Abstract
Background and Purpose
Atrial fibrillation is the most important risk factor for cardioembolic stroke. Thrombi form in the left atrial appendage rather than in the right. The causes of this different thrombogenicity are not well understood. The goal herein was to compare the activation of the anticoagulant protein C as well as the thrombomodulin and endothelial protein C/activated protein C receptor (EPCR) expression on the endocardium between right and left atria.
Methods
We harvested the atria of six monkeys (Macaca fascicularis) and quantified their ability to activate protein C ex vivo and we measured the thrombomodulin and EPCR expression by immunofluorescence.
Results
We found the ability to activate protein C decreased by half (P= 0.028), and there was lower expression of thrombomodulin in the left atrial endocardium than the right (52.5±19.9 and 72.1±18.8 arbitrary intensity units, mean ± standard deviation, P= 0.028). No differences were detected in EPCR expression.
Conclusions
Impaired protein C activation on the left atrial endocardium, due to low thrombomodulin expression may explain its higher thrombogenicity and play a role in cardioembolic stroke.
Keywords: Stroke, embolism, protein C anticoagulant system, thrombomodulin, endothelium
Atrial fibrillation (AF) is the most commonly sustained cardiac arrhythmia and the most important risk factor for cardioembolic stroke.1 The left atrium, particularly the left atrial appendage (LAA), is the most frequent location of the embolic thrombi even when the right atrium undergoes the same pathological process.2 The blood stasis at the LAA and the right atrial appendage (RAA) is similar3 and so this cannot explain the higher propensity of thrombi to be formed in the LAA. In the search for additional factors to explain the difference between the two atria in thrombogenicity, study of their endothelial phenotypes deserves consideration. Attention has recently focused on the functional consequences of endothelial heterogeneity among different vascular beds.4 Endothelium has been classically considered a thromboresistent surface due to the presence of receptors involved in several antithrombotic pathways. Among these, thrombomodulin and the endothelial protein C/activated protein C receptor (EPCR) play a crucial role in the protein C anticoagulant pathway.5 EPCR expression is high in large arteries and veins, but is virtually absent in capillaries.6 Thrombomodulin expression is particularly high in the pulmonary endothelium and low in the brain endothelium.7 Differences have also been reported within one vessel: in veins, both EPCR and thrombomodulin are expressed more in the valvular sinus endothelium as opposed to the vein lumenal endothelium.8 The association between protein C deficiency and thrombosis is well documented.9 For this reason we propose the hypothesis that the left atrium has a lower ability to activate protein C than the right one.
Methods
Collection of simian atria
The appendages of the left and right atria of each animal (Macaca fascicularis) were harvested. Details of the study are provided in Supplemental Materials.
Protein C activation assay on the atrial surfaces
The experiments were performed in the two hours following surgical removal of the atria. Two tissue slices 6 mm in diameter were cut from each atrial appendage using a circular biopsy punch (Stiefel, Spain) and placed separately at the bottom of a 48 well plate (Corning, NY, USA). The experiment was performed as described10 with minor modifications, which are described in Supplemental Materials. Activation was thrombomodulin and EPCR dependent (Supplemental Figure S1).
Immunofluorescence analysis of the atrial expression of thrombomodulin and EPCR
Images were analyzed as described in Supplemental Materials.
Statistical analysis
Data handling is described in Supplemental Materials.
Results and discussion
In six out of six cases, activation of protein C was higher on the RAA endocardium than on the LAA one. On average, generation of APC was twice as high on the right atrial surface (Figure 1A). Activated protein C (APC) not only plays a major anticoagulant role but it is also involved in a variety of cytoprotective mechanisms in response to inflammatory stimuli.11 It is well known that inflammation plays a role in the initiation and maintenance of AF as well as in thrombosis.11,12 Thus, the right atrium would be better prepared to prevent the formation of thrombi arising as a consequence of the AF. The fact that changes in APC circulating levels have been shown to influence the thrombotic risk reinforces this notion.13 Furthermore, low protein C levels have been recently associated with ischemic stroke, particularly the cardioembolic type, in the Atherosclerosis Risk in Communities Study.14
Figure 1. The protein C system in the endocardium of the RAA and LAA.
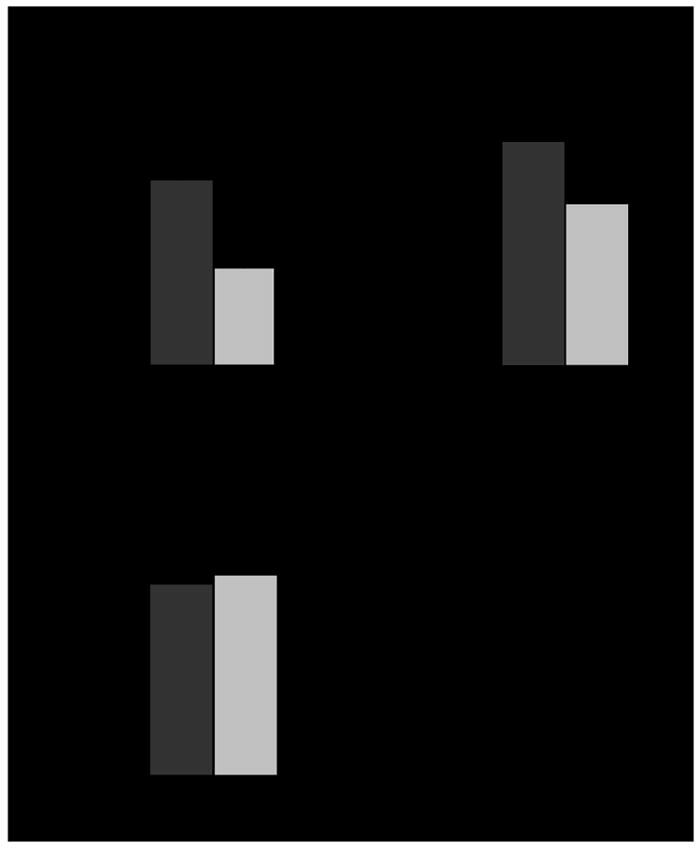
(A) Thrombin-dependent generation of APC was measured ex vivo on the endocardial surface of RAA and LAA samples of macaque origin (n = 6). The protein C activation assay was performed in duplicate. The mean ± SD of six independent experiments is represented. (B) Thrombomodulin and (C) EPCR expression on the endocardial surface of the RAA and LAA The mean ± SD of the tissue expression is represented in arbitrary intensity units (AIU). (D) The R2 Spearman’s correlation coefficient between the difference in the thrombomodulin expression (RAA–LAA) and the difference in the APC generation (RAA–LAA). Δ, RAA-LAA
In the search for an explanation for our results, we focused our attention on the two endothelial receptors involved in protein C activation. Thrombin must bind thrombomodulin for activation to proceed efficiently, but the cofactor role played by EPCR is also relevant.5 We therefore decided to study the expression of thrombomodulin and EPCR on the RAA and LAA endocardium of the same animals used for the protein C activation assays. As expected, thrombomodulin and EPCR were located on the cell surface as shown by confocal images (Figure 2A-B). Von Willebrand factor labeling served to ensure specific endothelial staining (Figure 2B). Again in six out of six animals thrombomodulin expression was higher in the RAA endocardium. The mean intensity values were 72.1±18.8 and 52.5±19.9 [mean ± standard deviation (SD)] for the RAA and LAA endocardium respectively (Figures 1B and 2A). EPCR expression did not differ between RAA and LAA (Figures 1C and 2A). The presence of thrombomodulin on the endothelial surface is crucial for APC generation. Therefore, these data dovetail with the finding that the RAA endocardium displays a higher capacity to activate protein C than its left counterpart. The strong positive correlation found between the RAA vs. LAA difference in APC generation capacity and the RAA vs. LAA difference in thrombomodulin expression (R2 = 0.764) further substantiates the notion that the higher expression of TM is responsible for the higher ability of RAA to generate APC (Figure 1D). The biological mechanisms that lead to the reduced thrombomodulin expression in the LAA are presently unknown, although they could involve differences in vascular beds expression, shedding, internalization or exposition to different local regulating factors.
Figure 2. Representative immunofluorescence and confocal images of the RAA and LAA expression of thrombomodulin and EPCR.
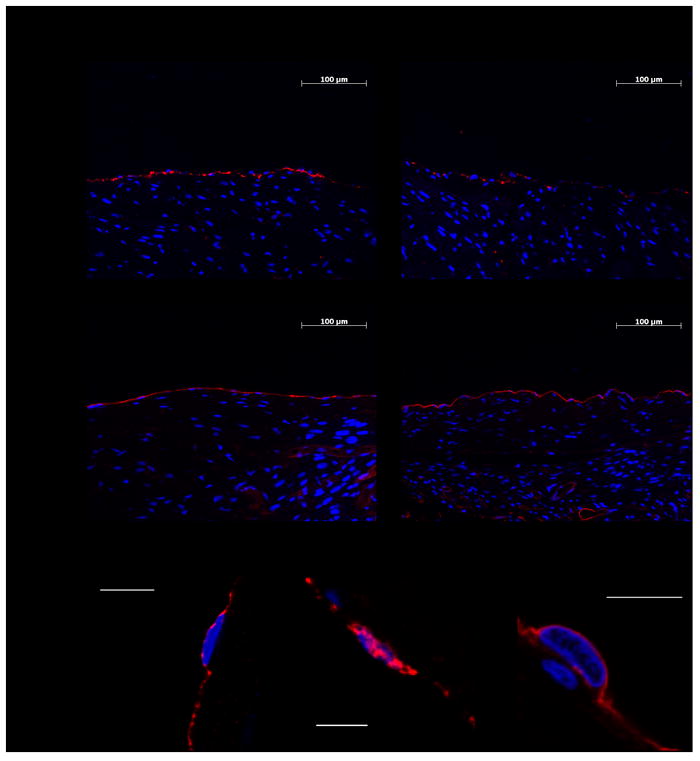
(A) Representative captures of thrombomodulin and EPCR on the endocardium of the RAA and LAA. Thrombomodulin and EPCR are red-stained. Blue DAPI was used to stain the nuclei. (B) Confocal microscopy captures of thrombomodulin (left), von Willebrand factor (middle) and EPCR (right), all red-colored, in representative endothelial cells. Von Willebrand factor staining was used as an endothelial positive control. Blue Topro-3 was used to stain the nuclei. The scale bars indicate 10 μm.
The main drawback of this study is that we analyzed non-human primate samples and, therefore application of these results to humans is not immediate. However, since the majority of the findings obtained in other studies dealing with the protein C system using non-human primates have been proven to apply to humans, we consider it reasonable to apply our results to the human setting.6,15
In conclusion, for the first time the ability of the left and right atrial endocardium to activate protein C has been compared. The right atrial endocardium exhibits a more thromboresistant phenotype, at least regarding this anticoagulant mechanism, which would help to explain why thrombi originate in the left atrium. Increasing thrombomodulin expression on the endocardium of the left atrium could provide a new approach to prevent cardioembolic stroke associated with AF.
Supplementary Material
Acknowledgments
We thank Carlos Ortiz de Solórzano and Miguel Galarraga for their excellent technical support with imaging analysis. We also thank José L. Lanciego and Gloria González for the simian tissue samples.
Sources of Funding This work was supported through the Unión Temporal de Empresas project CIMA and by grants from Instituto de Salud Carlos III (PI051178, PI081349 and Red Temática de Investigación RECAVA RD06/0014/0008 and RD06/0014/0004) and Education Department, Gobierno de Navarra (13.703). Jorge Cerveró was supported by a grant from the Sociedad Española de Trombosis y Hemostasia SETH/FETH.
Footnotes
Disclosures None.
References
- 1.Lip GY, Tse HF. Management of atrial fibrillation. Lancet. 2007;370:604–618. doi: 10.1016/S0140-6736(07)61300-2. [DOI] [PubMed] [Google Scholar]
- 2.Thambidorai SK, Murray RD, Parakh K, Shah TK, Black IW, Jasper SE, et al. ACUTE investigators. Utility of transesophageal echocardiography in identification of thrombogenic milieu in patients with atrial fibrillation (an ACUTE ancillary study) Am J Cardiol. 2005;96:935–941. doi: 10.1016/j.amjcard.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 3.de Divitiis M, Omran H, Rabahieh R, Rang B, Illien S, Schimpf R, et al. Right atrial appendage thrombosis in atrial fibrillation: its frequency and its clinical predictors. Am J Cardiol. 1999;84:1023–1028. doi: 10.1016/s0002-9149(99)00492-0. [DOI] [PubMed] [Google Scholar]
- 4.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 5.Esmon CT, Gu JM, Xu J, Qu D, Stearns-Kurosawa DJ, Kurosawa S. Regulation and functions of the protein C anticoagulant pathway. Haematologica. 1999;84:363–368. [PubMed] [Google Scholar]
- 6.Laszik Z, Mitro A, Taylor FB, Jr, Ferrell G, Esmon CT. Human protein C receptor is present primarily on endothelium of large blood vessels: implications for the control of the protein C pathway. Circulation. 1997;96:3633–3640. doi: 10.1161/01.cir.96.10.3633. [DOI] [PubMed] [Google Scholar]
- 7.Ishii H, Salem HH, Bell CE, Laposata EA, Majerus PW. Thrombomodulin, an endothelial anticoagulant protein, is absent from the human brain. Blood. 1986;67:362–365. [PubMed] [Google Scholar]
- 8.Brooks EG, Trotman W, Wadsworth MP, Taatjes DJ, Evans MF, Ittleman FP, et al. Valves of the deep venous system: an overlooked risk factor. Blood. 2009;114:1276–1279. doi: 10.1182/blood-2009-03-209981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koster T, Rosendaal FR, Briët E, van der Meer FJ, Colly LP, Trienekens PH, et al. Protein C deficiency in a controlled series of unselected outpatients: an infrequent but clear risk factor for venous thrombosis (Leiden Thrombophilia Study) Blood. 1995;85:2756–2761. [PubMed] [Google Scholar]
- 10.Hurtado V, Montes R, Gris JC, Bertolaccini ML, Alonso A, Martínez-González MA, et al. Autoantibodies against EPCR are found in antiphospholipid syndrome and are a risk factor for fetal death. Blood. 2004;104:1369–1374. doi: 10.1182/blood-2004-03-0793. [DOI] [PubMed] [Google Scholar]
- 11.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 12.Boos CJ, Anderson RA, Lip GY. Is atrial fibrillation an inflammatory disorder? Eur Heart J. 2006;27:136–149. doi: 10.1093/eurheartj/ehi645. [DOI] [PubMed] [Google Scholar]
- 13.España F, Vayá A, Mira Y, Medina P, Estellés A, Villa P, et al. Low level of circulating activated protein C is a risk factor for venous thromboembolism. Thromb Haemost. 2001;86:1368–1373. [PubMed] [Google Scholar]
- 14.Folsom AR, Ohira T, Yamagishi K, Cushman M. Low protein C and incidence of ischemic stroke and coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. J Thromb Haemost. 2009;7:1774–1778. doi: 10.1111/j.1538-7836.2009.03577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor FB, Jr, Peer GT, Lockhart MS, Ferrell G, Esmon CT. Endothelial cell protein C receptor plays an important role in protein C activation in vivo. Blood. 2001;97:1685–1688. doi: 10.1182/blood.v97.6.1685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


