Abstract
A reaction sequence involving the 1,6-conjugate addition of a nucleophile to a dienyl diketone followed by Nazarov cyclization is described. Several nucleophiles are identified as competent initiators for the sequence. A different reaction outcome is is observed when catalytic amounts of nucleophile are employed, involving elimination of the nucleophile after the electrocyclization.
The classical Nazarov cyclization involves Lewis acid-promoted generation of a pentadienyl cation from a divinyl ketone, which undergoes conrotatory 4π electrocyclization to deliver cyclopentenone derivatives.1 Recent advances in catalysis2 and capture of the oxyallyl cation intermediate3 have increased the synthetic utility of the reaction. It is also possible to generate the reactive pentadienyl cation using methods other than Lewis acid activation, including protonation of enol ethers,4 oxidation,5 and cyclopropanation.6 In this communication, the development of a 4π electrocyclization initiated by 1,6-conjugate addition of a nucleophile to a dienyl diketone is described. The two-step sequence is catalyzed by Lewis acids, is stereoselective, and affords high yields of α-hydroxycyclopentenones.
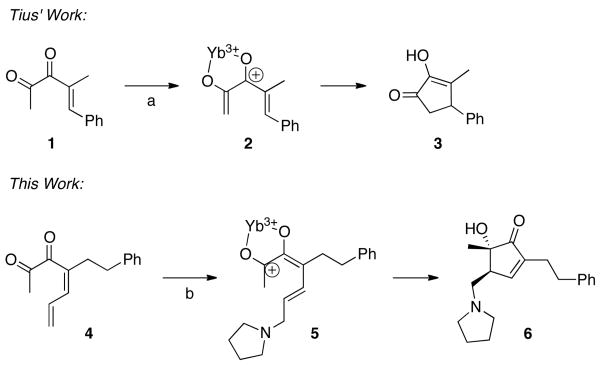
Tius has described the Nazarov cyclization of diketones of type 1 using Yb(OTf)3 and pyrrolidine as a base, which gives diosphenol 3 (Scheme 1).7, 8 We found that treatment of dienyl diketone 4 under similar reaction conditions resulted in a different cyclization, giving α-hydroxycyclopentenone 6, with incorporation of the pyrrolidine base. Further exploration of this interesting diastereoselective reaction was warranted.
Scheme 1. Two cyclization pathways of unsaturated α-diketones.
a) Yb(OTf)3 (catalytic), pyrrolidine, (1 equiv) DMSO, rt, 63%. b) Yb(OTf)3 (10 mol %), pyrrolidine (1 equiv), DMSO, rt, 83%.
Studies began with catalyst evaluation (Table 1). Although the reaction did occur in the absence of an acidic promoter, the rate was much slower (24 h vs 15 min) and no diastereoselectivity was observed (entry 1). Using 10 mol% of a protic or Lewis acid, full conversion was achieved in 15 min with very high diastereoselectivity (entries 2, 4, 5 and 6). In general, Lewis acidic transition metal complexes (entries 2-5) gave better chemical yields than the protic acid (TFA, entry 6).
Table 1. Optimization of the pyrrolidine-initiated cyclizationa.
 | ||||
|---|---|---|---|---|
| entry | catalyst | solvent | dr | yield (%) |
| 1b | -- | DMSO | 1:1 | 68 |
| 2 | Cu(OTf)2 (10 mol%) | DMSO | >20:1 | 99 |
| 3 | Sc(OTf)3 (10 mol%) | DMSO | 3:1 | 87 |
| 4 | Yb(OTf)3 (10 mol%) | DMSO | >20:1 | 83 |
| 5 | Y(OTf)3 (10 mol%) | DMSO | >20:1 | 99 |
| 6 | TFA (10 mol%) | DMSO | >20:1 | 74 |
| 7 | Cu(OTf)2 (2.5 mol%) | DMSO | 4:1 | 54 |
| 8 | Y(OTf)3 (2.5 mol%) | DMSO | 15:1 | 68 |
| 9 | Y(OTf)3 (1 mol%) | DMF | 9:1 | 81 |
| 10 | Y(OTf)3 (1 mol%) | CH2Cl2 | >20:1 | 71 |
| 11 | Y(OTf)3 (1 mol%) | THF | 9:1 | 89 |
| 12c | Y(OTf)3 (1 mol%) | THF | >20:1 | 99 |
Reaction Conditions: 4 (1 equiv.), pyrrolidine (1.2 equiv.), catalyst as listed. All reactions were conducted at 0.1M, for 15 min at rt except where otherwise noted.
The reaction required 24 h for completion.
With Et3N (1 equiv.) and LiCl (2 equiv.).
At lower catalyst loading (2.5 mol%), yields decreased, and Y(OTf)3 was found to be more effective than Cu(OTf)2 (entries 7 and 8). Experimentation with solvents revealed that CH2Cl2 gave the best selectivity (entry 10), while THF produced the highest chemical yield (entry 11). The reaction was further optimized by the addition of Et3N and LiCl (entry 12), which are thought to improve catalyst solubility.9
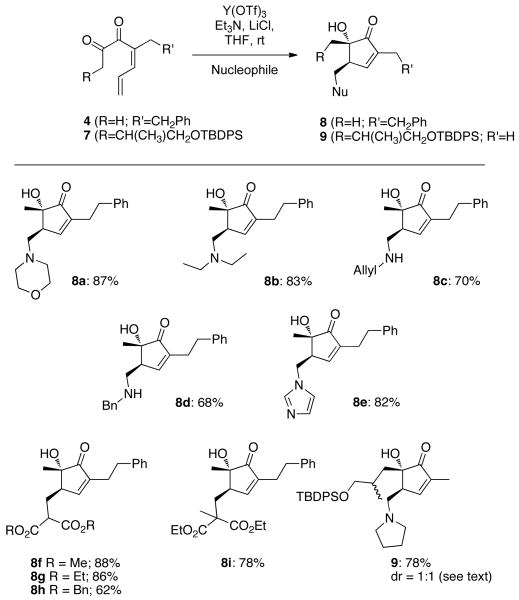
Efforts were then made to identify other viable nucleophiles for the reaction. Primary amines and cyclic and acyclic secondary amines did initiate the cyclization (8a-8e;Scheme 2). Cu(OTf)2 was also effective for these reactions, but yields were lower. Malonate derivatives also participated as nucleophiles, allowing for the installation of a versatile carbon based handle (8f-8i). Addition of malonate did not occur when Cu(OTf)2 was used as catalyst. In all of these reactions, only one diastereoisomer was detected. The more complex diketone 7 also underwent smooth cyclization to give pyrrolidine adduct 9, as a 1:1 mixture of diastereomers relative to the remote stereocenter (see supporting info). The stereochemistry of compounds 6 and 8c was assigned using X-ray crystallographic data, and in other cases the syn relationship between the α-methyl and β-methylene was detected by measurement of the nOe (see supporting information).
Scheme 2. Scope of the Cyclization.
a) Reaction Conditions: 4, Y(OTf)3 (1 mol%), Et3N (1 equiv.), LiCl (2 equiv.), amine or malonate nucleophile (1.2 equiv.), THF, rt b) Reaction Conditions: 7, Cu(OTf)2 (10 mol%), pyrrolidine (1 equiv.), DMSO, rt
By employing DMAP as the nucleophile, it was possible to achieve a different reaction outcome, obtaining dienone 10 instead of enones 8 (Table 2, entries 1 and 2). A stoichiometric amount was required for complete conversion (entry 2). Other nucleophiles known to be effective in the Morita-Baylis-Hillman reaction were also tested.10 The tertiary amines N,N-dimethylisopropylamine and N-methylmorpholine gave a 1:1 mixture of 10 and 11 in moderate yield (entries 3 and 4), while DBU and DABCO did not catalyze the reaction. Tertiary phosphines were also ineffective. Thiols 12 and 13, which are effective in the Rauhut-Currier reaction,11 did catalyze the cyclization, allowing complete conversion to 10 in 1 h. Interestingly, we also found that catalytic amounts of dimethyl malonate (DMM) in the presence of base also led to formation of product 10. The most effective base for the DMM-catalyzed process was DABCO (cf. entries 6-9, Table 2)
Table 2. Formation of Dienone 10a.
 | |||||
|---|---|---|---|---|---|
| entry | nucleophile | product (ratio 10:11) | baseb | time (h) | yield (%) |
| 1 | DMAP (10 mol%) | 10 only | -- | 48 | 45 |
| 2 | DMAP (1 equiv) | 10 only | -- | 48 | 75 |
| 3 | i-PrMe2N (10 mol%) | 1:1 | -- | 48 | 45 |
| 4 | N-methyl-morpholine (10 mol%) | 1:1 | -- | 48 | 55 |
| 5c, d | 12 (10 mol%) | 10 only | t-BuOK | 1 | 93 |
| 6c, d | 13 (10 mol%) | 10 only | t-BuOK | 1 | 82 |
| 7 | DMM (1 mol%) | 10 only | Et3N | 8 | 64 |
| 8 | DMM (1 mol%) | 1:1 | NaH | 1 | 94 |
| 9 | DMM (1 mol%) | 10 only | DBU | 1 | 50 |
| 10 | DMM (1 mol%) | 10 only | DABCO | 1 | 82 |
Reaction Conditions: 4, Y(OTf)3 (1 mol%), LiCl (2 equiv.), THF (0.1 M), at rt unless otherwise stated.
Ratio of base: nucleophile was 1:1.
Thiol premixed with base, -78 °C then 4.
No Lewis acid present. DBU=1,8-diazabicyclo[5.4.0]undec-7-ene; DABCO= 1,4-diazabicyclo[2.2.2] octane
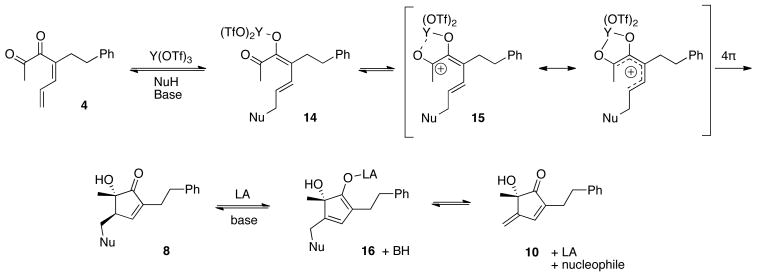
A proposed mechanism for the cyclization and subsequent elimination is presented in Scheme 3. The reaction begins with Lewis acid-promoted conjugate addition of the nucleophile to form intermediate 14. Activation of the ketone carbonyl produces pentadienyl cation 15, which undergoes 4π conrotatory cyclization to give products of type 8. The high diastereoselectivity is thought to result from the conrotatory electrocyclization of pentadienyl cation 15.12 Thus, Lewis acidic Y(OTf)3 is responsible for activation of first one carbonyl (for conjugate addition), and then the other (for electrocyclization). In the formation of compounds 10, intermediate 8 undergoes elimination through intermediate 16.
Scheme 3. Proposed Mechanism for Cyclization.
The reactions with dimethylmalonate (DMM) as nucleophile warrant further comment. Products 8f-8i are obtained using a stoichiometric amount of malonate (Scheme 2), while product 10 is obtained using catalytic DMM (Table 2, entry 9). A series of experiments were performed to better understand these observations. Subjection of 10 to DMM under the reaction conditions used in the Scheme 2 experiments gave 8f as a 1:1 mixture of diastereomers, presumably via intermediate 16. Also, if the reaction of 4 with DMM (Scheme 2) was run for a longer period, 8f with dr = 1:1 was isolated. When 8f (dr >20:1) was resubjected to the Scheme 2 reaction conditions, complete epimerization was observed after 2h (dr =1:1). Finally, treatment of 8f with 1 equivalent of diethylmalonate under the usual reaction conditions led to a 1:1 mixture of 8f and 8g, as mixtures of diastereomers. These experiments indicate that with malonate derivates as nucleophiles, the formation of 10 from 8 is reversible under the reaction conditions, and this equilibrium between 8 and 10 lies in favor of 8.
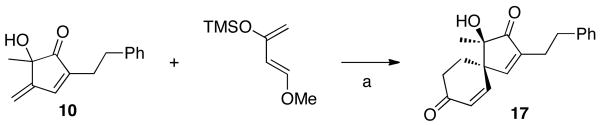
In the context of future application to natural product total synthesis, we were able to demonstrate that 10 is a useful building block for spirocycle formation. The Diels-Alder reaction of 10 with Danishefsky's diene provided 17 as a single diastereomer (Scheme 4), whose structure was confirmed by single X-ray crystal analysis (Figure 3).
Scheme 4. Cycloaddition of 10 with Danishefsky's Diene.
Reaction Conditions: a) Toluene, 180 °C, sealed tube, 24 h, then TFA (10 mol%) 10 min., rt, 80%.
In summary, a new method for the initiation of the Nazarov cyclization via conjugate addition to a dienyl diketone is described. The reaction efficiently proceeds to afford either the nucleophile adducts 8 or the elimination products 10. Further investigation into substrate scope is underway, as well as the development of an enantioselective variant of this reaction sequence.
Supplementary Material
Figure 1.
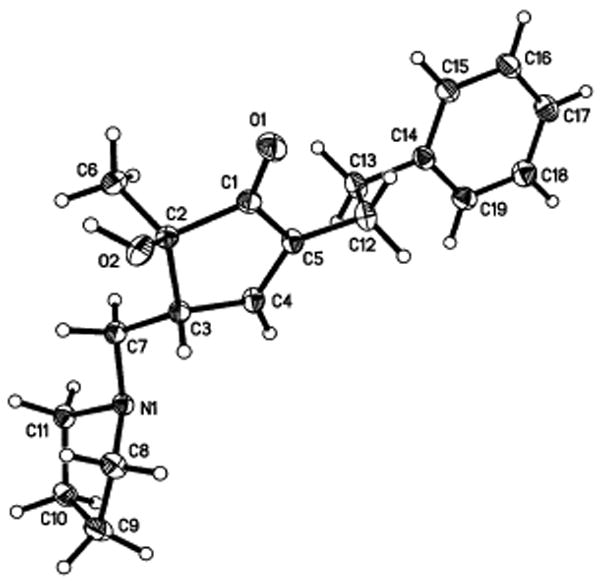
ORTEP drawing of 6.
Figure 2.
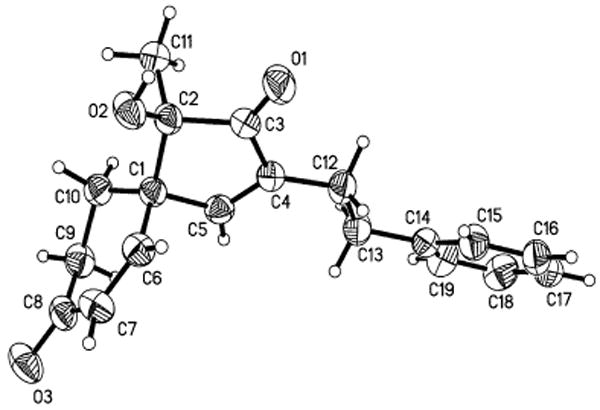
ORTEP drawing of 17.
Acknowledgments
We thank the National Institutes of Health (NIGMS R01 GM079364) and ARRA supplement (3R01GM079364-03S2) for funding this work. We are also grateful to Dr. William Brennessel for solving X-ray structures of 6, 7c and 17.
Footnotes
Supporting Information. Experimental procedure and characterization data for all new compounds, X-ray crystal structures, and CIF file for 6, 8c, and 17 are provided. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.a) Pellissier H. Tetrahedron. 2005;61:6479–6517. [Google Scholar]; b) Frontier AJ, Collison C. Tetrahedron. 2005;61:7577–7606. [Google Scholar]; c) Tius MA. Eur J Org Chem. 2005;2005:2193–2206. [Google Scholar]; d) Grant TN, Rieder CJ, West FG. Chem Commun. 2009:5675–5688. doi: 10.1039/b908515g. [DOI] [PubMed] [Google Scholar]; e) Nakanishi W, West FG. Curr Opin Drug Discov Dev. 2009;12:732–751. [PubMed] [Google Scholar]
- 2.For a review on the catalytic Nazarov cyclization, see: Vaidya T, Frontier AJ, Eisenberg R. ChemCatChem. 2011 in press.
- 3.a) Janka M, He W, Haedicke IE, Fronczek FR, Frontier AJ, Eisenberg R. J Am Chem Soc. 2006;128:5312–5313. doi: 10.1021/ja058772o. [DOI] [PubMed] [Google Scholar]; b) Marx VM, Burnell DJ. J Am Chem Soc. 2010;132:1685–1689. doi: 10.1021/ja909073r. [DOI] [PubMed] [Google Scholar]; c) Rieder CJ, Fradette RJ, West FG. Heterocycles. 2010;80:1413–1427. [Google Scholar]; d) Scadeng O, Ferguson MJ, West FG. Org Lett. 2010;13:114–117. doi: 10.1021/ol102651k. [DOI] [PubMed] [Google Scholar]; e) Marx VM, LeFort FM, Burnell DJ. Adv Synth Catal. 2011;353:64–68. [Google Scholar]
- 4.a) Prandi C, Deagostino A, Venturello P, Occhiato EG. Org Lett. 2005;7:4345–4348. doi: 10.1021/ol051464a. [DOI] [PubMed] [Google Scholar]; b) Wu YK, West FG. J Org Chem. 2010;75:5410–5413. doi: 10.1021/jo101112t. [DOI] [PubMed] [Google Scholar]
- 5.Spencer WT, Levin MD, Frontier AJ. Org Lett. 2010;13:414–417. doi: 10.1021/ol1027255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant TN, West FG. J Am Chem Soc. 2006;128:9348–9349. doi: 10.1021/ja063421a. [DOI] [PubMed] [Google Scholar]
- 7.Batson WA, Sethumadhavan D, Tius MA. Org Lett. 2005;7:2771–2774. doi: 10.1021/ol050970x. [DOI] [PubMed] [Google Scholar]
- 8.For other examples of this type of Nazarov cyclization see: Muxfeldt H, Weigele M, Rheenen VV. J Org Chem. 1965;30:3573–3574.Weinreb SM, Auerbach J. J Am Chem Soc. 1975;97:2503–2506.Uhrich EA, Batson WA, Tius MA. Synthesis. 2006;2006:2139–2142.Williams DR, Robinson LA, Nevill CR, Reddy JP. Angew Chem Int Ed. 2007;46:915–918. doi: 10.1002/anie.200603853.Bow WF, Basak AK, Jolit A, Vicic DA, Tius MA. Org Lett. 2009;12:440–443. doi: 10.1021/ol9025765.Basak AK, Shimada N, Bow WF, Vicic DA, Tius MA. J Am Chem Soc. 2010;132:8266–8267. doi: 10.1021/ja103028r.Cai Z, Harmata M. Org Lett. 2010;12:5668–5670. doi: 10.1021/ol102478h.
- 9.LiCl is known to aid in the solubility of copper (II) salts in THF: Tamura M, Kochi J. Synthesis. 1971:303.
- 10.Basavaiah D, Rao AJ, Satyanarayana T. Chem Rev. 2003;103:811–892. doi: 10.1021/cr010043d. [DOI] [PubMed] [Google Scholar]
- 11.Aroyan CE, Dermenci A, Miller SJ. J Org Chem. 2010;75:5784–5796. doi: 10.1021/jo101018t. [DOI] [PubMed] [Google Scholar]
- 12.For examples of cyclopentannulations of intermediates like 15 see: ref. 4a and Denmark SE, Habermas KL, Hite GA. Helv Chim Acta. 1988;71:168–194.Stone GB, Liebeskind LS. J Org Chem. 1990;55:4614–4622.Paquette LA, Morwick TM. J Am Chem Soc. 1997;119:1230–1241.Paquette LA. Eur J Org Chem. 1998:1709–1728.Pujanauski BG, Bhanu Prasad BA, Sarpong R. J Am Chem Soc. 2006;123:6786–6787. doi: 10.1021/ja061549m.Zou W. Res. 2004;339:2475–2485. doi: 10.1016/j.carres.2004.08.008.Caddick S, Cheung S, Doyle VE, Frost LM, Soscia MG, Delisser VM, Williams MRV, Etheridge ZC, Khan S, Hitchcock PB, Pairaudeau G, Vile S. Tetrahedron. 2001;57:6295–6303.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.