Abstract
Purpose
There is evidence for complement dysfunction in age-related macular degeneration (AMD). Complement activation leads to formation of the membrane attack complex (MAC), known to assemble on retinal pigment epithelial (RPE) cells. Therefore, the effect of sub-lytic MAC on RPE cells was examined with regard to pro-inflammatory or pro-angiogenic mediators relevant in AMD.
Methods
For sub-lytic MAC induction, RPE cells were incubated with an antiserum to complement regulatory protein CD59, followed by normal human serum (NHS) to induce 5% cell death, measured by a viability assay. MAC formation was evaluated by immunofluorescence and FACS analysis. Interleukin (IL)-6, -8, monocytic chemoattractant protein-1 (MCP-1), and vascular endothelial growth factor (VEGF) were quantified by enzyme-linked immunosorbent assay (ELISA). Intracellular MCP-1 was analysed by immunofluorescence, vitronectin by western blotting, and gelatinolytic matrix metalloproteinases (MMPs) by zymography.
Results
Incubation of RPE cells with the CD59 antiserum followed by 5% NHS induced sub-lytic amounts of MAC, verified by FACS and immunofluorescence. This treatment stimulated the cells to release IL-6, -8, MCP-1, and VEGF. MCP-1 staining, production of vitronectin, and gelatinolytic MMPs were also elevated in response to sub-lytic MAC.
Conclusions
MAC assembly on RPE cells increases the IL-6, -8, and MCP-1 production. Therefore, sub-lytic MAC might have a significant role in generating a pro-inflammatory microenvironment, contributing to the development of AMD. Enhanced vitronectin might be a protective mechanism against MAC deposition. In addition, the increased expression of gelatinolytic MMPs and pro-angiogenic VEGF may be associated with neovascular processes and late AMD.
Keywords: age-related macular degeneration, retinal pigment epithelial cells, membrane attack complex, complement
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in developed countries amongst the elderly. There is compelling evidence for a significant role of complement in AMD, particularly with regard to the complement factor H gene variant Y402H, which was demonstrated to have less complement-inhibitory activity.1 The altered complement factor H molecule was shown to be correlated with more than half of the diagnosed AMD cases,2, 3, 4 which highlights the importance of Y402H as a major genetic risk factor in the disease. Additionally, several proteins and their activation products involved in the complement cascade, including the membrane attack complex (MAC), were found in drusen, which are sub-retinal deposits and a hallmark of early stage AMD.5
The complement system, as part of the innate immune system, provides a first line of defence against infection through activation of leukocytes, opsonisation, and assembly of MAC, the terminal complex of the complement cascade. Upon complement activation, its individual components C5b, C6, C7, C8, and C9 combine to form a lytic pore on the surface of target cell membranes in response to activated complement, capable of inducing cell lysis and inflammatory processes, as well as activating various cell signalling pathways.6, 7 The formation of MAC is regulated by the membrane bound complement regulatory protein CD59, which controls activation of complement by inhibiting the incorporation of C9 into the forming complex, and thereby preventing assembly of a functional pore.8, 9 CD59 is strongly expressed in the normal human retina and in cultured retinal pigment epithelial (RPE) cells.10
AMD pathogenesis was shown to be associated with several inflammatory indicators, like an increase in white blood cell count, C-reactive protein levels, and intercellular adhesion molecule-1 amongst others.11, 12, 13 Specifically, RPE cells are thought to have a significant role in intraocular inflammation by secreting cytokines and chemokines.14, 15 Interleukins (IL)-6 and -8 have regulatory functions in the immune response,16 whereas monocytic chemoattractant protein (MCP)-1 attracts monocytes to inflammation sites, and drives their maturation towards macrophages.17 Augmented production of the pro-angiogenic vascular endothelial growth factor (VEGF) by the RPE has also been described.18
In addition, RPE cells also have the ability to produce vitronectin, another important constituent of drusen,19, 20 which interferes in complement-mediated cell lysis by preventing MAC formation.21 Gelatinolytic matrix metalloproteinases (MMP)-2 and -9 are also elevated in areas of new vessel formation, and are therefore associated with the development of choroidal neovascularisation.22
Currently, little is known about the molecular effects of MAC on RPE cells, and how these may contribute to the pathogenesis of AMD through the recruitment of monocytes and the modulation of subsequent macrophage function. In AMD, it is known that MAC assembles on RPE cells,23 and in early disease, the RPE may also be subject to sub-lytic assembly of MAC, which in other cell types has been shown to modulate intracellular signalling pathways, and thus alter normal cellular function.24, 25 This study, therefore, investigates whether sub-lytic MAC alters the ability of RPE cells to influence the local environment through cytokine and growth factor production, deposition of vitronectin or altered secretion of MMPs.
Methods
Cell culture and reagents
The human RPE cell line ARPE-1926 (ATCC number CRL-2302) was cultured in DMEM/F12 (1 : 1; Gibco, Paisley, UK) supplemented with 100 U/ml penicillin/streptomycin (PAA Laboratories GmbH, Pasching, Austria) and 10% foetal bovine serum (Gibco) at 37 °C, with a carbon dioxide content of 5%. The medium was changed twice a week. All reagents were obtained from Sigma (Steinheim, Germany), unless stated otherwise.
MAC deposition assay
Confluent ARPE-19 cells were serum-starved for 2 days, and incubated with a rabbit polyclonal anti-human CD59 antiserum (raised in house by Prof BP Morgan, Cardiff University, Cardiff, UK) for 1 h at 37 °C. Cells were washed and exposed to complement-containing normal human serum (NHS) or heat-inactivated (HI) NHS (45 min at 56 °C) as a complement deficient control at 37 °C for another hour. Afterwards, the cells were washed thoroughly, and incubated with serum-free media for 24 h. The amount of NHS selected for the MAC deposition assay was defined by calibration in a 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) viability assay, where sub-lytic doses of MAC were characterised by 5% cell death.27 Further, MAC formation was determined by FACS and immunofluorescence analysis.
MTT viability assay
After the aforementioned treatments of RPE cells, 100 μl/ml MTT (5 mg/ml thiazolyl blue in phosphate buffered saline (PBS) sterile filtered) was added to the media and incubated at 37 °C for about 3 h until formazan crystals appear. The reaction was stopped with 0.02 M SDS, and 50% dimethyl formamide. The optical density of the supernatants was measured photometrically at a wavelength of 570 nm after crystal dissolving. A positive control was created from cells treated with anti-CD59 and HI–NHS; a negative control for 100% cell death was performed by addition of 1% Triton X-100, 10 min before adding MTT.
Flow cytometry
RPE cells were washed and incubated in PBS and 1% bovine serum albumin for 30 min. A monoclonal mouse anti-C5b-9 antibody (clone αE11, Dako, Hamburg, Germany; 1 : 50) was applied for 1 h at 4 °C. The cells were washed three times, and incubated with a FITC-labelled secondary donkey anti-mouse IgG (Dako; 1 : 200) for 30 min at RT in the dark. The stained cells were washed again and detached with pre-warmed PBS and 15 nM EDTA at 37 °C. The cell suspension was then analysed by FACSCalibur (Becton Dickinson, Heidelberg, Germany), and the data were analysed with WinMDI 2.9 (http://facs.scripps.edu/software.html), and expressed as mean fluorescence intensity in a histogram.
Immunofluorescence
After assembly of sub-lytic MAC in glass-bottom dishes (MatTek, Ashland, MA, USA), cells were fixed with 4% paraformaldehyde in PBS at 4 °C for 20 min. Cells were blocked in PBS and 1% bovine serum albumin for 1 h and incubated with the anti-C5b-9 antibody, or a monoclonal anti-MCP-1 antibody (Dako; 1 : 50) at 4 °C overnight. The dishes were washed three times in PBS, and incubated with a FITC-conjugated rabbit anti-mouse IgG1 secondary antibody (Dako; 1 : 200) in PBS and 1% bovine serum albumin for 1 h at RT in the dark, and washed as described above. The nuclei were counterstained with Hoechst (1 mg/ml). The cells were washed, coated with mounting media, covered, and analysed by a Leica TSC-SP2 laser scanning confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Enzyme-linked immunosorbent assay (ELISA)
After MAC deposition, the serum-free media were harvested after 24 h, stored at −20 °C, and analysed for IL-6, -8, MCP-1 (human IL-6, -8, and MCP-1 OptEIA ELISA Sets; BD Bioscience, Heidelberg, Germany), and VEGF (human VEGF DuoSet; R&D Systems, Wiesbaden, Germany) by sandwich-ELISA.
SDS-PAGE and western blotting
Cells were lysed with 200 μl of 2% SDS, 25% glycerol, 12.5% 0.5 M Tris, 0.02% DTT, and 0.2% bromophenol blue, at 95 °C for 5 min. The cell lysate was homogenised and subjected to SDS-PAGE using a 10% acrylamide gel. The electrophoresis was performed at 120V for 2 h at 4 °C. A kaleidoscope protein standard (Bio-Rad Laboratories, Munich, Germany) enabled the estimation of the molecular weights. The proteins were transferred onto a nitrocellulose membrane at 350 mA for 50 min. The membrane was then blocked with 5% bovine serum albumin in PBS and 0.05% Tween overnight at 4 °C, and stained with a monoclonal mouse anti-vitronectin antibody (TaKaRa Bio Inc., Otsu, Japan; 1 : 2000) for 3 h. The membrane was then incubated with a monoclonal rabbit anti-mouse Immunoglobulin/Biotin Rabbit F(ab')2 (Dako, 1 : 3000) for 1 h, and with HRP (1 : 4000) for 20 min. Each step was followed by three washes with PBS–Tween. The bands on the membrane were visualised by a chemiluminescent reaction (Western Blotting Luminol Reagent Kit, Santa Cruz Biotechnology, Heidelberg, Germany), and exposed to x-ray film. The membrane was then stripped and re-probed with a monoclonal mouse α-tubulin antibody (clone TU-01; Exbio, Vestec, Czech Republic; 1 : 500). Densitometric analysis of obtained bands was performed by ImageJ (NIH freeware, Bethesda, MD, USA), and the data were normalised to α-tubulin.
Zymography
The protein concentration of the supernatants was measured with a DC Protein Assay Kit (Bio-Rad Laboratories) to load equal amounts of protein. For the detection of gelatinolytic active MMP, a resolving gel was used containing 0.1% gelatine. The media was diluted in 2% SDS, 25% glycerol, 12.5% 0.5 M Tris, and 0.2% bromophenol blue, and applied to the gel. A kaleidoscope protein standard and a human recombinant MMP-2 (R&D Systems) as a positive control were also loaded onto the gel. The enzymes were separated at 120V for about 2 h. The gel was then incubated in 2.5% Triton X-100 for 1 h, washed and incubated in 50 mM Tris, 10 mM calcium chloride, 0.02% sodium azide, and 1% Triton X-100, at 37 °C over night. The following day, the gel was washed and stained in 0.25% Coomassie brilliant blue, 10% acetic acid, 40% methanol in distilled water for 2 h, and destained in 10% acetic acid, and 40% methanol until white bands appeared. ImageJ and Microsoft Excel were used to analyse and plot the grey values of the different band sizes.
Statistical analysis
The obtained data were represented as mean and standard deviation. A two-tailed Student t-test was used to analyse differences between distinct groups. Significant differences were defined as P<0.05, and marked with an asterisk (*).
Results
Sub-lytic MAC formation on RPE cells
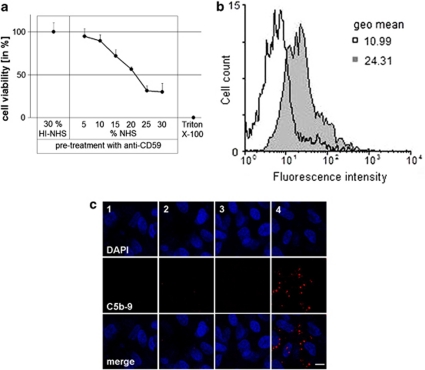
For the deposition of MAC, RPE cells were incubated with a polyclonal rabbit CD59 antiserum to permit complement activation and the assembly of MAC. The amount of NHS required to induce 5% cell death, reflecting sub-lytic amounts of MAC, was measured by an MTT viability assay. CD59 antiserum and increasing amounts of NHS led to a concentration-dependent increase in cell death, whereas treatment with anti-CD59 and HI–NHS had no effect on the cell viability. After pre-incubation with the antiserum, 5% NHS was used to provoke 5% cell death (Figure 1a).
Figure 1.
Verification of sub-lytic MAC assembly. (a) MTT viability assay measuring the amount of cell death after treating ARPE-19 cells with anti-CD59, and ascending concentrations of NHS for 1 h at 37 °C. For negative controls, cells were treated with anti-CD59/HI–NHS, and for positive controls with 1% Triton X-100. Data are plotted as means±s.d. (n=5). (b) The mean fluorescence intensity of C5b-9 using flow cytometry presented as a histogram in control (anti-CD59/HI–NHS; open histogram), and anti-CD59/NHS-treated cells (grey histogram) (n=4 for each treatment). (c) Representative images (n=4) from immunofluorescence staining for C5b-9 after MAC assembly on ARPE-19 cells. 1=HI–NHS alone, 2=NHS alone, 3=anti-CD59/HI–NHS, 4=anti-CD59/NHS. The scale bar indicates 10 μm.
The presence of MAC on ARPE-19 cells in vitro after sub-lytic MAC induction was determined by flow cytometry and immunofluorescence. Flow cytometry analysis revealed a significant increase in the mean fluorescence intensity for MAC, compared with the control anti-CD59/HI–NHS-treated cells. MAC formation was confirmed by the increase observed in the geometric mean from 10.99 in control cells to 24.31 in MAC-induced cells (Figure 1b). Immunofluorescence also revealed positive staining for C5b-9 after induction of sub-lytic MAC, whereas control treatments were negative for MAC staining (Figure 1c).
Sub-lytic MAC formation induces cytokine production in RPE cells
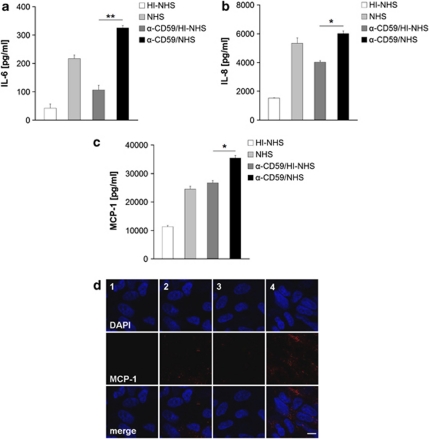
The media assayed by ELISA revealing that HI–NHS-treated control cells produced IL-8 (1522±11 pg/ml) and MCP-1 (11 312±365 pg/ml) constitutively, and to a minor degree IL-6 (42±8 pg/ml). After anti-CD59/HI–NHS treatment, the amount produced approximately doubled to 4018±106 pg/ml, 26 711±838 pg/ml, and 107±13 pg/ml for IL-8, MCP-1, and IL-6, respectively. NHS led to significant increases in the release of cytokines compared with HI–NHS. However, in response to MAC assembly (anti-CD59/NHS), the detected cytokine concentration was significantly higher with 6019±176 pg/ml for IL-8 (P<0.05), 35 367±923 pg/ml for MCP-1 (P<0.05), and 326±15 pg/ml for IL-6 (P<0.01; Figures 2a–c), when compared with all HI controls. Immunofluorescence analysis showed a punctate distribution of MCP-1 after MAC induction with anti-CD59/NHS, and to a much lesser extent with NHS alone. No staining was observed after treatment with anti-CD59/HI–NHS, or HI–NHS alone (Figures 2d).
Figure 2.
Cytokine production by RPE. Representative (a) IL-6, (b) IL-8, and (c) MCP-1 production after various treatments as measured in the cell culture supernatant by ELISA. Duplicate data are shown as mean±s.d. (n=3). (d) Representative immunofluorescent staining of MCP-1 following treatment with HI–NHS (1), NHS (2), anti-CD59/HI–NHS (3), and anti-CD59/NHS (4) out of 4 separate experiments. The scale bar indicates 10 μm. *P<0.05, **P<0.01.
Increased vitronectin expression in response to sub-lytic MAC
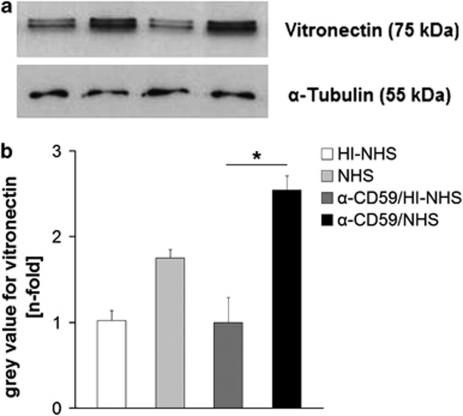
Proteins separated by SDS-PAGE, and western blot analysis from RPE cell lysates following the different treatments revealed a distinct band at ∼75 kDa corresponding to vitronectin. In ARPE-19 cells, in which sub-lytic MAC formation was induced, vitronectin was significantly elevated in comparison with HI–NHS alone, or anti-CD59/HI–NHS-treated control cells. NHS treatment on its own also led to a slightly higher production of vitronectin than the complement depleted controls, but still less than the effect of MAC assembly (Figures 3a and b).
Figure 3.
(a) Representative western blot for vitronectin using cell lysates of ARPE-19 cells after various treatments. (b) Semi-quantitative analysis of vitronectin western blots (n=4) normalised to α-tubulin. *P<0.05.
Sub-lytic MAC assembly on RPE cells induces VEGF secretion
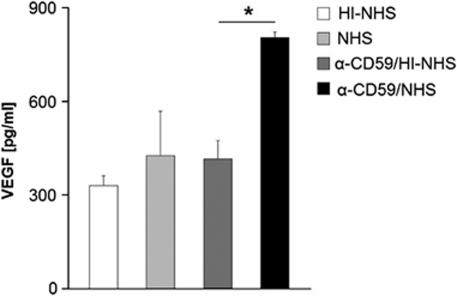
No differences in VEGF secretion from confluent ARPE-19 cells were detected following the various treatments. In contrast, sub-confluent RPE cells produced 805±17 pg/ml of VEGF following activation of MAC assembly, revealing a significant increase (P<0.05). VEGF secretion in response to the control treatments only resulted in values between 330 and 415 pg/ml (Figure 4).
Figure 4.
ELISA of representative VEGF production by sub-confluent ARPE-19 cells after treatment with anti-CD59/NHS, anti-CD59/HI–NHS, NHS alone, or HI–NHS alone. Duplicates are shown as mean±s.d., whereas the experiment was repeated two more times with comparable results. *P<0.05.
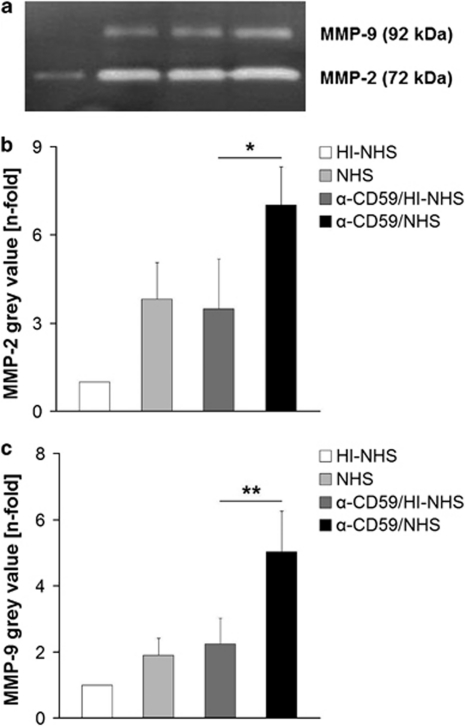
Increased MMP activity after inducing sub-lytic MAC
Secreted levels of the gelatinases MMP-2 and MMP-9 over 24 h into serum-free media were examined by zymography. Two bands at 72 and 92 kDa corresponding to MMP-2 and MMP-9 activity, respectively, were observed (Figure 5a). The constitutive activities of both MMP-2 and MMP-9 in supernatants of HI–NHS-treated control cells were low. In supernatants harvested from RPE cells treated with either NHS alone, or antiCD59/HI–NHS, the amount of both MMPs increased. However, when treated with anti-CD59/NHS to elicit MAC formation, there was a significant increase in both MMP-2 (Figure 5b; P<0.05) and MMP-9 (Figure 5c; P<0.01).
Figure 5.
(a) Representative zymography for gelatinolytic MMP following different treatments (n=5). Data of semi-quantitative analysis of (b) MMP-2 and (c) MMP-9 from zymographs after different treatments were presented as mean±s.d. *P<0.05, **P<0.01.
Discussion
The assembly of MAC on cell surfaces and the subsequent induction of cytolysis28 is a recognised feature of the innate immune response. However, in many nucleated cell types, assembly of MAC occurs without cell lysis due to the presence of complement regulatory proteins on the cell surface and active recovery processes, which remove the MAC before lysis can take place. In such cases, numerous non-lethal effects have been documented, many of which remain poorly understood. Previous studies have suggested that sub-lytic MAC formation can activate various signalling pathways, such as mobilisation of Ca2+, activation of receptor tyrosine kinases, phospholipase C, protein kinase C, phospholipase 2α, and extracellular signal-regulated kinases.24, 29 On endothelial cells, MAC has been shown to promote the expression of pro-inflammatory mediators30 and may thus function in mediating leukocyte recruitment.31 The functional consequences of sub-lytic MAC formation on RPE cells, however, have not been clearly defined, despite their potential importance in AMD. Furthermore, C-reactive protein, which is elevated in the choroid of AMD patients harbouring a risk-conferring variant of complement factor H, can bind to phospholipids exposed on MAC-activated cells,32 and impact further on RPE cell function. To address this issue, we have investigated the effect of sub-lytic MAC on RPE cells in vitro, with particular regard to the production of pro-inflammatory and pro-angiogenic mediators, as well as vitronectin and MMPs known to be relevant in the development and progression of AMD.
The application of the CD59 antiserum with HI–NHS had a discrete effect on RPE cells to produce the aforementioned cytokines, when compared with HI–NHS alone. However, this cytokine increase in response to anti-CD59 and HI–NHS was negligible, compared with HI–NHS alone, whereas the combination of anti-CD59 and NHS to induce sub-lytic MAC was highly significant, compared with all control treatments.
According to the immunofluorescence staining for MAC in this study, MAC induction could not be achieved by sole NHS. Still, NHS led to an increased release of IL-6, -8 and VEGF to a minor degree when compared with HI controls. These effects might be the outcome of heat-labile proteins, or specifically activation of C5a. This anaphylatoxin is another potent inflammatory factor of the complement cascade, and was shown earlier to stimulate ARPE-19 cells to produce IL-6, -8,33 and VEGF in vitro.34 We also discovered an augmented vitronectin production due to NHS in this regard. Nevertheless, the effects derived by MAC induction were always higher concerning IL-8 and VEGF secretion, or significantly higher when it came to produce IL-6, MCP-1, and vitronectin compared with NHS treatment alone, which may account for MAC-specific effects.
It has previously been reported that sub-lytic MAC on the surface of human umbilical vein endothelial cells promotes the expression and secretion of IL-8, MCP-1, and tumour necrosis factor-α, and thereby inducing a pro-inflammatory phenotype.35 On dendritic cells, it was shown to increase pro-inflammatory mediators such as IL-1β, IL-12, and tumour necrosis factor-α.36 The upregulated production of IL-6, -8, and MCP-1, which we observed in RPE cells following sub-lytic MAC induction, is therefore similar to that reported for other cell types. Moreover, these data are consistent with the development of a pro-inflammatory microenvironment in early AMD.37 The cytokines analysed in this study are locally produced by RPE cells in a variety of ocular inflammatory diseases,38, 39 and were also found increased in response to pro-inflammatory triggers like IL-ß1 in vitro.40 Therefore, dysfunction of the alternative pathway of the complement cascade in AMD may result in enhanced deposition of sub-lytic MAC on RPE cells,23 and subsequent induction of a pro-inflammatory phenotype.
The increase in vitronectin production in response to both MAC assembly and NHS is consistent with an earlier study where we reported that RPE cells produced elevated amounts of vitronectin after incubation with human complement-competent sera.41 Moreover, this finding is relevant to dry AMD, as vitronectin is a major component of drusen, a feature which correlates significantly with the early stage of AMD.20 As vitronectin regulates complement activation by inhibiting the formation of MAC,42 an increased amount of the protein suggests that a negative feed-back loop is activated to protect against uncontrolled complement activation.
Our observation that RPE-derived VEGF was increased after assembly of MAC has relevance to wet AMD. Interestingly, only sub-confluent cells produced elevated amounts of the growth factor, suggesting that when contact-inhibited and quiescent, they are refractory to triggers of enhanced VEGF expression. VEGF has been found to be produced constitutively by RPE cells,43 and was increased in response to MAC formation on primary RPE cells.44 Sustained low level secretion of VEGF is believed to be necessary for the normal development and maintenance of the choriocapillaris,45 whereas on the other hand, pathological increases in VEGF contribute to several ocular diseases including neovascularisation in wet AMD.46 Given that RPE cell–cell contacts may become disrupted in AMD, our findings therefore potentially link complement activation and sub-lytic MAC formation to increased VEGF secretion, and the neovascular processes observed in late stage disease.
MMP-2 and -9 were both augmented after inducing MAC assembly on ARPE-19 cells. An increased content of MMPs was shown to correlate with an increased appearance of choroidal neovascularisation in humans.47 Pathological angiogenesis was almost completely prevented in MMP-2/MMP-9 double-deficient mice,48 demonstrating the importance of these proteases in ocular angiogenesis. Furthermore, MMP-2 mRNA has been shown to be upregulated in experimental choroidal neovascularisation, and in MMP-2-deficient mice, such neovascularisation was attenuated.49 Thus, the increased MMP secretion we observed in response to sub-lytic MAC reveals another potential mechanism through which activated complement contributes to the development and progression of choroidal neovascularisation in wet AMD.
As for the essential role of these MMPs in the extracellular matrix (ECM) turnover,50 an increase in metalloproteinases may lead to a shift in ECM composition. Quantitative alterations in ECM components have previously been demonstrated to have a role in AMD.51 Lower expression of MCP-1 and intercellular adhesion molecule-1 as pro-inflammatory proteins led to reduced infiltration of inflammatory cells, and an accompanying reduction in ECM accumulation.52 Moreover, cytokines are known to be sequestered in the ECM and can be released by MMPs.53 The correlation between ECM and inflammatory proteins may be of importance in thickened BM as observed in both types of AMD.
These data demonstrate a number of functional consequences of sub-lytic MAC assembly on a cultured RPE cell line. MAC has been identified before in AMD patients within drusen, in Bruch's membrane,54 and on RPE overlying drusen in vivo.23 Therefore, this in vitro study may reflect the assembly of MAC, as it occurs in the human system, and increased secretion of pro-inflammatory and chemo-attractant cytokines by RPE cells, as well as the elevated amounts of vitronectin, may promote a role for sub-lytic MAC in the early stage of AMD. Further, the elevated growth factor VEGF, and raised production of gelatinolytic MMP is also consistent with a role for MAC assembly in the development of neovascularisation and late-stage AMD.
Acknowledgments
We would like to express our sincere appreciation to Grazyna Galatowicz at UCL Institute of Ophthalmology London and Maren Hennig at Ophtha-Lab Department of Ophthalmology Muenster for their help with the FACS analysis. This work was supported by DAAD and Voltmann Foundation. Funding: ‘DAAD', ‘Akademie des Sehens', and Voltmann Foundation.
The authors declare no conflict of interest.
Footnotes
Part of this study has previously been presented at a European congress.
References
- Skerka C, Lauer N, Weinberger AA, Keilhauer CN, Sühnel J, Smith R, et al. Defective complement control of factor H (Y402H) and FHL-1 in age-related macular degeneration. Mol Immunol. 2007;44 (13:3398–3406. doi: 10.1016/j.molimm.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci USA. 2005;43:582–589. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006;51:137–152. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JZ. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep. 2006;58 (3:353–363. [PubMed] [Google Scholar]
- Fosbrink M, Cudrici C, Tegla CA, Soloviova K, Ito T, Vlaicu S, et al. Response gene to complement 32 is required for C5b-9 induced cell cycle activation in endothelial cells. Exp Mol Pathol. 2009;86:87–94. doi: 10.1016/j.yexmp.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohana-Kashtan O, Ziporen L, Donin N, Kraus S, Fishelson Z. Cell signals transduced by complement. Mol Immunol. 2004;41:583–597. doi: 10.1016/j.molimm.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Yang P, Tyrrell J, Han I, Jaffe GJ. Expression and modulation of RPE cell membrane complement regulatory proteins. Invest Ophthalmol Vis Sci. 2009;50:3474–3481. doi: 10.1167/iovs.08-3202. [DOI] [PubMed] [Google Scholar]
- Miwa T, Song WC. Membrane complement regulatory proteins: insight from animal studies and relevance to human diseases. Int Immunopharmacol. 2001;1:445–459. doi: 10.1016/s1567-5769(00)00043-6. [DOI] [PubMed] [Google Scholar]
- Bora NS, Gobleman CL, Atkinson JP, Pepose JS, Kaplan HJ. Differential expression of the complement regulatory proteins in the human eye. Invest Ophthalmol Vis Sci. 1994;34:3579–3584. [PubMed] [Google Scholar]
- Shankar A, Mitchell P, Rochtchina E, Tan J, Wang JJ. Association between circulating white blood cell count and long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Am J Epidemiol. 2006;165 (4:375–382. doi: 10.1093/aje/kwk022. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N. Association between C-reactive protein and age-related macular degeneration. JAMA. 2004;291 (6:704–710. doi: 10.1001/jama.291.6.704. [DOI] [PubMed] [Google Scholar]
- Mullins RF, Skeie JM, Malone EA, Kuehn MH. Macular and peripheral distribution of ICAM-1 in the human choriocapillaris and retina. Mol Vis. 2006;12:224–235. [PubMed] [Google Scholar]
- Elner VM, Strieter RM, Elner SG, Baggiolini M, Lindley I, Kunkel SL. Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am J Pathol. 1990;136 (4:745–750. [PMC free article] [PubMed] [Google Scholar]
- Holtkamp GM, van Rossem M, de Vos AF, Willekens B, Peek R, Kijlstra A. Polarized secretion of IL-6 and IL-8 by human retinal pigment epithelial cells. Clin Exp Immunol. 1998;112 (1:34–43. doi: 10.1046/j.1365-2249.1998.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer JH, van Haren MA, de Vries-Knoppert WA, Baarsma GS, de Jong PV, Postema FJ, et al. Analysis of IL-6 levels in human vitreous fluid obtained from uveitis patients, patients with proliferative intraocular disorders and eye bank eyes. Curr Eye Res. 1992;11:181–186. doi: 10.3109/02713689208999530. [DOI] [PubMed] [Google Scholar]
- Ajuebor MN, Gibbs L, Flower RJ, Das AM, Perretti M. Investigation of the functional role played by the chemokine monocyte chemoattractant protein-1 in interleukin-1 induced murine peritonitis. Br J Pharmacol. 1998;125:319–326. doi: 10.1038/sj.bjp.0702071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Hageman GS, Mullins RF, Neitz M, Neitz J, Ozaki S, et al. Vitronectin gene expression in the adult human retina. Invest Ophthalmol Vis Sci. 1999;40:3305–3315. [PubMed] [Google Scholar]
- Hageman GS, Mullins RF, Russell SR, Johnson LV, Anderson DH. Vitronectin is a constituent of ocular drusen and the vitronectin gene is expressed in human retinal pigmented epithelial cells. FASEB J. 1999;13:477–484. doi: 10.1096/fasebj.13.3.477. [DOI] [PubMed] [Google Scholar]
- Podack ER, Mueller-Eberhard HJ. Isolation of human S-protein, an inhibitor of the membrane attack complex of complement. J Biol Chem. 1979;254:9808–9814. [PubMed] [Google Scholar]
- Steen B, Sejersen S, Berglin L, Seregard S, Kvanta A. Matrix metalloproteinases and metalloproteinase inhibitors in choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1998;39:2194–2200. [PubMed] [Google Scholar]
- Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- Cybulsky AV, Takano T, Papillon J, Bijian K, Guillemette J. Activation of the extracellular signal-regulated kinase by complement C5b-9. Am J Physiol Renal Physiol. 2005;289:593–603. doi: 10.1152/ajprenal.00066.2005. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang J, Guo G, Ruan Z, Jiang M, Wu S, et al. Induced B7-H1 expression on human renal tubular epithelial cells by the sublytic terminal complement complex C5b-9. Mol Immunol. 2009;46 (3:326–334. doi: 10.1016/j.molimm.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- Shankland SJ, Pippin JW, Couser WG. Complement (C5b-9) induces glomerular epithelial cell DNA synthesis but not proliferation in vitro. Kidney Int. 1999;56:538–548. doi: 10.1046/j.1523-1755.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- Niculscu F, Rus H. Mechanisms of signal transduction activated by sublytic assembly of terminal complement complexes on nucleated cells. Immunol Res. 2001;24:191–199. doi: 10.1385/ir:24:2:191. [DOI] [PubMed] [Google Scholar]
- Fosbrink M, Niculescu F, Rus H. The role of C5b-9 terminal complement complex in activation of the cell cycle and transcription. Immunol Res. 2005;31:37–46. doi: 10.1385/IR:31:1:37. [DOI] [PubMed] [Google Scholar]
- Kilgore KS, Schmid E, Shanley TP, Flory CM, Maheswari V, Tramontini NL, et al. Sublytic concentrations of the membrane attack complex of complement induce endothelial interleukin-8 and monocyte chemoattractant protein-1 through nuclear factor-kappa B activation. Am J Pathol. 1997;150:2019–2031. [PMC free article] [PubMed] [Google Scholar]
- Tramontini NL, Kuipers PJ, Huber CM, Murphy K, Naylor KB, Broady AJ, et al. Modulation of leukocyte recruitment and IL-8 expression by the membrane attack complex of complement (C5b-9) in a rabbit model of antigen-induced arthritis. Inflammation. 2002;26:311–319. doi: 10.1023/a:1021420903355. [DOI] [PubMed] [Google Scholar]
- Li YP, Mold C, Du Clos TW. Sublytic complement attack exposes C-reactive protein binding sites on cell membranes. J Immunol. 1994;152:2995–3005. [PubMed] [Google Scholar]
- Fukuoka Y, Strainic M, Medof ME. Differential cytokine expression of human retinal pigment epithelial cells in response to stimulation by C5a. Clin Exp Immunol. 2003;131:248–253. doi: 10.1046/j.1365-2249.2003.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortright DN, Meade R, Waters SM, Chenard BL, Krause JE. C5a, but not C3a, increases VEGF secretion in ARPE-19 human retinal pigment epithelial cells. Curr Eye Res. 2009;34 (1:57–61. doi: 10.1080/02713680802546658. [DOI] [PubMed] [Google Scholar]
- Kilgore KS, Flory CM, Miller BF, Evans VM, Warren JS. The membrane attack complex of complement induces interleukin-8 and monocyte chemoattractant protein-1 secretion from human umbilical vein endothelial cells. Am J Pathol. 1996;149:953–961. [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yang C, Jin N, Xie Z, Tang Y, Fei L, et al. Terminal complement complex C5b-9 treated human monocyte-derived dendritic cells undergo maturation and induce Th1 polarization. Eur J Immunol. 2007;37:167–176. doi: 10.1002/eji.200636285. [DOI] [PubMed] [Google Scholar]
- McGeer EG, Klegeris A, McGeer PL. Inflammation, the complement system and the diseases of aging. Neurobiol Aging. 2005;26:94–97. doi: 10.1016/j.neurobiolaging.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Biswas PS, Banerjee K, Kinchington PR, Rouse BT. Involvement of IL-6 in the paracrine production of VEGF in ocular HSV-1 infection. Exp Eye Res. 2006;82 (1:46–54. doi: 10.1016/j.exer.2005.05.001. [DOI] [PubMed] [Google Scholar]
- De Vos AF, Hoekzema R, Kijlstra A. Cytokines and uveitis, a review. Curr Eye Res. 1992;11 (6:581–597. doi: 10.3109/02713689209001814. [DOI] [PubMed] [Google Scholar]
- Kuppner MC, McKillop-Smith S, Forrester JV. TGF-beta and IL-1 beta act in synergy to enhance IL-6 and IL-8 mRNA levels and IL-6 production by human retinal pigment epithelial cells. Immunology. 1995;84 (2:265–271. [PMC free article] [PubMed] [Google Scholar]
- Wasmuth S, Lueck K, Baehler H, Lommatzsch A, Pauleikhoff D. Increased vitronectin production by complement-stimulated human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:5304–5309. doi: 10.1167/iovs.08-3326. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Masson D, Schäfer S, Peitsch M, Preissner KT. The heparin binding domain of S-protein/vitronectin binds to complement components C7, C8 and C9 and perforin from cytolytic T-cells and inhibits their lytic activities. Proc Natl Acad Sci USA. 1988;85:4814–4818. doi: 10.1021/bi00411a029. [DOI] [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Pe'er J, Gnessin H, Chan-Ling T, et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neuroci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Chen S, Ma M, Qian J, Ma X. Complement C5b-9 complex-induced alterations in human RPE cell physiology. Med Sci Monit. 2010;16 (1:BR17–BR23. [PubMed] [Google Scholar]
- Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D'Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci USA. 2006;106:18751–18756. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997;81:154–162. doi: 10.1136/bjo.81.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau KY, Sivaprasad S, Patel N, Donaldson TA, Luthert PJ, Chong NV. Plasma levels of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) in age-related macular degeneration. Eye. 2008;22:855–859. [PubMed] [Google Scholar]
- Lambert V, Munaut C, Jost M, Noel A, Werb Z, Foidart JM, et al. Matrix metalloproteinase-9 contributes to choroidal neovascularization. Am J Pathol. 2002;161:1247–1253. doi: 10.1016/S0002-9440(10)64401-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglin L, Sarman S, van der Ploeg I, Steen B, Ming Y, Itohara S, et al. Reduced choroidal neovascular membrane formation in matrix metalloproteinase-2-deficient mice. Invest Ophthalmol Vis Sci. 2003;44:403–408. doi: 10.1167/iovs.02-0180. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I.Extracellular matrix remodelling: the role of matrix metalloproteinases J Pathol 2003200(4448–464.Review. [DOI] [PubMed] [Google Scholar]
- Leibowitz HM, Krueger DE, Maunder LR, Milton RC, Kini MM, Kahn HA, et al. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973-1975. Surv Opthalmol. 1980;24 (Suppl:335–610. [PubMed] [Google Scholar]
- Li JJ, Lee SH, Kim DK, Jin R, Jung DS, Kwak SJ, et al. Colchicine attenuates inflammatory cell infiltration and extracellular matrix accumulation in diabetic nephropathy. Am J Physiol Renal Physiol. 2009;297 (1:F200–F209. doi: 10.1152/ajprenal.90649.2008. [DOI] [PubMed] [Google Scholar]
- Kresse H, Schönherr E.Proteoglycans of the extracellular matrix and growth control J Cell Physiol 2001189(3266–274.Review. [DOI] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]