Abstract
Purpose
The purpose of this study was to describe the long-term results of AlphaCor implantations, and to evaluate the main complications and risk factors.
Methods
Retrospective analysis of preoperative and follow-up data from 15 AlphaCor implantations. Analysis of outcomes, trends, and associations was performed and compared with data from published clinical trials and a literature review.
Results
The survival rate of the device at 1, 2, and 3 years was 87%, 58%, and 42%, respectively. Postoperative visual acuity ranged from hand movement to 0.8. The most significant complications were stromal melt (nine cases), optic deposition (three eyes), and retroprosthetic membrane formation (three eyes). The most common device-unrelated complication was trauma (three patients). All complications were managed without loss of the eye.
Conclusion
AlphaCor provides a treatment option for patients with corneal blindness in which a donor tissue graft would not succeed.
Keywords: AlphaCor, keratoprosthesis, outcomes, complications
Introduction
AlphaCor is a synthetic cornea made from poly(2-hydroxyethyl methacrylate) that has a peripheral region with interconnecting pores allowing biointegration with surrounding corneal tissue.1, 2 The two concentric regions are joined by means of an interpenetration of polymers across a junctional zone known as the interpenetrating polymer network (IPN).3 The entire device has a diameter of 7.0 mm, a thickness of 0.6 mm, and surface curvatures that result in appropriate refractive power when implanted. The device is presently available in two powers: AlphaCor-A (for aphakic patients) and AlphaCor-P (for phakic or pseudophakic patients). It is placed within a lamellar corneal pocket with tissue posterior to the optic being removed at the time of implantation (stage I surgery) and the tissue anterior to the optic being removed secondarily (stage II surgery).1, 2, 4 The porous skirt remains enclosed within the corneal stromal tissue with which it biointegrates because of cellular colonization and collagen deposition.
AlphaCor is the result of many years of laboratory and clinical research.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 Several complications, associations, and risk factors have been identified.21, 22, 23, 24, 25 The indications and surgical techniques have been evolving with experience.26, 27
This study aims to evaluate the long-term results, the main complications, and risk factors of AlphaCor implantations.
Materials and methods
The AlphaCor device, inclusion criteria, surgical techniques, and recommended management protocol have been described in details elsewhere, namely by Hicks et al.1, 2, 4, 20, 22, 23 Briefly, this keratoprosthesis (KPro) is approved for implantation into adults with an absence of current inflammation, and satisfactory tear film in cases of corneal blindness not treatable successfully by means of standard penetrating keratoplasty (PK) with donor corneal tissue. The study was conducted in conformance with the international ethics requirements, and all patients gave informed consent.
In this series, AlphaCor has been used in 15 eyes of 15 patients; 14 men (93%) and 1 woman (7%). The mean age of patients at the time of the surgery was 57 years, range 30–81 years. Patients typically had complex ocular histories with multiple pathologies: serious chemical burn in seven cases (47%), penetrating injury (dilaceratio bulbi) in two eyes (13%), bullous corneal dystrophies in four cases (27%), herpes simplex viral infection (HSV) in one eye (7%), and ocular cicatricial pemphigoid (OCP) in one case (7%) (Table 1). Mean preoperative visual acuity (VA) was hand movement (Table 2). Seven patients (47%) had bilateral blindness; in eight patients (53%), visual functions of the other eye were normal or significantly better than in the operated eye. Twelve patients (80%) had a history of prior failed PKs of the operated eye (mean 2.2; range 1–7). Three patients (20% one with OCP and two with bilateral serious chemical burn) had no previous donor PK in the operated eye, but underwent multiple failed PKs in the other eye. All cases showed 3–4 quadrants of deep vessels (Figures 1 and 2). Four eyes (27%) were phakic, five eyes (33%) were aphakic, and six eyes (40%) were pseudophakic (posterior chamber intraocular lenses (PC IOL) in five cases, and Morcher PC IOL in one case). Ten eyes (67%) had secondary glaucoma that had been previously managed by trabeculectomies with or without antimetabolites or cryocoagulation. One patient (7%) had bilateral pseudoexfoliative glaucoma. Intraocular pressure (IOP) was satisfactory controlled before AlphaCor surgery in all eyes. One patient with OCP was indicated for repeated electrolysis of trichiasis, and fornix and lid reconstruction surgery before AlphaCor implantation. All patients were educated about the importance of regular lid hygiene and received lubricating agents to improve the tear film.
Table 1. Patient data.
| Patient number | Age (in years) | Gender | Eye with AlphaCor |
Previous grafts |
Follow-up (months) | Survival (months) | Preoperative diagnoses | KPro type | Postoperative complications | Postoperative procedures | Reason for explantation | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OD | OS | |||||||||||
| 1 | 53 | M | OS | 3 | 7 | 67 | 47 | Failed graft, chemical burn | AlphaCor-P | Optic deposition, stromal melting | Device explantation, PK | Stromal melting, partial extrusion |
| 2 | 33 | M | OD | 1 | 0 | 54 | 20 | Failed graft, penetrating injury | AlphaCor-P | Secondary glaucoma, optic deposition, stromal melting | Cyclocryocoagulation, device explantation, PK | Stromal melting |
| 3 | 53 | M | OD | 3 | 0 | 54 | 54 | Failed graft, penetrating injury | AlphaCor-A | None | None | 0 |
| 4 | 46 | M | OD | 1 | 0 | 53 | 34 | Failed graft, chemical burn | AlphaCor-A | Optic deposition, stromal melting | Phototherapeutic keratectomy × 4, device explantation, PK | Stromal melting, partial extrusion |
| 5 | 51 | M | OS | 0 | 3 | 43 | 12 | Failed graft, chemical burn | AlphaCor-P | Retroprosthetic membrane formation, stromal melting | Amniotic membrane, scleral patch, device explantation, PK | Stromal melting, partial extrusion |
| 6 | 60 | M | OS | 5 | 0 | 43 | 15 | Chemical burn, leucoma corneae | AlphaCor-P | Trauma | Damaged device explantation, PK | Postoperative trauma |
| 7 | 39 | M | OS | 5 | 3 | 42 | 42 | Failed graft, chemical burn | AlphaCor-A | Retroprosthetic membrane formation, stromal melting | Conjunctival flap | 0 |
| 8 | 75 | M | OS | 4 | 0 | 41 | 41 | Ocular cicatricial pemphigoid | AlphaCor-P | None | Cataract surgery | 0 |
| 9 | 72 | M | OS | 1 | 0 | 37 | 5 | Chemical burn, leucoma corneae | AlphaCor-P | Trauma | Damaged device and IOL explantation, PK | Postoperative trauma |
| 10 | 73 | M | OS | 0 | 1 | 31 | 19 | Failed graft, postoperative keratopathy | AlphaCor-A | Stromal melting | Scleral patch, device explantation, PK | Stromal melting, partial extrusion |
| 11 | 81 | F | OD | 1 | 0 | 31 | 31 | Failed graft, postoperative keratopathy | AlphaCor-A | None | None | 0 |
| 12 | 42 | M | OD | 3 | 0 | 24 | 24 | Failed graft, chemical burn | AlphaCor-A | Stromal melting | Conjunctival flap | 0 |
| 13 | 72 | M | OS | 0 | 2 | 19 | 19 | Failed graft, corneal dystrophy | AlphaCor-P | Stromal melting | Amniotic membrane, conjunctival flap | 0 |
| 14 | 34 | M | OD | 3 | 0 | 19 | 0.24 | Failed graft, HZV keratitis | AlphaCor-P | Trauma and AlphaCor loss | Suture of the wound (anterior lamela) | Postoperative trauma |
| 15 | 68 | M | OS | 2 | 1 | 12 | 12 | Failed graft, corneal dystrophy | AlphaCor-P | Retroprosthetic membrane formation, stromal melting | Conjunctival flap, surgical discision of RM | 0 |
Abbreviations: AlphaCor-A, AlphaCor aphakic; AlphaCor-P, AlphaCor pseudophakic; BCVA, best-corrected visual acuity; F, female; HM, hand movement; IOL, intraocular lens; J, Jaeger chart; KPro, keratoprosthesis; M, male; OD, right eye; OS, left eye; PK, penetrating keratoplasty; RM, retroprosthetic membrane.
Table 2. Patient data.
| Patient number | Preoperative BCVA |
Postoperative BCVA/comments |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 months after I. st. | 12 months after I. st. | 18 months after I. st. | 24 months after I. st. | 30 months after I. st. | 36 months after I. st. | 42 months after I. st. | 48 months after I. st. | 54 months after I. st. | ||
| 1 | HM | 0.4; near J.1 | 0.4; near J.1 | 0.4; near J.1 | 0.4 | 0.15 | 0.3 | 0.15 | ||
| OpD | OpD, SM, exp | |||||||||
| 2 | HM | 0.01 | 0.03 | HM | ||||||
| RM, SM, exp. | ||||||||||
| 3 | HM | HM | HM | HM | HM | HM | HM | HM | HM | HM |
| TR | ||||||||||
| 4 | HM | 0.8; near J.1 | 0.8; near J.1 | 0.8; near J.1 | 0.125 | 0.02 | ||||
| OpD | OpD | OpD, SM, exp | ||||||||
| 5 | HM | HM | HM | |||||||
| RM, SM, exp | ||||||||||
| 6 | HM | 0.15; near J.1 | 0.1 | 0.1 | ||||||
| Glaucoma | Trauma, exp | |||||||||
| 7 | HM | HM | HM | HM | HM | HM | HM | HM | ||
| RM, SM | ||||||||||
| 8 | HM | 0.2; near J.3 | 0.1 | 0.1 | 0.3; near J.3 | 0.2; near J.3 | 0.3 | 0.1 | ||
| Cataract | Phaco+PC IOL | OpD | ||||||||
| 9 | HM | 0.25; near J.4 | ||||||||
| Exp 5 months after I.stage—trauma | ||||||||||
| 10 | HM | HM | HM | HM | ||||||
| Retinal pathology | SM, exp | |||||||||
| 11 | HM | 0.01 | 0.01 | HM | HM | HM | ||||
| Glaucoma | ||||||||||
| 12 | HM | 0.5; near J.2 | HM | HM | HM | |||||
| SM | ||||||||||
| 13 | HM | HM | HM | HM | ||||||
| Glaucoma | ||||||||||
| 14 | HM | 0.01 | ||||||||
| 1 week after I.stage—trauma | ||||||||||
| 15 | HM | 0.01 | 0.005 | |||||||
| RM | SM | |||||||||
Abbreviations: BCVA, best-corrected visual acuity; exp, explantation of the device; HM, hand movement; J., Jaeger chart; OpD, optic deposits; PC IOL, posterior chamber intraocular lens; RM, retroprosthetic membrane; SM, stromal melting; st., stage; TR, traumatic retinopathy.
Figure 1.
Patient 4 before stage I AlphaCor implantation.
Figure 2.
Patient 12 before stage I AlphaCor implantation.
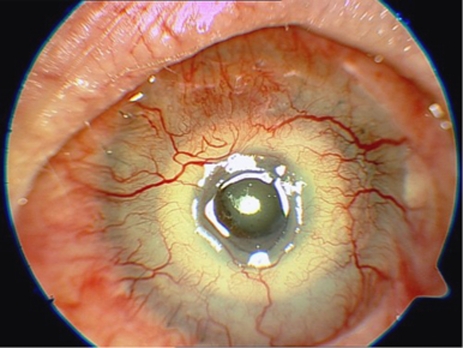
There are two stages to device implantation, separated by at least 3 months. In the first, a corneal lamellar pocket is created with a central opening in the posterior lamella, the device is positioned with its optic centered over the trephination, and the access wound is closed. In the stage II surgery, tissue anterior to the optic is removed to expose the device as a full-thickness corneal replacement centrally, whereas its skirt remains integrated within the stromal pocket. All surgeries were performed by two surgeons (NJ, PR) at the Department of Ophthalmology, University Hospital, Hradec Králové, except for the first stage of the first AlphaCor implantation that was provided for legislative reasons by Professor Bleckmann at Schlosspark-Klinik, Berlin, Germany. Stage I surgery was performed in all cases under general anesthesia using a traditional 180° entry wound in 12 cases (80%), and central trephination—‘within graft' technique in three cases (20%). Cataract surgery was performed concurrently with AlphaCor implantation in two cases (13%). Circular continuous capsulorhexis, extracapsular cataract extraction, bimanual irrigation/aspiration were performed through a central opening in the posterior lamella. In one eye, a PC IOL was implanted and in one case the eye was left aphakic and model AlphaCor-A was used. Stage II surgery was performed in nine cases, (60%) at 3–11 months (mean 4.9 months), after the stage I surgery under topical anesthesia. It was abandoned in five eyes (33%) because of thinning of the anterior lamella, and in one case (7%) because of the trauma and AlphaCor loss at 8 days after stage I surgery. There were no serious perioperative complications. Of the four cases that were phakic after AlphaCor implantation, one showed subsequent progression of lenticular opacity (Figure 3) and uneventful phacoemulsification with PC IOL implantation was performed at 18 months after stage II surgery (Figure 4).
Figure 3.
Patient 8 before cataract surgery at 18 months after stage II AlphaCor implantation.
Figure 4.
Patient 8 after cataract surgery at 18 months after stage II AlphaCor implantation.
Topical tobramycin (0.3%) or chloramphenicol (0.5%) and dexamethasone (0.1%) gtt (Tobradex gtt or Spersadex gtt) were administered five times a day as routine postoperative long-term medications. Lubrication drops (Tears Naturale II (ALCON-COUVREUR n.v., Puurs, Belgium), Vidisic gel) were applied in six cases. It was not possible to use topical medroxyprogesterone (1%) in our patients for legislative reasons. Disposable soft contact lenses (D55, WILENS or ACUVUE, Johnsons & Johnsons, Jacksonville, FL, USA) were used in 11 cases for postoperative refractive error correction and/or surface protection of the device. Oral doxycycline (100 mg twice a day) was administered in 15 patients. Intraocular pressure was estimated digitally, or using Tono-Pen XL (MEDTRONIC SOLAN, Jacksonville, FL, USA).
All patients were strongly encouraged to stop smoking and/or avoid exposure to cigarette or environmental smoke. They were also educated about the necessity of long-term treatment and protection of the operated eye. Follow-up period ranged from 12 to 67 months.
Results
Outcome data for every patient at every visit were collected for analysis, with no case lost to follow-up. Postoperative best-corrected VA (BCVA) ranged from hand movement to 0.8. The visual acuities post-implantation varied between visits depending upon cataract progression or optic deposits formation in some patients, and refractive correction with acuities up to 0.8 for distance and Jaeger no.1 for near vision being recorded (Table 2). Concurrent pathologies diagnosed before AlphaCor implantation that limited visual potential were present in 11 cases (73%), predominantly glaucoma causing defects in visual field in 10 eyes (67%), and traumatic retinopathy severely damaging vision in one patient (7%).
Complications can be categorized as anatomical complications related to stromal melting, optic complications related to deposition or surface spoliation, and device-unrelated complications, as summarized in Table 1.
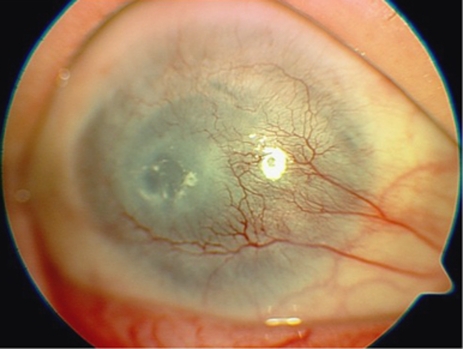
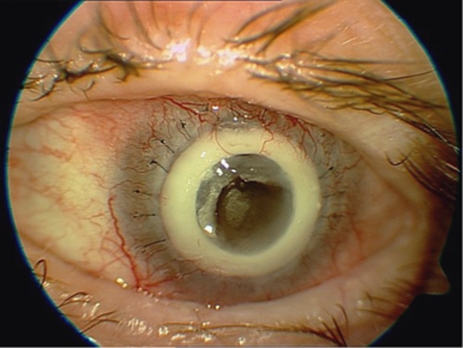
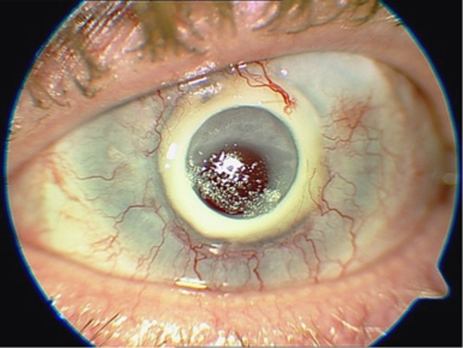
The commonest category of complication was that related to stromal melting adjacent or anterior to the device skirt. It is defined as any episode of stromal thinning or loss, whether or not the process subsequently stabilized. This was observed in nine cases (60% patients 1, 2, 4, 5, 7, 10, 12, 13, 15) (Figures 5 and 6). Four of these (27% patients 7, 12, 13, 15) have required conjunctival flap, two (13% patients 5, 13) amniotic membrane transplantations, and in two eyes (13% patients 5, 10) a scleral patch was used. Stromal melting culminated in device explantation and replacement with donor graft in five eyes (33% patients 1, 2, 4, 5, 10). No predisposing or risk factors for this type of complication was found.
Figure 5.
Stromal melting in patient 5.
Figure 6.
Stromal melting in patient 12.
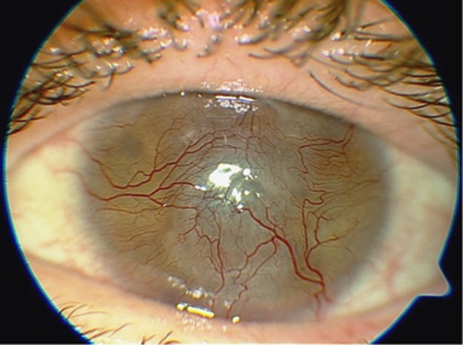
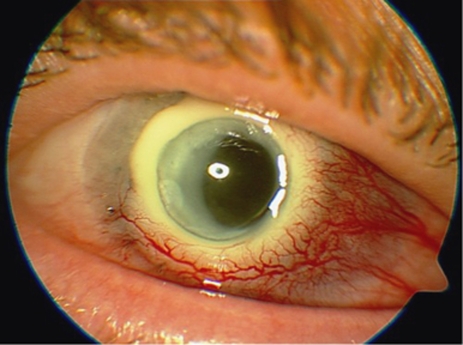
The other main category of complications relates to surface spoilation or deposition of substances within the hydrogel optic such that vision is reduced. There have been three cases (20% patients 1, 2, 4) of mild to moderate surface spoilation with the appearance of a contact lens ‘jelly-bump' type (Figure 7). These deposits require regular cleaning using OPTI-FREE SUPRACLENS (Alcon Laboratories, Inc., Fort Worth, TX, USA); in one case (7% patient 4) excimer laser abrasion was performed repeatedly. In one case (patient 1), deposits were observed on the optical surface of the device, at 2 years after implantation, progressing to an almost continuous crust on half of the optic periphery, with intervening clear areas, and severely affecting vision. At 4 years after implantation, stromal melting with leakage was diagnosed and explantation was performed with a new corneal graft.
Figure 7.
Optic deposits in patient 1.
Retroprosthetic membrane (fibrous closure of posterior lamellar opening) was observed in three eyes (patients 5, 7, 15), and was managed surgically in one eye (patient 15). No improvement of vision after removal of the central part of the retroprosthetic membrane was observed, because the patient developed hemophthalmus.
In one eye (patient 2), acute elevation of IOP was observed at 6 days after stage I surgery that did not respond to pharmacological treatment. Uneventful cyclocryocoagulation was performed, and the IOP was satisfactory controlled without medication. No other case of elevation IOP was observed in this series.
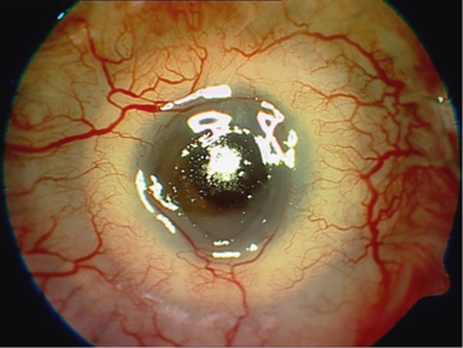
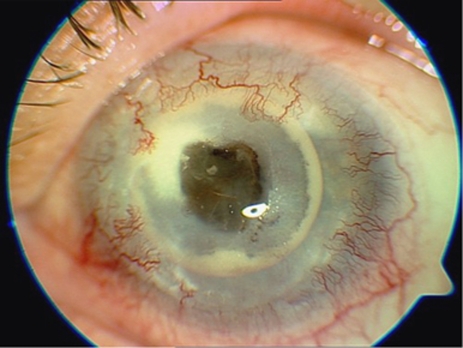
The commonest device-unrelated complication in our series was trauma. Two patients that were bilaterally blind before AlphaCor implantation (patients 6, 9) and have reached apparently very good postoperative visual results experienced serious injury with penetration of the device (Figure 8). In both cases, PK was performed and the damaged keratoprostheses were replaced with donor graft. One (patient 14) experienced penetrating injury in the surgical wound at 8 days after stage I surgery with AlphaCor loss. This case was managed by suturing of the wound (anterior lamella). All complications were managed without loss of the eye.
Figure 8.
Penetrating injury in patient 9.
No other complications such as endophthalmitis, glaucoma progression, retinal detachment, and inflammation were observed. The survival rate of the device at 1, 2, and 3 years was 87%, 58%, and 42%, respectively. It is necessary to emphasize that those two devices that were removed earlier than 12 months after implantation were explanted due to trauma (KPro-unrelated complication).
Discussion
In eyes with severely diseased corneas in which standard corneal transplantation has poor prognosis, an artificial cornea can provide good VA when successful. Keratoprosthesis surgery is carried out in very few centers, and devices design, surgical technique, and adjunctive therapies are evolving with experience.
AlphaCor artificial cornea was designed to address the need for an alternative to donor tissue, and to avoid the classic trial of sight-threatening KPro complications—progressive glaucoma, endophthalmitis, and retinal detachment.28, 29 It was intended to avoid reliance on donor tissue for its implantation while providing for reversibility to PK in the event of complications to minimize long-term risk to the eye.2 AlphaCor design features include its flexibility and form (analogous to a small donor corneal graft) that allows a relatively non-invasive implantation procedure. The design of the optic, when associated with an opening in the covering tissues after completion of stage II surgery, produces an acceptable visual field and allows intraocular examination. The IPN between the core and skirt creates a permanent and very strong junction, preventing aqueous leakage.3
The appropriate selection of patients for AlphaCor implantation is crucial for success. There should be severe, debilitating corneal disease-causing blindness, with a poor chance of success from primary or repeated donor PK. The majority of patients undergoing AlphaCor Kpro implantation at our department had multiple prior failed grafts. Only three high-risk cases (one patient with OCP and two with bilateral serious alkali burn) received the device without prior transplant, but all three had history of multiple failed-donor PK in the other eye. AlphaCor performs best in a reasonably normal ocular environment. This includes eyelid health, a good tear film, and an absence of active inflammation. Generally, three broad classes of potential AlphaCor recipient could be identified: (1) those with poor prognosis from donor PK, but with good prognosis for AlphaCor implantation both for anatomical and functional outcomes; (2) those with a poor prognosis from donor PK and also a relatively poor or uncertain prognosis in terms of final vision with artificial cornea due to previous glaucomatous damage or macular disease, but with a good prognosis for an anatomically satisfactory outcome without significant complications; (3) those with a greater risk of significant complications affecting not only final vision but also reducing the chance of successful long-term device retention. However, those categories are not strictly divided and as it was mentioned previously, the indications have been evolving with experience. History of HSV infection was previously considered as an exclusion factor for AlphaCor surgery, but new data proved that HSV is not a risk factor for melts.2 Ocular cicatrical pemphigoid is considered as a relative contraindication for AlphaCor implantation (category 3), but in our series, the patient with OCP has reached very good long-term outcomes with optimization of patient's condition before AlphaCor surgery (repeated electrolysis of trichiasis, and fornix, and lid reconstruction surgery).
The lamellar surgical procedure has been refined such that stage I surgery takes less than 1 h and does not require adjunctive tissue or devices. In our series there was no need to perform a full Gunderson flap or to use buccal mucosa, and no serious perioperative complications occurred during the stage I surgery. Cataract surgery was performed concurrently with AlphaCor implantation in two cases also without any complications. An earlier study by Hicks et al2 reported higher occurrence of perioperative complications (19.9%). An uneventful phacoemulsification with PC IOL implantation was performed in one eye with cataract progression with very good postoperative outcomes (Figures 3 and 4).
In our series, three patients (20%) experienced penetrating injury after AlphaCor implantation, despite they were strongly educated about the necessity of long-term protection of the operated eye. This high rate is rather alarming. We believe that there are patients with ‘higher inclination' to trauma, and not even previous disaster injury can teach them to protect themselves.
AlphaCor does not seem to exacerbate elevation of IOP. Glaucoma has been reported a common complication of rigid KPro,28 commonly requiring drainage devices and frequently resulting in loss of vision. AlphaCor retention is not affected by glaucoma or the presence of drainage devices, and AlphaCor implantation does not seem to worsen glaucoma control.2 In this series, only one case of acute elevation of IOP was observed at 6 days after stage I, and was satisfactorily treated using cyclocryocoagulation.
The most common postoperative complication was stromal melting adjacent or anterior to the device skirt. This was observed in nine cases (60%), and culminated in device explantation and replacement with donor graft in five eyes (33%). An important aspect of the response of the host tissue after KPro implantation is the production of enzymes. If this response is extensive, an increased risk of tissue melting and KPro loosening or extrusion would be expected. Coassin et al30 have noted strong expression of various inflammatory cytokines, and only a few inflammatory cells in the three AlphaCor devices explanted because of corneal melting during the late postoperative period. They proposed that these cytokines were expressed by the keratocytes themselves because it is well known that the injured epithelial cells can stimulate fibroblast myodifferentiation of the keratocytes through inflammatory cytokines. On the basis of these observations, they speculated that an epithelial defect overlying the skirt portion of a well-biointegrated device can initiate the cascade of events and can lead to a corneal melt and device extrusion. Not only proper biointegration of the artificial material in the corneal stroma but also achievement of complete and stable epithelization of the entire device is necessary for long-term retention of the AlphaCor KPro in situ.
Alternatives to AlphaCor implantation include other KPro, repeated donor PK, and more recent forms of ocular surface transplantation. Other KPro in use today include the Boston KPro, Osteo-Odonto-Keratoprosthesis (OOKP), KeraKlear KPro, and Pintucci Kpro—the last, however, being rarely used today. Indications for OOKP and Pintucci implantation are similar: namely, severe end-stage ocular surface disorders. The Boston and KeraKlear KPros (like the AlphaCor) are considered a treatment option for repeated graft failure, herpetic keratitis, ocular burns (acid and alkali), OCP, Stevens–Johnson syndrome, and other autoimmune diseases. Although direct comparison of all these devices is inappropriate because of variations in disease indications and severity, several articles reporting results with these KPros have been published.28, 29, 31, 32, 33, 34, 35, 36 In assessing these devices, visual outcomes and anatomic success in terms of KPro retention are especially important. Results with OOKP are promising, with good device retention, good visual performance, and relative paucity of device-related complications,35, 36 but it is relatively complicated 2(3)-stage procedure insisting return visits to operating room with delayed visual recovery. The Boston KPro is a one-stage procedure but usually requires a new corneal carrier graft for assembly of the polymethacrylate device. Interest in the Boston KPro has grown significantly and is currently the most commonly implanted KPro in the United States.37 A promising large multicenter Boston Type 1 study showed that at mean follow-up of 8.5 (0.03–24) months the retention rate was 95%.31 Eighty-three percent of cases were performed for graft rejection, chemical burn, or aphakic/pseudophakic bulous keratopathy, indications that differ from OOKP or Pintucci KPros series. Of these patients, 22.6% achieved vision of 20/40 or better. Bacterial endophthalmitis was not reported in this series, however it was reported in other studies.33 The KeraKlear KPro is a new foldable and injectable single-piece artificial cornea with no back plate or locking ring designed to create a clear window in an opacified cornea.37 The steps of the procedure are outlined for both full-thickness and lamellar technologies, but according to our experience with this KPro, the lamellar technique (non-penetrating) is the only one full-proof and safe method.
In conclusion, in our series, patients typically had complex ocular histories with multiple pathologies. Their corneal blindness was not treatable successfully by means of standard dPK implantation, and implantation of an artificial cornea was the only chance for them to improve vision. Although BCVA improved in the majority of patients after implantation, the incidence of complications was relatively high, especially in long-term follow-up (2 and more years after implantation). The most serious complications were stromal melts and optic deposition or surface spoilation. In our series, we have also observed three cases of trauma (device-unrelated complication). Each case has required individual assessment and management. Patient's compliance is of high importance in evaluating any prospective case for AlphaCor implantation. As with all Kpros, ongoing vigilance in follow-up is essential, and care of these patients is challenging and time consuming.
Acknowledgments
Supported in part by research project MZO 00179906 from the Ministry of Health, Prague, Czech Republic.
The authors declare no conflict of interest.
References
- Hicks CR, Crawford GJ. Indications and technique: AlphaCor artificial cornea. Tech Ophthalmol. 2003;1:151–155. [Google Scholar]
- Hicks CR, Crawford GJ, Dart JK, Grabner G, Holland EJ, Stulting RD, et al. AlphaCor clinical outcomes. Cornea. 2006;25:1034–1042. doi: 10.1097/01.ico.0000229982.23334.6b. [DOI] [PubMed] [Google Scholar]
- Chirila TV, Vijayasekaran S, Horne R, Chen YC, Dalton PD, Constable IJ, et al. Interpenetrating polymer network (IPN) as a permanent joint between the elements of a new type of artificial cornea. J Biomed Mater Res. 1994;28:745–753. doi: 10.1002/jbm.820280612. [DOI] [PubMed] [Google Scholar]
- Hicks CR, Crawford GJ, Tan DT, Snibson GR, Sutton GL, Downie N, et al. AlphaCor cases: comparative outcomes. Cornea. 2003;22:583–590. doi: 10.1097/00003226-200310000-00001. [DOI] [PubMed] [Google Scholar]
- Chirila TV, Thompson-Wallis DE, Crawford GJ, Constable IJ, Vijayasekaran S. Production of neocollagen by cells invading hydrogel sponges implanted in the rabbit cornea. Graefes Arch Clin Exp Ophthalmol. 1996;234:193–198. doi: 10.1007/BF00462032. [DOI] [PubMed] [Google Scholar]
- Crawford GJ, Constable IJ, Chirila TV, Vijayasekaran S, Thompson DE. Tissue interaction with hydrogel sponges implanted in the rabbit cornea. Cornea. 1993;12:348–357. doi: 10.1097/00003226-199307000-00012. [DOI] [PubMed] [Google Scholar]
- Crawford GJ, Chirila TV, Vijayasekaran S, Dalton PD, Constable IJ. Preliminary evaluation of a hydrogel core-and-skirt keratoprosthesis in the rabbit cornea. J Refract Surg. 1996;12:525–529. doi: 10.3928/1081-597X-19960501-19. [DOI] [PubMed] [Google Scholar]
- Fitton JH, Ziegelaar BW, Hicks CR, Clayton AB, Crawford GJ, Constable IJ, et al. Assessment of anticollagenase treatments after insertion of a keratoprosthetic material in the rabbit cornea. Cornea. 1998;17:108–114. doi: 10.1097/00003226-199801000-00016. [DOI] [PubMed] [Google Scholar]
- Hicks CR, Chirila TV, Clayton AB, Fitton JH, Vijayasekaran S, Dalton PD, et al. Clinical results of implantation of the Chirila keratoprosthesis in rabbits. Br J Ophthalmol. 1998;82:18–25. doi: 10.1136/bjo.82.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks CR, Chirila TV, Dalton PD, Clayton AB, Vijayasekaran S, Crawford GJ, et al. Keratoprosthesis: preliminary results of an artificial corneal button as a full-thickness implant in the rabbit model. Aust NZ J Ophthalmol. 1996;24:297–303. doi: 10.1111/j.1442-9071.1996.tb01597.x. [DOI] [PubMed] [Google Scholar]
- Hicks CR, Fitton JH, Chirila TV, Crawford GJ, Constable IJ. Keratoprostheses: advancing toward a true artificial cornea. Surv Ophthalmol. 1997;42:175–189. doi: 10.1016/s0039-6257(97)00024-6. [DOI] [PubMed] [Google Scholar]
- Hicks CR, Lou X, Platten S, Clayton AB, Vijayasekaran S, Fitton HJ, et al. Keratoprosthesis results in animals: an update. Aust NZ J Ophthalmol. 1997;25 (Suppl 1:S50–S52. doi: 10.1111/j.1442-9071.1997.tb01756.x. [DOI] [PubMed] [Google Scholar]
- Hicks CR, Vijayasekaran S, Chirila TV, Platten ST, Crawford GJ, Constable IJ. Implantation of PHEMA keratoprostheses after alkali burns in rabbit eyes. Cornea. 1998;17:301–308. [PubMed] [Google Scholar]
- Vijayasekaran S, Fitton JH, Hicks CR, Chirila TV, Crawford GJ, Constable IJ. Cell viability and inflammatory response in hydrogel sponges implanted in the rabbit cornea. Biomaterials. 1998;19:2255–2267. doi: 10.1016/s0142-9612(98)00128-8. [DOI] [PubMed] [Google Scholar]
- Vijayasekaran S, Hicks CR, Chirila TV, Fitton JH, Clayton AB, Lou X, et al. Histologic evaluation during healing of hydrogel core-and-skirt keratoprostheses in the rabbit eye. Cornea. 1997;16:352–359. [PubMed] [Google Scholar]
- Ziegelaar B, Fitton JH, Clayton AB, Platten ST, Steer J, Chirila TV. The modulation of cellular responses to poly(2-hydroxyethyl methacrylate) hydrogel surfaces: phosphorylation decreases macrophage collagenase production in vitro. J Biomater Sci Polym Ed. 1998;9:849–862. doi: 10.1163/156856298x00190. [DOI] [PubMed] [Google Scholar]
- Lou X, Vijayasekaran S, Chirila TV, Maley MA, Hicks CR, Constable IJ. Synthesis, physical characterization, and biological performance of sequential homointerpenetrating polymer network sponges based on poly(2-hydroxyethyl methacrylate) J Biomed Mater Res. 1999;47:404–411. doi: 10.1002/(sici)1097-4636(19991205)47:3<404::aid-jbm16>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Hicks C, Crawford G, Chirila T, Wiffen S, Vijayasekaran S, Lou X, et al. Development and clinical assessment of an artificial cornea. Prog Ret Eye Res. 2000;19:149–170. doi: 10.1016/s1350-9462(99)00013-0. [DOI] [PubMed] [Google Scholar]
- Hicks CR, Werner L, Vijayasekaran S, Mamalis N, Apple DJ. Histology of AlphaCor skirts: evaluation of biointegration. Cornea. 2005;24:933–940. doi: 10.1097/01.ico.0000160969.50706.7f. [DOI] [PubMed] [Google Scholar]
- Hicks CR, MaeVie O, Crawford GJ, Constable IJ. A risk score as part of an evidence-based approach to the selection of corneal replacement surgery. Cornea. 2005;24:523–530. doi: 10.1097/01.ico.0000153103.27399.e6. [DOI] [PubMed] [Google Scholar]
- Hicks CR, Crawford GJ. Melting after keratoprosthesis implantation: the effects of medroxyprogesterone. Cornea. 2003;22:497–500. doi: 10.1097/00003226-200308000-00001. [DOI] [PubMed] [Google Scholar]
- Hicks CR, Crawford GJ, Tan DT, Snibson GR, Sutton GL, Gondhowiardjo TD, et al. Outcomes of implantation of an artificial cornea. AlphaCor: effects of prior ocular herpes simplex infection. Cornea. 2002;21:685–690. doi: 10.1097/00003226-200210000-00010. [DOI] [PubMed] [Google Scholar]
- Hicks CR, Chirila TV, Werner L, Crawford GJ, Apple DJ, Constable IJ. Deposits in artificial corneas: risk factors and prevention. Clin Experiment Ophthalmol. 2004;32:185–191. doi: 10.1111/j.1442-9071.2004.00781.x. [DOI] [PubMed] [Google Scholar]
- Chirila TV, Morrison DA, Hicks CR, Gridneva Z, Barry CJ, Vijayasekaran S. In vitro drug-induced spoliation of a keratoprosthetic hydrogel. Cornea. 2004;23:620–629. doi: 10.1097/01.ico.0000121703.74077.ea. [DOI] [PubMed] [Google Scholar]
- Hicks CR, Hamilton S. Retroprosthetic membranes in AlphaCor patients: risk factors and prevention. Cornea. 2005;24:692–698. doi: 10.1097/01.ico.0000154380.13237.ea. [DOI] [PubMed] [Google Scholar]
- Crawford GJ, Eguchi H, Hicks CR. Two cases of AlphaCor surgery performed using a small incision technique. Clin Experiment Ophthalmol. 2005;33:10–15. doi: 10.1111/j.1442-9071.2005.00937.x. [DOI] [PubMed] [Google Scholar]
- Eguchi H, Hicks CR, Crawford GJ, Tan DT, Sutton GR. Cataract surgery with AlphaCor. J Cataract Refract Surg. 2004;30:1486–1491. doi: 10.1016/j.jcrs.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Netland PA, Terada H, Dohlman CH. Glaucoma associated with keratoprosthesis. Ophthalmology. 1998;105:751–757. doi: 10.1016/S0161-6420(98)94034-9. [DOI] [PubMed] [Google Scholar]
- Nouri M, Terada H, Alfonso EH, Foster CS, Durand ML, Dohlman CH. Endophthalmitis after keratoprosthesis: incidence, bacterial causes, and risk factors. Arch Ophthalmol. 2001;119:484–489. doi: 10.1001/archopht.119.4.484. [DOI] [PubMed] [Google Scholar]
- Coassin M, Zhang C, Gree WR, Aquavella JV, Akpek EK. Histopathologic and immunologic aspects of AlphaCor artificial corneal failure. Am J Ophthalmol. 2007;144:699–704. doi: 10.1016/j.ajo.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Zerbe BR, Belin MW, Ciolino JB. Boston Type 1 Keratoprosthesis Study Group. Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology. 2006;113:1779–1784. doi: 10.1016/j.ophtha.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Bradle JC, Hernandez EG, Schwab IR, Mannis MJ. Boston type 1 keratoprosthesis: The University of California Davis Experience. Cornea. 2009;28:321–327. doi: 10.1097/ICO.0b013e31818b8bfa. [DOI] [PubMed] [Google Scholar]
- Chew HF, Ayres DB, Hammersmith KM, Rapuano CJ, Laibson PR, Myers JS, et al. Boston keratoprostesis outcomes and complications. Cornea. 2009;28:989–996. doi: 10.1097/ICO.0b013e3181a186dc. [DOI] [PubMed] [Google Scholar]
- Liu C, Paul B, Tandon R, Lee E, Fong K, Mavrikakis I, et al. The Osteo-Odonto-Keratoprosthesis (OOKP) Semin Ophthalmol. 2005;20:113–128. doi: 10.1080/08820530590931386. [DOI] [PubMed] [Google Scholar]
- Falcinelli G, Falsini B, Taloni M, Colliardo P, Falcinelli G. Modified osteo-odonto-keratoprosthesis for treatment of corneal blindness: long term anatomical and functional outcomes in 181 cases. Arch Ophthalmol. 2005;123:1319–51329. doi: 10.1001/archopht.123.10.1319. [DOI] [PubMed] [Google Scholar]
- Tan DTH, Tay ABG, Theng JTS, Lye KW, Parthasarathy A, Por YM, et al. Keratoprosthesis surgery for end-stage corneal blindness in Asian eyes. Ophthalmology. 2008;3:503–510. doi: 10.1016/j.ophtha.2007.04.065. [DOI] [PubMed] [Google Scholar]
- Pineda R, Shiuey Y. The keraklear artificial cornea, a novel keratoptosthesis. Tech Ophthalmlol. 2009;7:101–104. [Google Scholar]