderate aGVHD, but the downside of the GVL effect is an increased risk of cGVHD. Therefore, it is important to control the degree of GVHD. It is known that host rejection and GVHD occur even in HLA-matched donor–recipient pairs, because of mismatches in the minor histocompatibility antigens.6, 8, 9
We found that, if dry eye occurred at all, it usually became severe among male BMT recipients whose donor was female (OR, 7.6). Conversely, there was much less progression to severe dry eye in female BMT recipients of donor tissue from males. These findings are consistent with previous reports on the severity of systemic GVHD, in which male HSCT recipients of female donor tissue had a significantly higher probability of developing systemic GVHD compared with the recipients of other recipient–donor gender combinations.6, 7, 8, 9 These results indicate that dry eye well reflects systemic cGVHD, suggesting that dry eye could be a hallmark of cGVHD based on the assessment of dry eye parameters compared with pre-BMT (Figure 1). There was no difference in the overall incidence of dry eye among the four groups. However, there was a significant difference in the percentage of severe dry eye/total dry eye between the two gender mismatched groups, suggesting that gender-related factors affect the severity of dry eye after BMT (Figure 2). Particularly, male BMT recipients from female donors have possibility of progressing of severer dry eye.
Copelan20 reported that minor antigens encoded by genes on the Y chromosome account for the higher incidence of GVHD and lower rate of patient relapse among male recipients of marrow transplants from female donors than among male recipients of transplants from male donors. Miklos et al proposed that, because female donor T cells have not been exposed to unique epitopes on the Y chromosome, thymic maturation does not delete the T cells capable of recognizing the H-Y antigens.21 Besides the T cells, antibodies against the H-Y antigen made by donor B cells probably also contribute to GVHD.21, 22 There are some reports that male recipients whose female donors had previous pregnancies or blood transfusions are at increased risk of developing GVHD,23, 24, 25 apparently because of B-cell sensitization to the H-Y antigen. In this study, the previous donor pregnancies and transfusions was not checked. Although many factors contribute to the severity, there is a possible that our study supports these earlier findings, showing a higher percentage of cGVHD-associated dry eye progressing to the severe form in male recipients of bone marrow from a female donor.
We also pooled the groups to compare all gender-matched pairs with all gender-mismatched pairs. We found the incidence of dry eye in the gender-mismatched pairs to be slightly higher than in gender-matched pairs, although the difference was not significant (Figure 3). This finding also agrees with previous studies in the medical literature, in which the occurrence of GVHD in gender-mismatched pairs was higher than in gender-matched pairs.5 However, some other reports have not shown a significant difference in GVHD incidence between gender-mismatched and gender-matched pairs.24, 26 In this study, we found no significant differences in patient age in these two groups, ruling out age as a contributing factor. However, the diagnosis, stage of the disease, and genetic relatedness between the donor and the recipient, which we did not take into account, all seem to affect the incidence of GVHD.
The only significant difference was the percentage of patients with dry eye who developed severe dry eye, and this difference reached significance only in the group III vs group IV comparison. However, patient refusal of the Schirmer test with nasal stimulation may have distorted the data from this test (Table 3). We therefore, double checked the severity level of dry eye according to the DEWS report 2007.
Our study indicates that there was a significant association between severe dry eye and female to male BMT. We are unaware of any previous report on the severity of dry eye correlating with a gender-donor mismatch, although we searched PubMed and MEDLINE reviews. Until now, little attention has been paid to the possible risk for severe dry eye after HSCT associated with donor–recipient gender mismatch. Our study indicates that careful observation is needed for male BMT recipients of female donors. As dry eye well reflects systemic cGVHD, suggesting that dry eye could be a hallmark of cGVHD. As severe dry eye can lead to blindness and poor QOL, early detection to prevent dry eye progression is important.
In this study, we investigated the effect of donor–recipient mismatch on dry eye and severe dry eye incidence, without taking into consideration the relatedness of donor and recipient, previous donor pregnancies, or the recipient's disease diagnosis or stage. Further study will be required to understand the relative impact of these various factors, and will lead to a better understanding of cGVHD.

Acknowledgments
This study was supported in part by grants from the Japanese Ministry of Education, Science, Sports, and Culture (no. 20592058).
The authors declare no conflict of interest.
References
- Arai S, Vogelsang GB. Management of graft-versus-host disease. Blood Rev. 2000;14:190–204. doi: 10.1054/blre.2000.0137. [DOI] [PubMed] [Google Scholar]
- Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- Sullivan KM.Graft-vs-host diseaseIn: Blume KG, Forman SJ, Applebaum FR (eds).Thomas' Hematopoietic Cell Transplantation3rd edn.Blackwell: Malden, MA; 2004635–664. [Google Scholar]
- Ogawa Y, Kuwana M. Dry eye as a major complication associated with chronic graft-versus-host disease after hematopoietic stem cell transplantation. Cornea. 2003;22:S19–S27. doi: 10.1097/00003226-200310001-00004. [DOI] [PubMed] [Google Scholar]
- Bross DS, Tutschka PJ, Farmer ER, Beschorner WE, Braine HG, Mellitis ED, et al. Predictive factors for acute graft-versus-host disease in patients transplanted with HLA-identical bone marrow. Blood. 1984;63:1265–1270. [PubMed] [Google Scholar]
- Randolph SSB, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood. 2004;103:347–352. doi: 10.1182/blood-2003-07-2603. [DOI] [PubMed] [Google Scholar]
- Gale RP, Bortin MM, Bekkum DW, Biggs JC, Dicke KA, Gluckman E, et al. Risk factors for acute graft-versus-host disease. Br J Haematol. 1987;67:397–406. doi: 10.1111/j.1365-2141.1987.tb06160.x. [DOI] [PubMed] [Google Scholar]
- Stern M, Brand R, Witte T, Sureda A, Rocha V, Passweg J, et al. Female-versus-male alloreactivity as a model for minor histocompatibility antigens in hematopoietic stem cell transplantation. Am J Transplant. 2008;8:2149–2157. doi: 10.1111/j.1600-6143.2008.02374.x. [DOI] [PubMed] [Google Scholar]
- Gratwohl A, Hermans J, Niederwieser D, Biezen A, Houwelingen HC, Apperley J. Female donors influence transplant-related mortality and relapse incidence in male recipients of sibling blood and marrow transplants. Hematol J. 2001;2:363–370. doi: 10.1038/sj.thj.6200117. [DOI] [PubMed] [Google Scholar]
- Toda I, Tsubota K. Practical double vital staining for ocular surface evaluation. Cornea. 1993;12:366–367. doi: 10.1097/00003226-199307000-00015. [DOI] [PubMed] [Google Scholar]
- Tsubota K, Toda I, Yagi Y, Ogawa Y, Ono M, Yoshino K. Three different types of dry eye syndrome. Cornea. 1994;13 (3:202–209. doi: 10.1097/00003226-199405000-00002. [DOI] [PubMed] [Google Scholar]
- Shimmura S, Ono M, Shinozaki K, Toda I, Takamura E, Mashima Y, et al. Sodium hyaluronate eyedrops in the treatment of dry eyes. Br J Ophthalmol. 1995;79:1007–1011. doi: 10.1136/bjo.79.11.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995;21:221–232. [PubMed] [Google Scholar]
- van Bijsterveld OP. Diagnostic tests in the Sicca syndrome. Arch Ophthalmol. 1969;82:10–14. doi: 10.1001/archopht.1969.00990020012003. [DOI] [PubMed] [Google Scholar]
- Ban Y, Ogawa Y, Goto E, Uchino M, Terauchi N, Seki M, et al. Tear function and lipid layer alterations in dry eye patients with chronic graft-vs-host disease. Eye. 2009;23:202–208. doi: 10.1038/eye.2008.340. [DOI] [PubMed] [Google Scholar]
- Tsubota K. The importance of the Schirmer test with nasal stimulation. Am J Ophthalmol. 1991;111:106–108. doi: 10.1016/s0002-9394(14)76908-9. [DOI] [PubMed] [Google Scholar]
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant. 2010;45:1388–1395. doi: 10.1038/bmt.2009.359. [DOI] [PubMed] [Google Scholar]
- Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–387. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- Miklos DB, Kim HT, Zorn E, Hochberg EP, Guo L, Mattes-Ritz A, et al. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004;103:353–359. doi: 10.1182/blood-2003-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers ME, Pepe MS, Longton G, Doney KC, Monroe D, Witherspoon RP, et al. Previous donor pregnancy as a risk factor for acute graft-versus-host disease in patients with aplastic anaemia treated by allogeneic marrow transplantation. Br J Haematol. 1990;74 (4:492–496. doi: 10.1111/j.1365-2141.1990.tb06340.x. [DOI] [PubMed] [Google Scholar]
- Kollman C, Howe CWS, Anasetti C, Antin JH, Davies SM, Filipovich AH, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98:2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- Carlens S, Ringden O, Remberger M, Lonnqvist B, Hagglund H, Klaesson S, et al. Risk factors for chronic graft-versus-host disease after bone marrow transplantation: a retrospective single centre analysis. Bone Marrow Transplant. 1998;22:755–761. doi: 10.1038/sj.bmt.1701423. [DOI] [PubMed] [Google Scholar]
- Przepiorka D, Smith TL, Folloder J, Khouri I, Ueno NT, Mehra R, et al. Risk factors for acute graft-versus-host disease after allogeneic blood stem cell transplantation. Blood. 1999;94:1465–1470. [PubMed] [Google Scholar]