Abstract
Purpose
To evaluate the efficacy and safety of topical 0.005% tacrolimus eye drop for treatment of refractory vernal keratoconjunctivitis (VKC).
Methods
This prospective study included 20 eyes of 10 patients with refractory VKC, who had active symptomatic disease despite conventional medications including topical steroids. After discontinuing all other medications, patients were treated with topical 0.005% tacrolimus eye drop four times a day. Changes in subjective symptoms and objective signs after treatment were evaluated, and development of possible complications was assessed.
Results
Mean age of patients was 21.3±7.4 years and mean duration of VKC was 12.1±5.8 years. After starting tacrolimus eye drop, patients were followed for a mean duration of 10.7±3.7 months (range, 6–15 months). All symptoms including itching, redness, photosensitivity, foreign body sensation, and mucus discharge improved after the treatment; itching was the first symptom to show dramatic relief. In addition, there was improvement in objective signs including conjunctival hyperaemia, conjunctival papillary hypertrophy, giant papillae, limbal hypertrophy, corneal punctate epithelial erosions, and corneal pannus; conjunctival hyperaemia was the first sign to show improvement. No patient required addition of other medications including steroids for further relief. Any attempt to discontinue tacrolimus eye drop was associated with recurrence of patients' symptoms and signs, necessitating continued use of the medication during the entire follow-up time. No ocular complication related to tacrolimus was noted.
Conclusion
Topical 0.005% tacrolimus eye drop seemed to be a safe and effective treatment for steroid-resistant refractory VKC; however, long-term use was needed to control the disease.
Keywords: allergy, giant papillae, tacrolimus, vernal keratoconjunctivitis
Introduction
Vernal keratoconjunctivitis (VKC) is a chronic, bilateral, seasonally exacerbated, allergic inflammation of the ocular surface, involving tarsal and/or bulbar conjunctiva. It is more common in children and young adults who have an atopic background. Although VKC has traditionally been considered as a classical IgE-mediated disease (type I hypersensitivity), recent findings implicate more complex pathogenesis with particular involvement of Th2 lymphocytes.1
For treatment of VKC, a variety of medications have been used which include anti-histamines, mast-cell stabilisers, and non-steroidal anti-inflammatory drugs. Nevertheless, topical steroids are the mainstay of treatment for moderate–severe forms of VKC.2, 3 Some cases, however, remain still symptomatic despite treatment with topical steroids. Furthermore, prolonged use of topical steroids may be associated with various complications, such as glaucoma, cataract, and secondary infections.2 To avoid steroid-related complications, immunomodulatory agents such as topical cyclosporine A have recently been used for treatment of VKC.4, 5, 6
Tacrolimus (formerly, FK-506) is another immunomodulatory agent, which is similar to cyclosporine A in mechanism but with much higher potency (up to 100 times).7 It suppresses T-cell activation, T helper cell-mediated B-cell proliferation, and formation of cytokines, especially interleukin-2. In ophthalmology, tacrolimus has mainly been used to suppress immune reactions in corneal and limbal transplantations,8, 9, 10, 11, 12 uveitis,13, 14, 15 and allergic eye disease.16, 17, 18 However, there is no commercially available ophthalmic preparation for tacrolimus.
Topical tacrolimus with concentrations of 0.02–0.1% in ointment form has successfully been used for treatment of atopic keratoconjunctivitis (AKC),18, 19, 20 giant papillary conjunctivitis,21 and VKC.22, 23 In a recent study, 0.1% ophthalmic suspension of tacrolimus has been used for treatment of AKC and VKC with only 4 weeks of follow-up.24 However, the long-term effects of the treatment remain unknown.
In this study, we investigated the efficacy and safety of a much lower concentration of topical tacrolimus eye drop in patients with refractory VKC, who had more than 6 months of follow-up after the treatment.
Materials and methods
This prospective study included 20 eyes of 10 patients with active VKC, which was refractory to conventional treatments. These patients were consecutively enrolled in the study from a tertiary eye care facility from February through May 2009. The study was approved by Institutional Review Board of Farabi Eye Hospital, Tehran, Iran.
VKC was diagnosed with a typical history of chronic, bilateral conjunctivitis, and with the patient exhibiting symptoms and signs of itching, redness, mucus discharge, papillae on the upper tarsal conjunctiva, and/or limbal changes. All these patients had active disease with no significant improvement despite being already treated with conventional medications such as anti-histamines, mast-cell stabilisers, non-steroidal anti-inflammatory drugs, and topical steroids. Despite all the medications, these patients still complained of significant redness, itching, photosensitivity, foreign body sensation, and conjunctival discharge. To rule out the inadequate medical regimen as the cause for persistent active disease, all cases received a 2-week course of topical steroid (0.1% betamethasone eye drop); only patients with persistent symptoms and signs of VKC were included in the study. Patients who had received any form of immunosuppressive therapy including cyclosporine A eye drop were excluded.
Before starting treatment with tacrolimus eye drop and at each visit thereafter, all patients were given a questionnaire regarding the symptoms of itching, redness, photosensitivity, foreign body sensation, and mucus discharge. The patients were asked to use a grading scale of scale 0 (none), scale 1 or mild (occasional symptoms), scale 2 or moderate (frequent symptoms), and scale 3 or severe (constant symptoms) to report the severity of each individual symptom. In addition, they underwent a complete ophthalmic examination including measurement of best spectacle-corrected visual acuity (BSCVA), slit-lamp biomicroscopy and photography, fluorescein staining, and applanation tonometry. Before starting topical tacrolimus eye drop, all patients were asked to discontinue all other medications including steroids for 1 week.
Advantages and disadvantages of the treatment were fully explained for the patients and/or their parents and then informed consent was obtained from each participant or his/her parents. The treatment included topical 0.005% tacrolimus eye drop four times a day. Tacrolimus eye drop was reconstituted by adding balanced salt solution to tacrolimus vial for injection (Prograf, Astellas Pharma Inc., Dublin, Ireland) under sterile condition in the local laboratory to achieve a 0.005% concentration. The patients were visited 3 days after starting the medication and every 2–4 weeks thereafter. In each visit after treatment, the above-mentioned questionnaire and ophthalmic examination were repeated; the patients were also specifically questioned about the discomfort associated with the use of tacrolimus eye drop.
Efficacy of the treatment was evaluated by assessing the changes in patients' symptoms and signs, and by the patient's need for additional medications to have more relief. For each patient, consecutive slit lamp photographs were compared by two independent observers to evaluate the changes in conjunctival hyperaemia, papillary hypertrophy, giant papillae, limbal hypertrophy, corneal punctate epithelial erosion, corneal pannus formation, and corneal stromal clarity. Each sign was graded as scale 0 (none), scale 1 (mild), scale 2 (moderate), or scale 3 (severe). In the case of discrepancy between the observers, a third opinion was sought. Improvement of each symptom or sign was defined as at least 1-score reduction in severity compared with values before the treatment. ANOVA test and paired t-test were used to statistically analyse the changes in mean score of symptoms and signs after treatment with topical 0.005% tacrolimus eye drop; P-values of 0.05 or less were considered as statistically significant.
Results
In all, 20 eyes of 10 patients (nine males and one female) with refractory VKC were included in this study. These patients had a mean age of 21.3±7.4 years (range, 11–38 years), and all had bilateral involvement of VKC for a mean duration of 12.1±5.8 years (range, 5–25 years) (Table 1). All patients had active perennial symptomatic disease despite previous use of various medications including anti-histamines, mast-cell stabilisers, and topical steroids. The most prominent symptom in all patients was itching, but they also complained of various degrees of other symptoms, including redness, photosensitivity, foreign body sensation, and mucus discharge (Table 2). Although all eyes had papillary hypertrophy, giant papillae were found in eight eyes. Furthermore, 8 eyes had limal hypertrophy and 12 eyes had various degrees of corneal involvement by punctate epithelial erosions, pannus formation or stromal opacity (Table 3).
Table 1. Characteristics of patients with refractory VKC before treatment with topical 0.005% tacrolimus eye drop and their final visual acuity after the treatment.
| Case no. | Gender/age (years) | VKC duration (years) |
BSCVA |
Follow-up (months) | |
|---|---|---|---|---|---|
| Before R-L | Final R-L | ||||
| 1 | M/23 | 15 | 20/30–20/30 | 20/25–20/25 | 15 |
| 2 | F/24 | 17 | 20/30–20/30 | 20/30–20/30 | 14 |
| 3 | M/16 | 7 | 20/25–20/25 | 20/25–20/25 | 13 |
| 4 | M/26 | 15 | 20/200–20/100 | 20/70–20/50 | 10 |
| 5 | M/38 | 25 | 20/50–20/40 | 20/30–20/30 | 10 |
| 6 | M/11 | 7 | 20/25–20/30 | 20/25–20/30 | 11 |
| 7 | M/19 | 8 | 20/25–20/30 | 20/25–20/30 | 10 |
| 8 | M/18 | 8 | 20/30–20/30 | 20/25–20/20 | 9 |
| 9 | M/22 | 9 | 20/20–20/20 | 20/20–20/20 | 9 |
| 10 | M/16 | 10 | 20/25–20/25 | 20/25–20/25 | 6 |
Abbreviations: BSCVA, best spectacle-corrected visual acuity; L, left; R, right; VKC, vernal keratoconjunctivitis.
Table 2. Mean score of symptoms in patients with refractory VKC before and after treatment with topical 0.005% tacrolimus eye drop.
| Symptom | Before | 3 days | 1 month | Final follow-up | P-value |
|---|---|---|---|---|---|
| Itching | 2.90±0.31 | 0.20±0.42 | 0 | 0 | <0.001 |
| Redness | 2.00±0.82 | 2.00±0.82 | 0.70±0.48 | 0.50±0.53 | <0.001 |
| Photosensitivity | 1.80±0.63 | 1.50±0.53 | 0.30±0.48 | 0.10±0.32 | <0.001 |
| Foreign body sensation | 1.80±0.79 | 1.60±0.52 | 0.20±0.42 | 0.20±0.42 | <0.001 |
| Mucus discharge | 1.50±0.85 | 1.50±0.85 | 0 | 0 | <0.001 |
Table 3. Mean score of objective signs in patients with refractory VKC before and after treatment with topical 0.005% tacrolimus eye drop.
| Sign | Before | 3 days | 1 month | Final follow-up | P-value |
|---|---|---|---|---|---|
| Conjunctival hyperaemia | 2.60±0.52 | 2.60±0.52 | 0.80±0.42 | 0.60±0.52 | <0.001 |
| Papillary hypertrophy | 2.70±0.48 | 2.70±0.48 | 1.30±0.67 | 0.60±0.52 | <0.001 |
| Giant papillary conjunctivitis | 1.10±1.45 | 1.10±1.45 | 0.40±0.52 | 0.20±0.42 | 0.05 |
| Limbal hypertrophy | 0.90±1.29 | 0.90±1.29 | 0.80±1.13 | 0.20±0.42 | 0.04 |
| Corneal punctate epithelial erosions | 1.10±1.20 | 1.10±1.20 | 0 | 0 | 0.02 |
| Corneal pannus | 0.80±1.23 | 0.80±1.23 | 0.50±0.85 | 0.30±0.48 | 0.10 |
| Corneal stromal opacity | 0.50±1.10 | 0.50±1.10 | 0.50±1.10 | 0.40±0.84 | 0.34 |
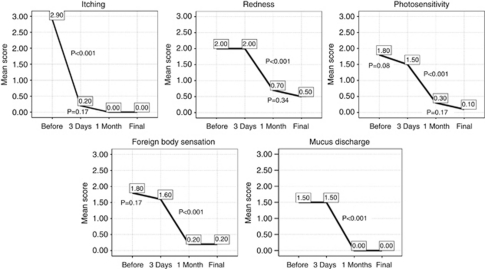
After starting 0.005% tacrolimus eye drop, the patients were followed for a mean duration of 10.7±3.7 months (range, 6–15 months). Changes in mean score of symptoms after treatment have been shown in Table 2 and Figure 1. All symptoms significantly improved after treatment with tacrolimus eye drop. Itching was the first symptom to show dramatic relief; while before treatment, nine patients had severe itching and one had moderate itching, 3 days after stating tacrolimus eye drop eight patients had no itching and two other patients had only mild itching, which was relieved by 1 month. By 1 month after treatment, the residual symptoms included mild redness in seven patients, mild photosensitivity in three patients and mild foreign body sensation in two patients (Table 2). The patients remained mostly symptom-free while under treatment during follow-up, with only mild symptoms of redness, photosensitivity, and foreign body sensation in 5, 1, and 2 patients, respectively, at the end of follow-up. Under treatment with tacrolimus, no patient needed to use any additional medications such as topical anti-histamines, mast-cell stabilisers, or topical steroids to have more relief.
Figure 1.
Mean score of symptoms in patients with refractory VKC before and after treatment with topical 0.005% tacrolimus eye drop.
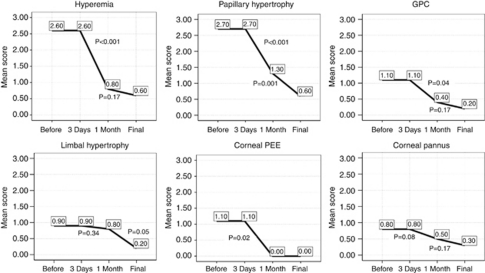
In addition to relief of symptoms, there was improvement in objective signs after starting tacrolimus eye drop (Table 3 and Figure 2). Conjunctival hyperaemia was the first sign to show improvement; it improved in all eyes and while before treatment hyperaemia was severe in 12 eyes and moderate in 8 eyes, by 1 month 4 eyes had no hyperaemia and 16 eyes showed mild hyperaemia (Figures 3a–d). Conjunctival hyperaemia was not deteriorated during the course of treatment, and at the end of follow-up 12 eyes had only mild residual hyperaemia. In addition, conjunctival papillary hypertrophy showed improvement in all eyes (Figures 3e–f). Of eight eyes with moderate or severe giant papillae, all showed improvement after treatment as early as 2 weeks in three eyes (Figure 4); by 1 month all these eyes had mild giant papillae, which disappeared in four eyes by the end of follow-up. There was improvement in limbal hypertrophy (8/8 eyes), corneal punctate epithelial erosions (10/10 eyes), and corneal pannus (8/8 eyes) after treatment (Figures 5a–f). Of four eyes with corneal stromal opacity, two eyes showed 1-score improvement (Figures 5g and h). At the end of follow-up, BSCVA improved by more than two Snellen lines in two eyes, by two lines in two eyes, and by one line in four other eyes (Table 1).
Figure 2.
Mean score of objective signs in patients with refractory VKC before and after treatment with topical 0.005% tacrolimus eye drop (GPC, giant papillary conjunctivitis; PEE, punctate epithelial erosion).
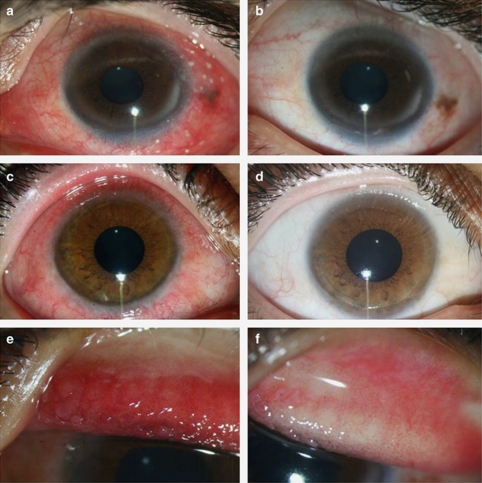
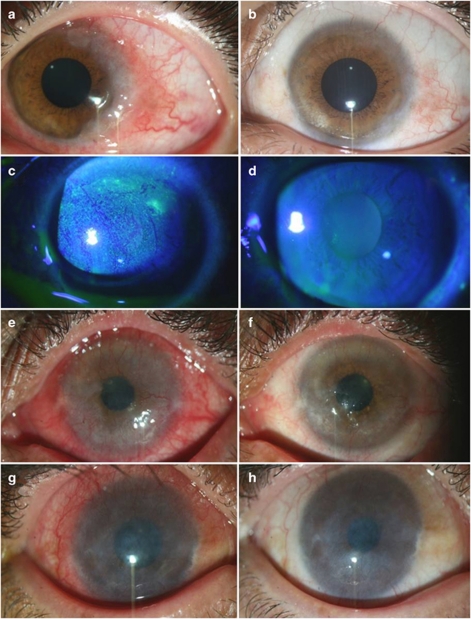
Figure 3.
Improvement of conjunctival hyperaemia and conjunctival papillary hypertrophy after treatment with topical 0.005% tacrolimus eye drop in refractory VKC. In these two eyes with severe conjunctival hyperaemia (a and c), there was remarkable improvement with mild hyperaemia (b) or no hyperaemia (d) by 1 month after treatment. This eye with severe conjunctival papillary hypertrophy (e) showed improvement after the treatment (f).
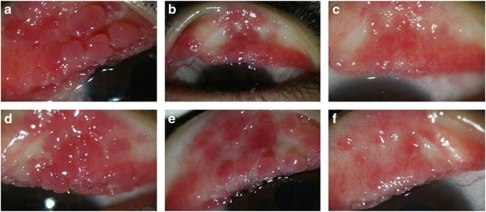
Figure 4.
Improvement of giant papillae after treatment with topical 0.005% tacrolimus eye drop in refractory VKC. In these two eyes with giant papillae (a and d), there was improvement after 2 weeks of treatment (b and e) with further improvement by 1 month after starting tacrolimus (c and f).
Figure 5.
Improvement of limbal hypertrophy, corneal punctate epithelial erosions, corneal pannus and corneal stromal opacity after treatment with topical 0.005% tacrolimus eye drop in refractory VKC. In this eye with conjunctival hyperaemia and limbal hypertrophy (a), there was significant improvement after treatment (b). Another eye with diffuse corneal punctate epithelial erosions (c) showed smooth corneal surface with no erosion after treatment (d). In this eye with severe conjunctival hyperaemia and corneal pannus formation (e), hyperaemia and corneal pannus decreased after treatment (f). In this eye with active VKC and corneal stromal opacity (g), there was decreased corneal stromal opacity after treatment with increased BSCVA from 20/200 to 20/70 (h).
Any attempt to discontinue tacrolimus eye drop was associated with recurrence of patients' symptoms and signs. But with restarting the treatment, there was immediate improvement of manifestations. Therefore, all patients received the medication during the entire follow-up time. No patient complained of any discomfort or burning on instillation of the eye drop. No patient needed to discontinue the medication due to any adverse effect. Intraocular pressure remained normal in all patients. No ocular complication related to tacrolimus eye drop was seen in any patient.
Discussion
This study showed that 0.005% tacrolimus eye drop was a safe and effective treatment for patients with VKC refractory to conventional medications including topical steroids. All patients showed dramatic improvement of inflammatory symptoms and signs without developing any significant adverse effect. No patient needed to use any additional medication including topical steroids to obtain further relief, showing the role of this medication as a steroid-sparing agent. However, long-term use of the medication was necessary to control the disease. Further studies are needed to evaluate the optimal dosage and concentration of topical tacrolimus and the long-term safety of this treatment.
Tacrolimus is a potent immunosuppressive agent, which inhibits several immune reactions involved in the pathogenesis of VKC.7 In our study, a very low concentration of topical tacrolimus (0.005%) was used as an eye drop to treat patients with refractory VKC. Previously, systemic tacrolimus in the form of oral preparation has successfully been used in refractory severe AKC.25, 26 Furthermore, to treat allergic disease, some studies have used either a commercially available 0.03–0.1% ‘skin' ointment applied to eyelids17, 18, 20, 27 or fornix,21 or they have used their own-made 0.02–0.1% ointment for the eye.22, 23 Because it is more convenient to use an eye drop rather than an ointment, Sengoku et al28 used 0.01–1% eye drop in an animal study of ocular allergy and Ohashi et al24 used an 0.1% ophthalmic suspension for a clinical study. The latter was formulated to be an isotonic aqueous suspension using polyvinyl alcohol as dispersive agent. In the present study, tacrolimus eye drop was just reconstituted by adding balanced salt solution, which can conveniently be performed under sterile condition by local pharmacy. Although no reduced efficacy of the treatment was noted during the study, further research is needed to evaluate the stability of the compound and the optimal method for reconstituting the eye drop.
Even with this very low concentration of the topical tacrolimus, rapid and dramatic improvement of symptoms was noted in patients with refractory VKC. By 3 days after the treatment, itching was mostly relieved in all patients and 80% had no itching at all (Table 2 and Figure 1). Most other symptoms were also remarkably relieved by 1 month after starting tacrolimus eye drop. Similar findings have also been noted in other studies. Vichyanond et al22 applied topical 0.1% tacrolimus ointment for 4 weeks in 10 patients with recalcitrant VKC. All patients responded rapidly to the treatment; mean score of symptoms reached 31% of their baseline at the end of the first week and after 4 weeks the mean symptom score was 20% of baseline. In another study, Miyazaki et al23 used 0.02% tacrolimus ointment for five patients with AKC and one patient with VKC. They noticed marked improvement of their symptoms within 2–4 weeks. Furthermore, in a multicentre, randomised clinical trial, Ohashi et al24 applied 0.1% ophthalmic suspension of tacrolimus twice-daily for 4 weeks in 21 patients with AKC and 7 patients with VKC. Compared with placebo group, the treated eyes showed marked improvement in symptoms after 4 weeks of treatment.
Parallel to improvement of symptoms, objective signs in our cases showed improvement soon after starting the treatment (Table 3 and Figure 2). Conjunctival hyperaemia was the first sign to show improvement with marked reduction of hyperaemia within 1 month of treatment, which continued to show improvement during the therapy course (Figures 3a–d). In addition to improved conjunctival papillary hypertrophy in all eyes (Figures 3e and f), giant papillae were found to have improvement after treatment as early as 2 weeks in 3/8 eyes (Figure 4); by 1 month all these eyes had mild giant papillae, which disappeared in four eyes by the end of follow-up. In one case report, Kymionis et al21 reported that 0.03% tacrolimus skin ointment applied twice a day in a boy with giant papillary conjunctivitis due to VKC resulted in resolution of giant papillae within 15 days, with no evidence of these papillae after 1 month of treatment. Ohashi et al24 also showed significant improvement of the giant papillae after 4 weeks of treatment with 0.1% tacrolimus ophthalmic suspension.
In addition to conjunctival signs, limbal hypertrophy and corneal signs such as corneal punctate epithelial erosions, corneal pannus, and to some degrees corneal stromal opacity showed improvement (Figure 5). Although resolved corneal shield ulcer after treatment with topical tacrolimus has been reported previously,24 no case in our series had this form of corneal involvement. Improvement in corneal findings in our series was associated with improvement of BSCVA in 8 (44%) out of 18 eyes with initial BSCVA less than 20/20. This improved visual acuity reflects the improvment in corneal surface and also the resulting changes in corneal vascularisation and opacity.
In our series, any attempt to discontinue the tacrolimus eye drop resulted in recurrence of allergic symptoms and signs. Therefore, patients needed to be under treatment for their entire course. In some previous studies, topical tacrolimus has been used just for 4 weeks in VKC with no follow-up after cessation of the treatment.22, 24 In the study by Miyazaki et al,23 treatment with topical tacrolimus was continued for 7 months. Although improvement of clinical findings has been reported after topical tacrolimus, there is paucity of data regarding the optimal duration of the treatment. In some studies, topical tacrolimus has been used for up to 42 months in patients with AKC.18, 20 In our study, all patients had perennial disease and after starting treatment with tacrolimus they did not need to use additional medications such as anti-histamines or mast cell stabilisers. It may be possible to discontinue tacrolimus or to reduce the dosage in patients with less severe disease, with or without addition of less potent agents during the time of year when the allergy has not been exacerbated. For example, Kymionis et al21 showed that after 3 months of tapered treatment for giant papillary conjunctivitis there was significant improvement that did not recur during 6 months after cessation of tacrolimus.
Although burning sensation upon application of topical tacrolimus has been reported before,20, 21, 24 no patient in our study experienced any burning or discomfort. The low concentration of the medication and no additional compound other than balanced salt solution in our preparation may be the reason for this. During a mean follow-up time of more than 10 months (range, 6–15 months), no patient developed any side effect attributed to tacrolimus. However, due to its local immunosuppressive effects, topical tacrolimus may potentially be associated with some complications. Activation of herpes simplex dendritic keratitis29 and development of molluscum contagiosum20 have been reported before. Further studies are required to evaluate the long-term safety of this medication.
The main limitation of our study is the small sample size. In addition, we did not include a control group. However, knowing that our patients had already been treated with conventional medications including topical steroids signifies the role of this immunomodulatory agent in treatment of patients with VKC. Future studies are needed to compare the results of topical tacrolimus with other immunomodulatory agents such as topical cyclosporine A in severe allergic disease, and also to use other outcome measures such as conjunctival scrapings to evaluate the efficacy of treatment. With these limitations in mind, it seems that topical 0.005% tacrolimus eye drop is a safe and effective treatment for patients with refractory VKC and it can also be used as a steroid-sparing agent to avoid the complications associated with prolonged use of steroids. Future studies will shed light to this important aspect of treatment of severe allergic eye disease.
The authors declare no conflict of interest.
Footnotes
This paper was presented at ASCRS Meeting, Boston, April 2010.
References
- Bielory L, Frohman LP. Allergic and immunologic disorders of the eye. J Allergy Clin Immunol. 1992;89 (1 Part 1:1–15. doi: 10.1016/s0091-6749(05)80033-8. [DOI] [PubMed] [Google Scholar]
- Bonini S, Bonini S, Lambiase A, Marchi S, Pasqualetti P, Zuccaro O, et al. Vernal keratoconjunctivitis revisited: a case series of 195 patients with long-term follow-up. Ophthalmology. 2000;107 (6:1157–1163. doi: 10.1016/s0161-6420(00)00092-0. [DOI] [PubMed] [Google Scholar]
- Tabbara KF. Ocular complications of vernal keratoconjunctivitis. Can J Ophthalmol. 1999;34 (2:88–92. [PubMed] [Google Scholar]
- BenEzra D, Pe'er J, Brodsky M, Cohen E. Cyclosporine eye drops for the treatment of severe vernal keratoconjunctivitis. Am J Ophthalmol. 1986;101 (3:278–282. doi: 10.1016/0002-9394(86)90819-6. [DOI] [PubMed] [Google Scholar]
- Secchi AG, Tognon MS, Leonardi A. Topical use of cyclosporine in the treatment of vernal keratoconjunctivitis. Am J Ophthalmol. 1990;110 (6:641–645. doi: 10.1016/s0002-9394(14)77061-8. [DOI] [PubMed] [Google Scholar]
- Bleik JH, Tabbara KF. Topical cyclosporine in vernal keratoconjunctivitis. Ophthalmology. 1991;98 (11:1679–1684. doi: 10.1016/s0161-6420(91)32069-4. [DOI] [PubMed] [Google Scholar]
- Sawada S, Suzuki G, Kawase Y, Takaku F. Novel immunosuppressive agent, FK506. In vitro effects on the cloned T cell activation. J Immunol. 1987;139 (6:1797–1803. [PubMed] [Google Scholar]
- Kobayashi C, Kanai A, Nakajima A, Okumura K. Suppression of corneal graft rejection in rabbits by a new immunosuppressive agent, FK-506. Transplant Proc. 1989;21 (1 Part 3:3156–3158. [PubMed] [Google Scholar]
- Nishi M, Herbort CP, Matsubara M, Morishita M, Nishimura M, Nieda M, et al. Effects of the immunosuppressant FK506 on a penetrating keratoplasty rejection model in the rat. Invest Ophthalmol Vis Sci. 1993;34 (8:2477–2486. [PubMed] [Google Scholar]
- Sloper CM, Powell RJ, Dua HS. Tacrolimus (FK506) in the management of high-risk corneal and limbal grafts. Ophthalmology. 2001;108 (10:1838–1844. doi: 10.1016/s0161-6420(01)00759-x. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhou Q, Zeng J, Xu JT, Zhao SB, Wang YP.The study of FK506 eye drops preventing and treating immune rejection on limbal allograft transplantation Zhonghua Yan Ke Za Zhi 200339(9550–554.(in Chinese). [PubMed] [Google Scholar]
- Yalçindag FN, Incel O, Ozdemir O. Effectiveness of tacrolimus in high-risk limbal allo-graft transplantation. Ann Ophthalmol (Skokie) 2008;40 (3–4:152–156. [PubMed] [Google Scholar]
- Kawashima H, Fujino Y, Mochizuki M. Effects of a new immunosuppressive agent, FK506, on experimental autoimmune uveoretinitis in rats. Invest Ophthalmol Vis Sci. 1988;29 (8:1265–1271. [PubMed] [Google Scholar]
- Mochizuki M, Ikeda E, Shirao M, Fujito S, Yoshimura K, Shimada N. Preclinical and clinical study of FK506 in uveitis. Curr Eye Res. 1992;11 (Suppl:87–95. doi: 10.3109/02713689208999516. [DOI] [PubMed] [Google Scholar]
- Sloper CM, Powell RJ, Dua HS. Tacrolimus (FK506) in the treatment of posterior uveitis refractory to cyclosporine. Ophthalmology. 1999;106 (4:723–728. doi: 10.1016/S0161-6420(99)90156-2. [DOI] [PubMed] [Google Scholar]
- Iwamoto H, Yoshida H, Yoshida O, Fukushima A, Ueno H. Inhibitory effects of FK506 on the development of experimental allergic/immune-mediated blepharoconjunctivitis in Lewis rats by systemic but not by topical administration. Graefes Arch Clin Exp Ophthalmol. 1999;237 (5:407–414. doi: 10.1007/s004170050252. [DOI] [PubMed] [Google Scholar]
- Mayer K, Reinhard T, Reis A, Böhringer D, Sundmacher R.FK 506 ointment 0.1%—A new therapeutic option for atopic blepharitis. Clinical trial with 14 patients Klin Monbl Augenheilkd 2001218(11733–736.German. [DOI] [PubMed] [Google Scholar]
- Rikkers SM, Holland GN, Drayton GE, Michel FK, Torres MF, Takahashi S. Topical tacrolimus treatment of atopic eyelid disease. Am J Ophthalmol. 2003;135 (3:297–302. doi: 10.1016/s0002-9394(02)01982-7. [DOI] [PubMed] [Google Scholar]
- Virtanen HM, Reitamo S, Kari M, Kari O. Effect of 0.03% tacrolimus ointment on conjunctival cytology in patients with severe atopic blepharoconjunctivitis: a retrospective study. Acta Ophthalmol Scand. 2006;84 (5:693–695. doi: 10.1111/j.1600-0420.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- Zribi H, Descamps V, Hoang-Xuan T, Crickx B, Doan S. Dramatic improvement of atopic keratoconjunctivitis after topical treatment with tacrolimus ointment restricted to the eyelids. J Eur Acad Dermatol Venereol. 2009;23 (4:489–490. doi: 10.1111/j.1468-3083.2008.02933.x. [DOI] [PubMed] [Google Scholar]
- Kymionis GD, Goldman D, Ide T, Yoo SH. Tacrolimus ointment 0.03% in the eye for treatment of giant papillary conjunctivitis. Cornea. 2008;27 (2:228–229. doi: 10.1097/ICO.0b013e318159afbb. [DOI] [PubMed] [Google Scholar]
- Vichyanond P, Tantimongkolsuk C, Dumrongkigchaiporn P, Jirapongsananuruk O, Visitsunthorn N, Kosrirukvongs P. Vernal keratoconjunctivitis: result of a novel therapy with 0.1% topical ophthalmic FK-506 ointment. J Allergy Clin Immunol. 2004;113 (2:355–358. doi: 10.1016/j.jaci.2003.10.065. [DOI] [PubMed] [Google Scholar]
- Miyazaki D, Tominaga T, Kakimaru-Hasegawa A, Nagata Y, Hasegawa J, Inoue Y. Therapeutic effects of tacrolimus ointment for refractory ocular surface inflammatory diseases. Ophthalmology. 2008;115 (6:988–992.e5. doi: 10.1016/j.ophtha.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Ohashi Y, Ebihara N, Fujishima H, Fukushima A, Kumagai N, Nakagawa Y, et al. A randomized, placebo-controlled clinical trial of tacrolimus ophthalmic suspension 0.1% in severe allergic conjunctivitis. J Ocul Pharmacol Ther. 2010;26 (2:165–174. doi: 10.1089/jop.2009.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf T, Luqmani N, Sumich P, Cook S, Tole D. Systemic tacrolimus in the treatment of severe atopic keratoconjunctivitis. Cornea. 2006;25 (10:1147–1149. doi: 10.1097/01.ico.0000240091.11854.14. [DOI] [PubMed] [Google Scholar]
- Anzaar F, Gallagher MJ, Bhat P, Arif M, Farooqui S, Foster CS. Use of systemic T-lymphocyte signal transduction inhibitors in the treatment of atopic keratoconjunctivitis. Cornea. 2008;27 (8:884–888. doi: 10.1097/ICO.0b013e318172fbb1. [DOI] [PubMed] [Google Scholar]
- Nivenius E, van der Ploeg I, Jung K, Chryssanthou E, van Hage M, Montan PG. Tacrolimus ointment vssteroid ointment for eyelid dermatitis in patients with atopic keratoconjunctivitis. Eye (Lond) 2007;21 (7:968–975. doi: 10.1038/sj.eye.6702367. [DOI] [PubMed] [Google Scholar]
- Sengoku T, Sakuma S, Satoh S, Kishi S, Ogawa T, Ohkubo Y, et al. Effects of FK506 eye drops on late and delayed-type responses in ocular allergy models. Clin Exp Allergy. 2003;33 (11:1555–1560. doi: 10.1046/j.1365-2222.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- Joseph MA, Kaufman HE, Insler M. Topical tacrolimus ointment for treatment of refractory anterior segment inflammatory disorders. Cornea. 2005;24 (4:417–420. doi: 10.1097/01.ico.0000151507.49565.6e. [DOI] [PubMed] [Google Scholar]