Abstract
A new heterobifunctional (succinimidyl carbonate, SC)-activated poly(ethylene glycol) (PEG) with a reversible 1,6-elimination linker and a terminal alkyne for "click" chemistry was synthesized with high efficiency and low polydispersity. The α-alkyne-ω-hydroxyl PEG was first prepared using trimethylsilyl-2-propargyl alcohol as an initiator for ring-opening polymerization of ethylene oxide followed by mild deprotection with tetrabutylammonium fluoride. The hydroxy end was then modified with diglycolic anhydride to generate α-alkyne-ω-carboxylic acid PEG. The reversible 1, 6-elimination linker was introduced by conjugation of a hydroxymethyl phenol followed by activation with N,N'-disuccinimidyl carbonate to generate the heterobifunctional α-alkyne-ω-SC PEG. The terminal alkyne is available for "click" conjugation to azido ligands via 1,3-dipolar cycloaddition, and the succinimidyl carbonate will form a reversible conjugate to amines (e.g. in proteins) that can release the unaltered amine after base or enzyme catalyzed cleavage of the 1,6-linker.
Keywords: PEGylation, heterobifunctional PEG
1. Introduction
Poly(ethylene glycol) (PEG) is often employed as a solubilizer for poorly water soluble drugs. The conjugation of PEG, or PEGylation, is known to increase in vivo blood circulatory time, reduce immunogenicity, and improve stability and pharmacological properties of both small molecule and large biological drugs, including peptides, proteins, viruses, enzymes, and DNAs. The majority of these systems utilize monofunctional PEG with only one reactive terminal group, such as a hydroxy, amine, thiol, aldehyde, carboxylic acid, or activated variants. Targeted drug delivery systems using PEGylated therapeutics require two reactive termini on the PEG, i.e. α,ω-heterobifunctional PEG; one bound to the therapeutic and the other available for conjugation to targeting ligands, such as peptides, antibodies, or proteins. Unfortunately, most commercially available PEGs are not suitable for high extents of PEGylation because they form irreversibly conjugates that may hinder activity of the therapeutic cargo. Furthermore, delicate targeting antibodies must be conjugated under aqueous conditions, and most available linkers are not suitable for heterogeneous conjugation in aqueous milieu.
The 1,3-dipolar cycloaddition reaction (termed "click" chemistry) between alkyne and organic azides, which yields the corresponding 1,2,3-triazoles, is highly chemoselective and can be performed under mild reaction conditions in aqueous buffers within a wide pH range. Several publications have reported the application of click chemistry to label proteins (1,2,3), modify viruses (4), synthesize a "clickable" PEG-dendrimer (5), and introduce biological ligands onto the surface of liposomes (6) and Au nanoparticles (7). Recently Kataoka and coworkers reported an azide-terminated PEG that could be used in click reactions with alkyne-bearing ligands (8).
A reversible 1,6-elimination PEG linker was reported by Greenwald et al. (9,10), and lysozyme was conjugated onto the PEG via the ε-amino group of lysine residues. Under physiological conditions, the unaltered lysozyme was released after enzymatic or hydrolytic cleavage of the 1,6-linker.
Heterobifunctional PEGs combining a "clickable" termini and reversible amine conjugation offer novel possibilities for the PEGylation and targeting of both small molecule and large bimolecular drugs.
2. Materials and Methods
All chemicals were obtained from Fisher Scientific (Pittsburgh, PA) or Sigma-aldrich (St. Louis, MO) and used as received unless stated otherwise. Ethylene oxide was purchased from Balchem Corporation (New Hampton, NY), refrigerated, and used within 3 months of receipt. Dichloromethane (DCM) and tetrahydrofuran (THF) were distilled under argon immediately before use. All reactions were conducted under an atmosphere of dry argon in flamed glassware. NMR spectra were taken on a 400-MHz Bruker and chemical shifts δ are reference to TMS in ppm.
Synthesis of α-alkyne-ω-hydroxyl PEG (1)
The following procedure is for 4,500 Da PEG; other lengths were synthesized similarly. Freshly sublimated naphthalene (3 mmol), potassium (3 mmol), and THF (20 mL) were mixed in a dry 250-mL two-neck flask. After 24 h, the solution turned to dark green and was then diluted with THF (40 mL). The 3-trimethylsilyl-2-propargyl alcohol (TMSP, 3 mmol) was dried overnight over calcium hydride, vacuum distilled, and added into the flask. The solution was stirred for 24 h and transitioned to lime green as the initiator was formed. Ethylene oxide was freshly distilled over calcium hydride and added via a cold syringe. The reaction proceeded for 72 h at 20°C. The produced α-trimethylsilyl-propargyl-ω-hydroxyl PEG was precipitated from THF in cold diethyl ether three times and dried under vacuum. The alkyne termini was deprotected by addition of tetrabutylammonium fluoride (Bu4NF•3H2O or TBAF, 1.6 mmol) to a solution of α-trimethylsilyl-propargyl-ω-hydroxyl PEG (1.5 mmol) in 35 mL of THF with stirring for 3 h at 20°C (11). The solution was concentrated under reduced pressure and filtered through a short silica pad to remove TBAF. The yield of polymer 1 after purification was 48% (3.4 g).
Synthesis of α-alkyne-ω-carboxylic acid PEG (2)
Diglycolic anhydride (5.6 mmol) and 4-dimethylaminopyridine (DMAP, 5.6 mmol) were added to a solution of 1 (0.70 mmol) in DCM (32 mL) and stirred at 20°C for 24 h. The solution was concentrated under reduced pressure, and polymer 2 was precipitated in cold diethyl ether, filtered, and crystallized from DCM (ca. 2 mL) with isopropyl alcohol (IPA) (ca. 10 mL). The yield after purification was 95%. 1H NMR (CDCl3): δ 3.48 (t, J = 2.5 Hz, CHCCH2–), 3.60–3.77 (br s, –CH2CH2–), 3.83 (d, J = 3.5 Hz, CHCCH2–), 4.22 (t, J = 3.2 Hz, –CH2CH2OOC–), 4.23 (s, –OCH2COOH), 4.32 (s, –OOCCH2O–).
Synthesis of α-alkyne-ω-thiazolidine-2-thione PEG (3)
The carboxyl terminal of 2 was activated with a thiazolidine-2-thione by addition of 2-mercaptothiazoline (2-MT, 2.1 mmol) and DMAP (2.1 mmol) to a solution of 2 (0.68 mmol) in DCM (31 mL), chilling on ice to 0°C, and then adding 1-[3-(dimethylamino) propyl]-3-ethylcarbodiimide hydrochloride (EDC, 2.1 mmol). After 20 min, the solution was warmed to 20°C and stirred for 24 h. The polymer 3 was precipitated from DCM with ice cold diethyl ether, filtered, and crystallized from IPA. The yield of 3 was 87%. 1H NMR (CDCl3): δ 3.42 (t, J = 7.7 Hz, –NCH2CH2S–), 3.46 (t, CHCCH2–), 3.58–3.76 (br s, –CH2CH2–), 3.82 (d, J = 3.5 Hz, CHCCH2–), 4.22 (t, J = 3.2 Hz, –CH2CH2OOC–), 4.28 (s, –OCH2CON–), 4.31 (s, –OOCCH2O–), 4.63 (t, J = 7.3 Hz, –NCH2CH2S–).
Synthesis of 4-hydroxymethyl-2,6-dimethylphenol (4)
A solution of 2,6-xylenol (70 mmol), 37% formaline (140 mmol), and NaOH (70 mmol) was mixed at 20° C for 18 h, forming a crystalline mass. Acetic acid (100 mmol) in 50 mL water was added into the mixture, and the orange/yellow solids were removed by filtration. The filtrate was extracted four times with 25 mL of DCM giving the colorless crystalline 4 (1.5 g, 18% yield) after vacuum drying (12). 1H NMR (acetone-d6): δ 2.19 (s, 6H, ArCH3), 3.81 (s, 1H, ArCH2OH), 4.44 (s, 2H, ArCH2OH), 6.90 (s, 2H, Ar protons), 7.09 (s, 1H, ArOH).
Synthesis of α-alkyne-ω-dimethylphenyl alcohol PEG (5)
To a solution of polymer 3 (0.55 mmol) in 25 mL of DCM was added 4 (3.3 mmol) and DMAP (3.3 mmol). After stirring at 20°C for 24 h, the solution was concentrated under reduced pressure and precipitated in ice cold diethyl ether, filtered, and crystallized from IPA. The yield of polymer 5 after purification was 87%. 1H NMR (CDCl3): δ 2.13 (s, 6H, ArCH3), 3.48 (t, J = 2.5 Hz, CHCCH2–), 3.60–3.77 (br s, –CH2CH2–), 3.83 (d, J = 3.5 Hz, CHCCH2–), 4.19 (t, J = 3.2 Hz, –CH2CH2OOC–), 4.25 (s, –OCH2COOH), 4.27 (s, –OOCCH2O–), 4.64 (s, ArCH2OH), 7.07 (s, Ar protons).
Synthesis of α-alkyne-ω-(succinimidyl carbonate, SC) PEG (6)
Pyridine (PyD, 1.9 mmol) and disuccinimidyl carbonate (DSC, 1.9 mmol) were added to a solution of polymer 5 (0.47 mmol) in 21 mL of DCM and 8.9 mL of DMF, and mixed at 20°C for 24 h. The mixture was concentrated under reduced pressure and precipitated in ice cold diethyl ether three times, filtered, and crystallized from IPA to yield 2 g of polymer 6 (94 % yield). 1H NMR (CDCl3): δ 2.15 (s, ArCH3), 2.81 (s, –CH2CH2– of NHS), 3.48 (t, J = 2.5 Hz, CHCCH2–), 3.60–3.77 (br s, –CH2CH2– of PEG), 3.83 (d, J = 3.5 Hz, CHCCH2–), 4.19 (t, J = 3.2 Hz, –CH2CH2OOC–), 4.25 (s, –OCH2COOH), 4.27 (s, –OOCCH2O–), 5.33 (s, ArCH2OCO–), 7.07 (s, Ar protons).
Synthesis of azidoacetic acid
Azidoacetic acid was synthesized according to a reported procedure (13). In a 100-mL round-bottom flask, a solution of 0.5-M NaN3 (1.43 g, 22 mmol) in freshly distilled DMSO (44 mL) was stirred for 24 h at 20°C under argon. Bromoacetic acid (2.78 g, 20 mmol) was added into the above NaN3/DMSO solution, and the reaction was monitored by a Fourier transform infrared spectroscopy (Nicolet 510P FT-IR Spectrometer, Waltham, MA). The reaction mixture was quenched with 100 mL of H2O and extracted three times with diether ether (60 mL); the organic layer was then washed with H2O (2 × 100 mL) and brine (100 mL), dried over MgSO4, filtered, and rotavaped to yield a light yellow liquid. 1H NMR (CDCl3): δ 3.97 (s, –COCH2–), 10.82 (s, HOCO–). The characteristic absorption was found at 2,117 cm−1, which was a vibration of the N3 group in the FTIR spectra.
Synthesis of α-IR820-ω-SC PEG conjugates (8)
The IR820 diamine was produced by mixing IR 820 (85 mg, green solid, 0.1 mmol) and 1,3-diaminopropane (0.89 g, 12 mmol) in anhydrous DMF (5 mL). The reaction was monitored by thin layer chromatography (TLC) and the product was purified on silica gel (EtOAc:MeOH 1:1). The IR820 diamine (13 mg, 14.65 nmol) in 0.5 mL of anhydrous DMF was reacted with azidoacetic acid (1.4 mg, 13.95 nmol) in the presence of DMAP (0.85 mg, 6.98 nmol) and N,N'-dicyclohexylcarbodiimide (3.45 mg, 16.74 nmol) at 20°under argon. The product IR820 azide 7 was dried under vacuum and used without further purification. According to the "click" method of Rostovtsev et al. (14), the Huisgen 1,3-dipolor cycloaddition of IR820 azide 7 (13 mg, 13.40 nmol) and alkyne-terminal PEG 6 (Mn 5000, 59 mg, 12.18 nmol) proceeded in the presence of 1 mol% copper(II) sulfate and 5 mol% sodium ascorbate in a mixture of water and tert-butyl alcohol (2:1, v/v) at room temperature overnight. The reaction mixture was dialyzed against H2O (1,000 MWCO; Pierce, Rockford, IL) until no color was present in the dialysate. The resulting α-IR820-ω-SC PEG conjugate (compound 8) was lyophilized and stored at −20°C in a desiccator.
Polymer analysis
Molecular weights and distributions were determined using a Shimadzu 2010HCT system with a Shimadzu RID-10A refractive index detector and the Shimadzu EZStart 7.4 software. Samples (100 µL) were applied to a Shodex GPC LF804 column using N,N-dimethylformamide with 10 mM LiCl as the mobile phase (0.8 mL/min, 40°C). The calibration curve was generated with PEG standards from Scientific Polymer Products (Ontario, NY).
Analysis of α-IR820-ω-SC PEG conjugates
The excitation and emission wavelengths of α-IR820-ω-SC PEG conjugates (1 mg/mL in MeOH) were determined on a Shimadzu RF-5301PC spectrofluorophotometer. A Shimadzu 2010HCT system coupled with a Shimadzu RID-10A refractive index detector and a Waters 2475 multi wavelength fluorescence detector along with a Shodex GPC LF804 column was used to confirm the successful conjugation of the IR820 azide and alkyne-terminal PEG.
3. Results and Discussion
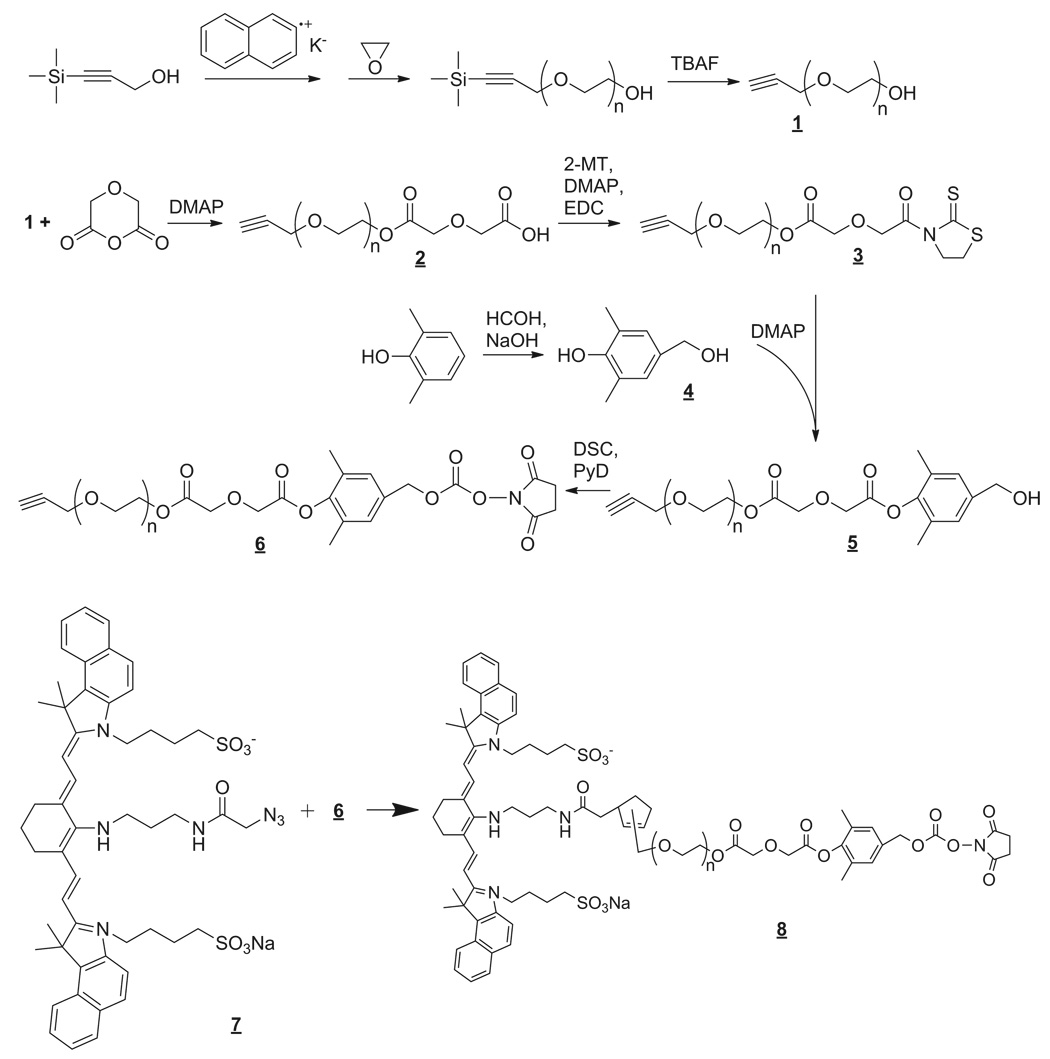
The alkyne PEG was synthesized by the anionic ring-opening polymerization of ethylene oxide initiated by potassium trimethylsilyl-propynyl-olate, which was formed in situ by the reaction between 3-trimethylsilyl-2-propargyl alcohol and potassium naphthalene. The trimethylsilyl terminal of the PEG chain can be deprotected with tetrabutylammonium fluoride, leaving alkyne exposed as one terminal, which can undergo 1,3-dipolar cycloaddition to azides (Figure 1). The gel permeation chromatography (GPC) analysis revealed that the resulting α-alkyne-ω-hydroxyl PEG had a number average molecular weight (Mn) of 4,546, and a polydispersity index (PI, Mw/Mn) of 1.045 (Table 1).
Figure 1. Synthesis of reversible "click" PEG and IR820 conjugates.
Table 1. Molecular weight and polydispersities of α-alkyne-ω-SC PEG by gel permeation chromatography.
| 5 K | 16 K | 80 K | ||||
|---|---|---|---|---|---|---|
| Mn | PI | Mn | PI | Mn | PI | |
| α-alkyne-ω-SC PEG | 4853 | 1.124 | 16113 | 1.206 | 80105 | 1.231 |
In order to modify polymer 1, the hydroxyl end was conjugated with diglycolic anhydride to generate α-alkyne-ω-carboxylic acid PEG (polymer 2). The single peak at δ 4.23 (s, –OCH2COOH) on the NMR spectra demonstrated the successful conversion of hydroxyl end to carboxylic acid terminal. In order to introduce the 1,6-elimilation moiety into the PEG, the carboxyl terminal of 2 was first converted into a thiazolidine-2-thione followed by the acylation of the phenolic hydroxyl group on 4-hydroxymethyl-2, 6-dimethylphenol (compound 4). The acylation reaction selectively occurred at the phenolic –OH because under basic conditions, the phenolate anion was more nucleophilic compared to the primary benzyl alcohol (15). The PEG benzyl alcohol (compound 5) was activated by disuccinimidyl carbonate in the presence of pyridine as base. The activated α-alkyne-ω-SC PEG (compound 6) can easily couple to amino-containing ligands such as proteins via a carbamate bond under mild reaction conditions (16).
Physical characterizations of the produced α-alkyne-ω-SC PEGs are given in Table 1. Three different molecular weights of 5, 16 and 80 kDa were synthesized with narrow polydispersities (PI < 1.25). As the molecular weight of the PEG linker increased, the PI slightly increased. This was most likely due to side reactions during ring-opening polymerization and chain breakage during repeated precipitation and crystallization steps.
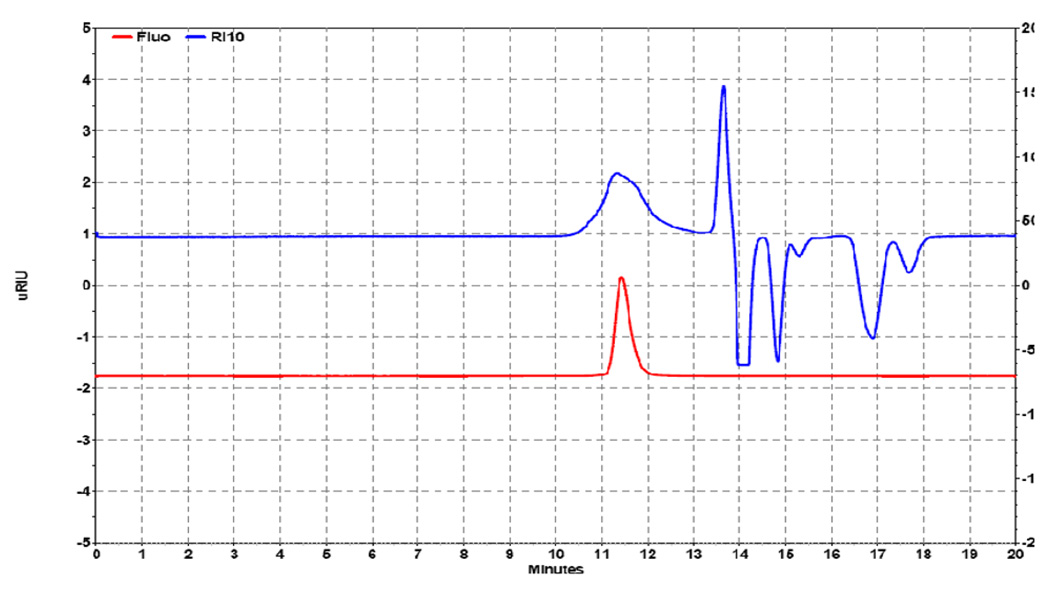
The reported PEG linker has functional termini of alkyne and succinimidyl carbonate. The SC terminus can form carbamate conjugates with the primary amines of proteins, i.e. amine or lysine or the N-terminus, while the alkyne terminus can undergo 1,3-dipolar cycloaddition, i.e. "click" reaction, with azido ligands to form 1,2,3-triazoles, such as modified targeting proteins, antibodies, or peptides. The model fluorescent dye IR820 azide (compound 7) was "click" conjugated to α-alkyne-ω-SC PEG (Mn 5000) in aqueous solution using an in situ formed copper (I) salt as the catalyst. The α-IR820-ω-SC PEG conjugate (compound 8) had excitation and emission wavelengths at 585 nm and 605 nm, respectively, indicating the successful conjugation of IR820 azide and α-alkyne-ω-SC PEG since the free IR820 had a maxima excitation of 710 nm and emission of 820 nm. GPC analysis of α-IR820-ω-SC PEG conjugates (Figure 2) showed conjugation of IR820 azide to alkyne-terminal PEG as indicated by the fluorescent peak coeluting with the polymer (RI detection) at around 11.5 min; by contrast, no fluorescent peak was observed for the unconjugated α-alkyne-ω-SC PEG (data not shown).
Figure 2. Gel chromatography analysis of α-IR820-ω-SC PEG conjugates.
The 5 K PEG conjugate RI peak (top, 11.5 min) corresponds to the fluorescence peak (bottom, 11.5 min) indicating "click" conjugation of the dye and polymer.
In vivo, the reversable PEG linker system is known to cleave at a predictable rate by enzymatic cleavage, followed by a rapid 1,6-benzyl elimination reaction to release intact proteins or drugs (9,10,15,17). The hydrolytic rate of the PEG revisable linker can be regulated by modifying the chemistry of the phenol ring, for example, the introduction of the steric hindrance via the 2,6-dimethyl substitution on the phenol ring resulted in a longer plasma t1/2 of PEG-amphotericin B and PEG-Daunorubicin, compared with no substitution on the phenol ring (15,18). In addition, the release rate of the conjugated proteins and drugs was closely related with the number of PEG strands used. It was reported that in the PEG-lysozyme system, intact lysozyme was released more rapidly from the single PEG strand conjugates than the multistrand conjugates (9,10).
Biopharmaceuticals such as proteins, antibodies, and peptides had a 24% market share in 2007, with 18 drugs reaching "blockbuster" status with $1 billion-plus sales (19). PEGylation has become an important tool to improve residence time and stability of biotech drugs in the body (20); however, the site of PEG conjugation cannot be controlled if multiple sites are available, which may negatively impact drug efficacy. Reversible linkers can overcome this limitation by restoring the native molecule after cleavage. In addition, most biologicals require relatively high concentrations since they are competing for binding sites with endogenous ligands, so the addition of targeting ligands to diseased tissues may further improve efficacy. The reported heterobifunctional, reversible "click" poly(ethylene glycol) may be useful as a single agent for both protection and targeting of biologicals.
Acknowledgements
This work was supported by awards from the National Institutes of Health (R21 CA132033 and P20 RR015563) and the American Cancer Society (Research Scholar Grant RSG-08-133-01-CDD). In addition, partial support for this project and its authors was provided by the University of Kansas Cancer Center (Research Pilot Award) and the University of Kansas (General Research Fund Award).
References
- 1.Deiters A, Cropp TA, Mukherji M, Chin JW, Anderson JC, Schultz PG. Adding amino acids with novel reactivity to the genetic code of Saccharomyces cerevisiae. J Am Chem Soc. 2003;125:11782–11783. doi: 10.1021/ja0370037. [DOI] [PubMed] [Google Scholar]
- 2.Deiters A, Cropp TA, Summerer D, Mukherji M, Schultz PG. Site-specific PEGylation of proteins containing unnatural amino acids. Bioorg Med Chem Lett. 2004;14:5743–5745. doi: 10.1016/j.bmcl.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 3.Link AJ, Tirrell DA. Cell surface labeling of Escherichia coli via copper(I)-catalyzed [3+2] cycloaddition. J Am Chem Soc. 2003;125:11164–11165. doi: 10.1021/ja036765z. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. Bioconjugation by copper(I)-catalyzed azide-alkyne [3+2] cycloaddition. J Am Chem Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Megia E, Correa J, Riguera R. "Clickable" PEG-dendritic block copolymers. Biomacromolecules. 2006;7:3104–3111. doi: 10.1021/bm060580d. [DOI] [PubMed] [Google Scholar]
- 6.Hassane FS, Frisch B, Schuber F. Targeted liposomes: Convenient coupling of ligands to preformed vesicles using "click chemistry". Bioconjug Chem. 2006;17:849–854. doi: 10.1021/bc050308l. [DOI] [PubMed] [Google Scholar]
- 7.Brennan JL, Hatzakis NS, Tshikhudo TR, Dirvianskyte N, Razumas V, Patkar S, Vind J, Svendsen A, Nolte RJ, Rowan AE, Brust M. Bionanoconjugation via click chemistry: The creation of functional hybrids of lipases and gold nanoparticles. Bioconjug Chem. 2006;17:1373–1375. doi: 10.1021/bc0601018. [DOI] [PubMed] [Google Scholar]
- 8.Hiki S, Kataoka K. A facile synthesis of azido-terminated heterobifunctional poly(ethylene glycol)s for "click" conjugation. Bioconjug Chem. 2007;18:2191–2196. doi: 10.1021/bc700152j. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Greenwald RB, McGuire J, Yang K, Shi C. Drug delivery systems employing 1,6-elimination: Releasable poly(ethylene glycol) conjugates of proteins. Bioconjug Chem. 2001;12:163–169. doi: 10.1021/bc000064z. [DOI] [PubMed] [Google Scholar]
- 10.Greenwald RB, Yang J, Zhao H, Conover CD, Lee S, Filpula D. Controlled release of proteins from their poly(ethylene glycol) conjugates: Drug delivery systems employing 1,6-elimination. Bioconjug Chem. 2003;14:395–403. doi: 10.1021/bc025652m. [DOI] [PubMed] [Google Scholar]
- 11.Cai CZ, Vasella A. Oligosaccharide analogs of polysaccharides. Part 3. A new protecting group for alkynes: orthogonally protected dialkynes. Helv Chim Acta. 1995;78:732–757. [Google Scholar]
- 12.Massy DJR, McKillop A. Carboxyalkylthiomethylation of phenols. Synthesis. 1989:253–255. [Google Scholar]
- 13.Alvarez SG, Alvarez MT. A practical procedure for the synthesis of alkyl azides at ambient temperature in dimethyl sulfoxide in high purity and yield. Synthesis. 1997:413–414. [Google Scholar]
- 14.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective "ligation" of azides and terminal alkynes. Angew Chem Int Ed Engl. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Greenwald RB, Pendri A, Conover CD, Zhao H, Choe YH, Martinez A, Shum K, Guan S. Drug delivery systems employing 1,4- or 1,6-elimination: poly(ethylene glycol) prodrugs of amine-containing compounds. J Med Chem. 1999;42:3657–3667. doi: 10.1021/jm990166e. [DOI] [PubMed] [Google Scholar]
- 16.Miron T, Wilchek M. A simplified method for the preparation of succinimidyl carbonate polyethyleneglycol for coupling to proteins. Bioconjug Chem. 1993;4:568–569. doi: 10.1021/bc00024a022. [DOI] [PubMed] [Google Scholar]
- 17.Greenwald RB, Pendri A, Conover CD, Lee C, Choe YH, Gilbert C, Martinez A, Xia J, Wu DC, Hsue M. Camptothecin-20-PEG ester transport forms: the effect of spacer groups on antitumor activity. Bioorg Med Chem. 1998;6:551–562. doi: 10.1016/s0968-0896(98)00005-4. [DOI] [PubMed] [Google Scholar]
- 18.Conover CD, Zhao H, Longley CB, Shum KL, Greenwald RB. Utility of poly(ethylene glycol) conjugation to create prodrugs of amphotericin B. Bioconjug Chem. 2003;14:661–666. doi: 10.1021/bc0256594. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence S. Billion dollar babies – biotech drugs as blockbusters. Nat Biotechnol. 2007;25:380–382. doi: 10.1038/nbt0407-380. [DOI] [PubMed] [Google Scholar]
- 20.Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov Today. 2005;10:1451–1458. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]