Sir,
We report the clinical and electrophysiological findings in a case of Oguchi disease carrying a homozygous nonsense mutation in SAG (c.874C>T, p.Arg292X). The SAG gene encodes S-antigen, a visual/beta arrestin abundant in rod photoreceptors. S-antigen binds to light-activated rhodopsin preventing further interaction with transducin during the recovery phase of phototransduction. Mutations in SAG are primarily associated with Oguchi disease.1
Case report
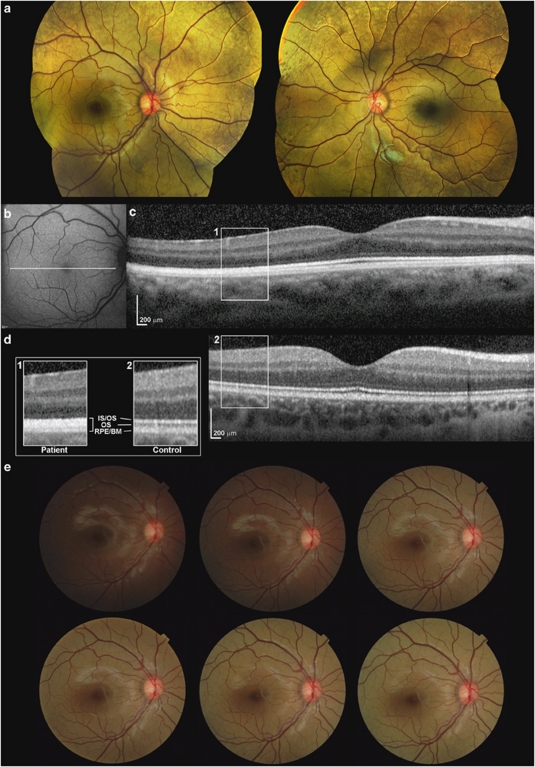
A 7-year-old girl of South Asian origin was referred for evaluation of congenital nyctalopia. Visual acuity was 0.12 logMAR in each eye. Fundus examination revealed widespread golden discolouration and peripheral RPE mottling (Figure 1a). Fundus autofluorescence imaging was normal (Figure 1b). Spectral-domain optical coherence tomography (SD-OCT; Spectralis, Heidelberg Engineering, Heidelberg, Germany) is shown in Figure 1c. In the foveola, three hyper-reflective bands representing the inner segment/outer segment junction of the photoreceptors, the cone outer segment tips, and the RPE/Bruch's membrane complex are visible. Outside the fovea, the hypo-reflective band corresponding to the outer segments is not apparent. The findings were consistent on three different tests over a 1-year interval. SD-OCT of an age-matched control is also shown in Figure 1d.
Figure 1.
(a) Colour fundus photography of both eyes showing typical golden fundus reflex and pigment mottling in the far periphery. Discolouration is less profound in the macular area. (b) Fundus autofluorescence imaging of the right eye demonstrating no abnormality. (c) Axial cross-sectional image of the proband's right macula obtained using SD-OCT. In the parafovea, SD-OCT failed to detect the hyporeflective band (outer segments; OS) that is observed between the hyper-reflective layers associated with the inner/outer segment junction (IS/OS), and the RPE/Bruch's membrane complex (RPE/BM). (d) SD-OCT of the right eye of a 9-year-old control individual. Scans in c and d are to scale and acquired using the same SD-OCT protocol. Panel a with enlarged images of boxed regions (1, patient; 2, control) shows outer retina in detail. (e) Colour fundus photography of the posterior pole of the right eye using a non-mydriatic camera. After overnight (12-h) dark adaptation, a series of images were obtained over a 20-min interval. Disappearance of the golden reflex can be seen in the first image taken (top left). The golden colour gradually reappears after 10–15 flashes. Bottom right image is taken after 20 min and 32 flashes.
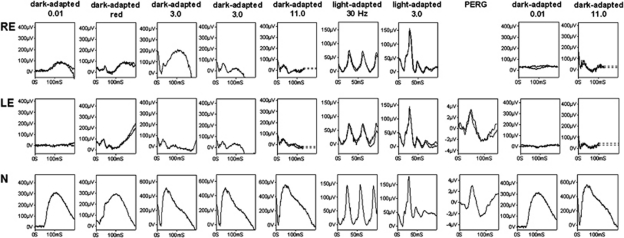
International-standard full-field electroretinograms (ERGs) were consistent with severe generalised rod photoreceptor dysfunction. Prolonged dark adaptation resulted in partial ERG recovery, in keeping with abnormally slow rod dark adaptation, but with marked desensitisation following a single bright flash (Figure 2). Generalised cone function was normal. Pattern ERG revealed no evidence of macular dysfunction.
Figure 2.
Full-field ERGs from the right (RE) and left (LE) eyes of the patient and normal examples (N) for comparison. After 25-min dark adaptation, left eye rod ERGs (0.01 cd s per m2) were undetectable and bright flash ERGs (3.0 and 11.0 cd s per m2) had a waveform that resembled the early component of the red flash ERG, consistent with a dark-adapted cone system origin.7 After overnight dark adaptation of the right eye, ERGs showed partial recovery but a second bright flash (3.0 cd s per m2; inter-stimulus interval 60 s) resulted in marked ERG attenuation. Light-adapted ERGs (3.0 cd s per m2; 30 Hz and 2 Hz) revealed no evidence of generalised cone system dysfunction. The pattern ERG (PERG; left eye only) revealed no evidence of macular dysfunction. After photopic testing ERGs were repeated following an additional 20-min dark adaptation of both eyes.
To test for the Mizuo-Nakamura phenomenon, the patient's right eye was dark-adapted overnight and images were obtained with a non-mydriatic camera (TRC-NW65, Topcon, Tokyo, Japan). Fundus appearance was initially normal in the dark-adapted eye but after 10–15 flashes, the golden sheen reappeared (Figure 1e).
Comment
Oguchi disease is caused by mutations in either SAG or GRK1, a gene encoding rhodopsin kinase.1 Mutated GRK1 alleles are considered the commonest cause of Oguchi in South Asians and only one Indian family has been reported carrying SAG mutation.2
SD-OCT findings similar to the proband have been previously described in two non-genetically confirmed Oguchi cases;3, 4 this outer retinal appearence has been attributed to microstructural changes,3, 4 and could indicate increased reflectivity in the light-adapted state. Additional outer retinal attenuation demonstrated in one of these cases, a 31-year-old man,3 was not evident in our case.
A retinal sheen similar to Oguchi disease can be associated with RS1 mutation, and partial or complete ERG recovery following prolonged dark adaption can occur in RDH5 or RLBP1-related disease.1, 5 Rapid ERG attenuation to successive flashes can result from RGS9/R9AP mutation,6 but the combination of normal cone function, delayed rod ERG dark adaptation and marked rod desensitisation to a bright flash is distinctive for Oguchi disease.
Acknowledgments
We acknowledge the following sources of funding: British Retinitis Pigmentosa Society, Fight for Sight, Alexander S Onassis Public Benefit Foundation, Moorfields Eye Hospital Special Trustees, National Institute for Health Research UK (Moorfields Eye Hospital and Institute of Ophthalmology, London, UK), Foundation Fighting Blindness (USA).
The authors declare no conflict of interest.
References
- Dryja TP. Molecular genetics of Oguchi disease, fundus albipunctatus, and other forms of stationary night blindness: LVII Edward Jackson Memorial Lecture. Am J Ophthalmol. 2000;130:547–563. doi: 10.1016/s0002-9394(00)00737-6. [DOI] [PubMed] [Google Scholar]
- Maw M, Kumaramanickavel G, Kar B, John S, Bridges R, Denton M. Two Indian siblings with Oguchi disease are homozygous for an arrestin mutation encoding premature termination. Hum Mutat. 1998. pp. S317–S319. [DOI] [PubMed]
- Hashimoto H, Kishi S. Shortening of the rod outer segment in Oguchi disease. Graefes Arch Clin Exp Ophthalmol. 2009;24:1561–1563. doi: 10.1007/s00417-009-1114-6. [DOI] [PubMed] [Google Scholar]
- Takada M, Otani A, Ogino K, Yoshimura N. Spectral-domain optical coherence tomography findings in the Mizuo–Nakamura phenomenon of Oguchi disease. Retina. 2011;31:626–628. doi: 10.1097/IAE.0b013e318206cd52. [DOI] [PubMed] [Google Scholar]
- Robson AG, Mengher LS, Tan MH, Moore AT. An unusual fundus phenotype of inner retinal sheen in X-linked retinoschisis. Eye (Lond) 2009;23:1876–1878. doi: 10.1038/eye.2008.358. [DOI] [PubMed] [Google Scholar]
- Nishiguchi KM, Sandberg MA, Kooijman AC, Martemyanov KA, Pott JW, Hagstrom SA, et al. Defects in RGS9 or its anchor protein R9AP in patients with slow photoreceptor deactivation. Nature. 2004;427:75–78. doi: 10.1038/nature02170. [DOI] [PubMed] [Google Scholar]
- McBain VA, Egan CA, Pieris SJ, Supramaniam G, Webster AR, Bird AC, et al. Functional observations in vitamin A deficiency: diagnosis and time course of recovery. Eye (Lond) 2007;21:367–376. doi: 10.1038/sj.eye.6702212. [DOI] [PubMed] [Google Scholar]