Abstract
OBJECTIVE
Previous studies have noted a specific association between type 1 diabetes and insufficient levels of vitamin D, as well as polymorphisms within genes related to vitamin D pathways. Here, we examined whether serum levels or genotypes of the vitamin D–binding protein (VDBP), a molecule key to the biologic actions of vitamin D, specifically associate with the disorder.
RESEARCH DESIGN AND METHODS
A retrospective, cross-sectional analysis of VDBP levels used samples from 472 individuals of similar age and sex distribution, including 153 control subjects, 203 patients with type 1 diabetes, and 116 first-degree relatives of type 1 diabetic patients. Single nucleotide polymorphism (SNP) typing for VDBP polymorphisms (SNP rs4588 and rs7041) was performed on this cohort to determine potential genetic correlations. In addition, SNP analysis of a second sample set of banked DNA samples from 1,502 type 1 diabetic patients and 1,880 control subjects also was used to determine genotype frequencies.
RESULTS
Serum VDBP levels were highest in healthy control subjects (median 423.5 µg/mL [range 193.5–4,345.0; interquartile range 354.1–]586), intermediate in first-degree relatives (402.9 µg/mL [204.7–4,850.0; 329.6–492.4]), and lowest in type 1 diabetic patients (385.3 µg/mL [99.3–1,305.0; 328.3–473.0]; P = 0.003 vs. control subjects). VDBP levels did not associate with serum vitamin D levels, age, or disease duration. However, VDBP levels were, overall, lower in male subjects (374.7 µg/mL [188.9–1,602.0; 326.9–449.9]) than female subjects (433.4 µg/mL [99.3–4,850.0; 359.4–567.8]; P < 0.0001). It is noteworthy that no differences in genotype frequencies of the VDBP polymorphisms were associated with serum VDBP levels or between type 1 diabetic patients and control subjects.
CONCLUSIONS
Serum VDBP levels are decreased in those with type 1 diabetes. These studies suggest that multiple components in the metabolic pathway of vitamin D may be altered in type 1 diabetes and, collectively, have the potential to influence disease pathogenesis.
Various pathways and characteristics of vitamin D metabolism, such as vitamin D analogs and polymorphisms in the vitamin D receptor, as well as genes encoding specific vitamin D enzymes, recently have been associated with type 1 diabetes (1). For example, reduced serum vitamin D concentrations have been noted for those with type 1 diabetes (2–4), but the disease specificity ascribed to these reductions recently has been questioned (5). Indeed, vitamin D deficiency does not seem to be an uncommon event (6). Vitamin D therapy (active form) can modulate the development of disease in the nonobese diabetic (NOD) mouse model of autoimmune diabetes (7,8), and a variety of trials have tested whether vitamin D supplementation has the capacity to modify the development of this disease (2). To that question, a meta-analysis of trials seeking such a therapeutic benefit suggests that vitamin D supplementation can reduce disease risk (9,10).
This said, despite our current understanding of the vitamin D pathway, including its capacity to modulate the immune system (6), the causal relationship between impaired vitamin D constituents and the development of type 1 diabetes remains uncertain. This is largely attributed to the intricate nature of vitamin D metabolic processes, as well as the extensive biological effects exhibited by its components. Therefore, understanding the influence of the vitamin D pathway on the pathogenesis of type 1 diabetes requires a systematic examination into the distinct roles of its various components.
One essential component of the vitamin D pathway is the polymorphic vitamin D–binding protein (VDBP), also known as group-specific component (Gc). Aside from its main function of vitamin D transport and preservation, VDBP has been shown to scavenge actin, bind fatty acids, activate macrophages, stimulate osteoclasts, enhance the chemotactic activity of C5-derived peptides, and associate with immune cell surfaces, such as T and B cells (11). Even after ligand binding, 98–99% VDBP binding sites remain unoccupied, which suggests a function beyond vitamin D transport (11). Although several studies have associated specific VDBP gene polymorphisms with the presence of diabetes (i.e., type 1 and type 2 diabetes) (12,13), we sought to confirm this association and identify differences in VDBP levels in patients with type 1 diabetes.
RESEARCH DESIGN AND METHODS
Banked serum samples from a total of 472 individuals in Florida who participated in studies on the natural history of type 1 diabetes were grouped into the following cohorts: control subjects (n = 153, mean age 22.7 years, SD 11.1; 68 [44%] were male, and 141 [92%] were white); patients with type 1 diabetes (n = 203, mean age 16.9, SD 8.8; mean disease duration 4.0 years, SD 6.7; 107 [53%] were male, and 183 [90%] were white); and first-degree relatives of those with type 1 diabetes (n = 116, age 26.7 ± 16.8 years, 57 [50%] were male, and 91 [80%] were Caucasian). Of this study group, DNA from 53 control subjects, 81 patients, and 38 relatives were further analyzed by single nucleotide polymorphism (SNP) genotyping for VDBP polymorphisms (SNPs rs4588 and rs7041). Previously banked DNA samples from a second study cohort consisting of 1,502 patients and 1,880 healthy control subjects, collected at the Georgia Health Sciences University, obtained from a national population from the U.S., also were genotyped. All samples were collected under informed consent with the approval of the University of Florida and Georgia Health Sciences University’s institutional review boards.
VDBP levels.
VDBP levels were quantified in duplicate with a commercial EIA kit (Alpco; Salem, NH) using 10 µL of banked serum from each subject. Levels of VDBP were interpolated from a standard curve after reading the absorbance on an M5 Spectramax plate reader using Softmax Pro 4.8 software (Molecular Devices, Sunnyvale, CA). The intra- and interassay coefficients of variation for this assay were 5.0 and 12.7%, respectively. The published normal reference range for VDBP levels is 300–600 µg/mL (11). Correlation analysis also was performed for a subset of the VDBP serum samples (n = 386), which had previously been measured for 25-OH vitamin D levels (5). This subset included 152 control subjects, 141 type 1 diabetic patients, and 93 first-degree relatives.
SNP genotyping methods.
For the initial Florida dataset, 200 ng of genomic DNA were used to amplify an 809-bp fragment with primers 5′-CAAGTCTTATCACCATCCTG-3′ and 5′-GCCAAGTTACAATAACAC-3′, as previously published (12). The amplicons were gel-purified using the Gene Clean Turbo kit (MP Biomedicals; Aurora, OH) to ensure no inhibition of restriction enzymes. For verification of the rs4588 and the rs7041 SNPs, PCR products were digested with StyI and HaeIII, respectively, and the genotypes were determined by gel electrophoresis on a 1.5–2% agarose gel. For the dataset collected under the auspices of the University of Georgia, the SNPs were genotyped using TaqMan PCR genotyping, with modifications, as previously described (14). All primers and probes used in this study were designed and validated by Applied Biosystems (Foster City, CA). Amplification reactions were performed in a 5-µL final volume in optical 384-well plates. PCR was carried out with 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C using an ABI 9700 real-time PCR system (Applied Biosystems). To validate the TaqMan assays, five SNPs also were genotyped using standard amplified restriction fragment–length polymorphism analysis.
Statistical analysis.
Analysis of multiple, unpaired group comparisons was achieved using the nonparametric Kruskal-Wallis test. The Dunn post-test was used for multiple testing corrections if the Kruskal-Wallis test was significant. The association of age and disease duration with VDBP levels was analyzed by linear regression. To determine the relationship between VDBP levels and sex, the nonparametric Mann-Whitney test was used. All analyses were performed using Prism software, version 5.00 (GraphPad Software, Inc., San Diego, CA). The association between each VDBP SNP and type 1 diabetes was assessed by calculating the odds ratios (ORs) separately for each genotype, as well as for total allelic frequency (i.e., heterozygous and homozygous minor allele combined). The Pearson χ2 and Fisher exact tests were used to test the differences in genotype and allele (respectively) distribution between patients and control subjects. Statistical significance was defined as P < 0.05. We used the Breslow-Day test to examine heterogeneity in the ORs between subsets stratified for age at onset, sex, and HLA risk status. Hardy-Weinberg equilibrium of the genotypic frequencies among case and control subjects was examined separately.
RESULTS
Levels of serum VDBP associated with type 1 diabetes.
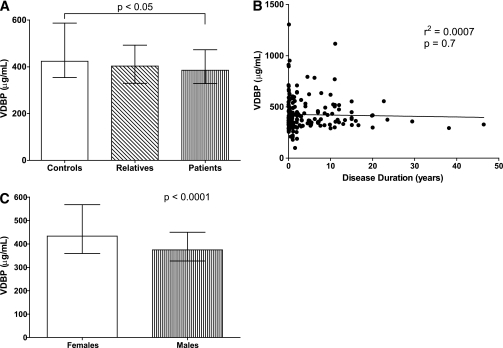
Serum VDBP levels for the three study groups were as follows: healthy control subjects (median 423.5 µg/mL [range 193.5–4345.0; interquartile range 354.1–586.7]); first-degree relatives (402.9 µg/mL [204.7–4850.0; 329.6–492.4]); and type 1 diabetic patients (385.3 µg/mL [99.3–1305.0; 328.3–473.0]) (Fig. 1A). Median VDBP serum levels were significantly lower in patients with type 1 diabetes than in control subjects (P = 0.003). Because of the lack of availability, serum VDBP levels were not measured for the Georgia samples.
FIG. 1.
Serum levels of VDBP. A: VDBP levels in control subjects (n = 153), first-degree relatives (n = 116), and type 1 diabetic patients (n = 203). B: There is no association between VDBP levels and disease duration by linear regression analysis. C: Serum VDBP in male (n = 233) and female (n = 238) subjects and the total of all study groups. Medians with interquartile ranges are shown.
In finding that VDBP levels were significantly lower in the presence of type 1 diabetes, we then questioned whether the duration of disease influenced VDBP levels. Linear regression analysis indicated that disease duration did not associate with VDBP levels (Fig. 1B).
Serum VDBP levels associate with sex but not age or serum 25-OH vitamin D levels.
It previously has been shown that sex influences VDBP levels (15). Therefore, we performed a sex analysis with regard to VDBP levels to identify any correlations within our study participants. The 472 study participants were distributed into two near-identical sex-based cohorts of 238 female and 233 male subjects. Serum VDBP levels were significantly higher in female subjects (433.4 µg/mL [range 99.32–4850.0; interquartile range 359.4–567.8]) versus male subjects (374.7 µg/mL [188.9–1602.0; 326.9–449.9]; P < 0.0001) (Fig. 1C). Sex distribution between type 1 diabetic patients, relatives, and control subjects was not significantly different (P = NS), reducing the likelihood that group composition contributed to the aforementioned association between type 1 diabetes and serum VDBP levels. We next sought to determine whether age influenced serum levels of VDBP. Linear regression analysis revealed that age did not associate with VDBP levels in our entire study population (P = 0.164, r2 = 0.004) or as a function of sex (Supplementary Fig. 1).
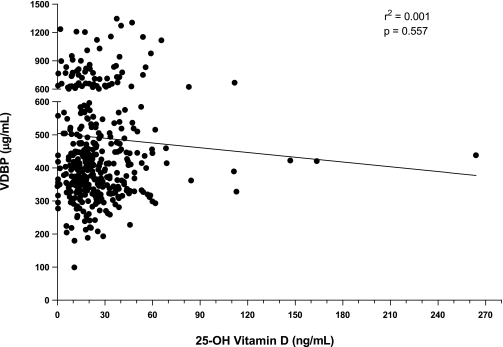
We previously noted that individuals with type 1 diabetes had insufficient serum levels of 25-OH vitamin D, although this finding was not specific for those with the disease (5). To determine whether a correlation exists between serum 25-OH vitamin D and VDBP levels, we analyzed VDBP levels on banked serum from a large subset (n = 386) of the 25-OH vitamin D cohort. Of interest, we found no association (P = 0.557, r2=0.001) between these two analytes, irrespective of study group (152 control subjects, 141 patients, and 93 relatives) (Fig. 2).
FIG. 2.
Linear regression of serum 25-OH vitamin D levels and VDBP levels. There was no significant correlation between these two parameters in the study cohort (n = 386).
VDBP genotypes do not associate with type 1 diabetes.
The rs4588 and rs7041 VDBP genetic variants were analyzed in the type 1 diabetic patients and control subjects whose DNA had been banked at the Georgia Health Sciences University for studies regarding the genetics of this disease (16). Case and control subjects were found to be in Hardy-Weinberg equilibrium for the two SNPs analyzed (Supplementary Table 1). There were no significant differences in the allele and genotype frequencies of the VDBP rs4588 and rs7041 genetic variants among patients with type 1 diabetes and control subjects (Table 1).
TABLE 1.
Association analysis of VDBP SNPs rs7041 (G>T) and rs4588 (C>A) with type 1 diabetes
| SNP | Type 1 diabetic patients | Control subjects | OR (95% CI) | P |
|---|---|---|---|---|
| rs7041 | ||||
| Genotype | ||||
| GG | 441 (30.33) | 579 (31.67) | 1.00 (reference) | |
| GT | 723 (49.72) | 884 (48.36) | 1.07 (0.92–1.26) | 0.3774 |
| TT | 290 (19.94) | 365 (19.97) | 1.04 (0.86–1.27) | 0.6755 |
| GT + TT | 1,013 (69.67) | 1,249 (68.33) | 1.07 (0.92–1.24) | 0.4086 |
| rs4588 | ||||
| Genotype | ||||
| CC | 723 (50.45) | 929 (51.58) | 1.00 (reference) | |
| CA | 578 (40.33) | 734 (40.76) | 1.01 (0.87–1.17) | 0.8745 |
| AA | 132 (9.21) | 138 (7.66) | 1.23 (0.95–1.59) | 0.1163 |
| CA + AA | 710 (49.55) | 872 (48.42) | 1.05 (0.91–1.20) | 0.5235 |
Data are n (%), unless otherwise indicated.
We next tested whether the association between these SNPs is dependent upon other covariates, such as the sex of the subjects, age of disease onset (i.e., early onset being under 18 years of age), or HLA-DQB1 genotypes. No significant association was detected when patients were stratified by sex or age of disease onset (Supplementary Table 2). A heterogeneity test further showed no difference in ORs between male and female subjects or between early- and late-onset subsets (Supplementary Table 3).
We then determined whether the high-risk HLA-DQB1 genotype was associated with the SNPs. All subjects were classified into two subsets, a high-risk DQB1 genotype subset (i.e., 0201/0201, 0302/0302, or 0201/0302) and a low-risk DQB1 genotype subset (i.e., all others). There was no significant association of the rs4588 and rs7041 SNPs in either the low- or high-risk HLA-DQB1 subsets after correcting for multiple testing (Supplementary Table 2). Because the P values for these tests are heavily influenced by the smaller number of control subjects carrying the high-risk HLA genotypes, we performed heterogeneity tests to determine whether the ORs differed between the high- and low-risk subsets. We found no significant difference between the ORs of the HLA-DQB1 risk subsets (Supplementary Table 3).
DISCUSSION
The major finding in our study adds further credence to the concept that the vitamin D pathway may play a significant role in the development of type 1 diabetes. Although previous studies have associated the rs4588 and rs7041 VDBP SNPs with the disease (12,13), we were unable to support that conclusion in this large-scale study. The potential reasons for this discrepancy are many (e.g., heterogeneity in study populations as a function of genetics and/or disease phenotype, sample size, etc.) and have, over the years, been a common occurrence in studies of type 1 diabetes genetics. However, we were able to associate the phenotype of lower VDBP levels with type 1 diabetes. Although the exact consequence of this association remains unknown, the fact that VDBP has immunomodulatory characteristics and is responsible for transport of vitamin D metabolites supports, in theory, a model whereby the impact of reduced serum levels may be significant enough to lend itself, directly or indirectly, to the autoimmune destruction of pancreatic β-cells in the disease. Our findings also suggest that other means by which VDBP levels are influenced, beyond the VDBP SNPs tested here, must exist and will certainly be subject to future investigations.
A recently published study assessing VDBP levels in 100 healthy, middle-aged and older participants found that women had higher mean VDBP levels than men, yet no associations were observed between VDBP levels and age, body weight, BMI, fat mass, or fat percentage (15). While the aforementioned study measured VDBP levels among individuals older than middle age, the majority of our samples were derived from individuals much younger than middle age; however, we were able to confirm sex differences in the younger study cohort.
Despite our having noted this interesting correlation, additional studies are required to address its biological significance. For example, it could be argued that that the presence of reduced VDBP levels in the type 1 diabetic study group may not have a biologically significant effect on the transport of vitamin D metabolites (i.e., reduced transport) because VDBP circulates at a much higher concentration than its ligands (17). Another consideration to this point is the finding that even though VDBP levels were reduced in those with type 1 diabetes (in comparison with control subjects), unlike what was seen in our previous studies of 25-OH vitamin D, the mean values were within the normal range. In addition, as mentioned previously, in a previous study, we found that 25-OH vitamin D levels did not specifically associate with type 1 diabetes but were low in similar proportion among those with and without the disease (5). In the current investigation, we did not find a significant correlation between those previously measured 25-OH vitamin D levels and serum VDBP in the same patients. We interpret this to imply that lower VDBP levels in type 1 diabetes are independent of 25-OH vitamin D, which is one of its ligands.
Although our study is novel in its simultaneous assessment of 25-OH vitamin D, VDBP, and genetic polymorphisms, we note a very recently published study in which type 1 diabetic patients were noted to have exaggerated urinary loss of VDBP (18). However, that study did not find a significant difference in plasma VDBP levels, which may, in part, be attributed to their smaller cohort size. Although we did not measure urine levels of VDBP in our study, it is possible that exaggerated VDBP urinary loss may be a factor contributing to lower serum VDBP in type 1 diabetic patients. This said, our study failed to observe a relationship between type 1 diabetes disease duration and VDBP levels. Namely, if this were to be a factor in type 1 diabetic patients who are prone to proteinuria (i.e., those with extended disease duration), one might have anticipated reduced VDBP in those with extended disease duration. However, a vast majority of our type 1 diabetic subjects were of limited disease duration, and future efforts addressing this issue would do well to examine a large number of patients of extended disease duration, including those with and without proteinuria, to adequately address this concept. Other important notions for future efforts include assessments for the potential role of ethnicity, geography, pregnancy, and use of oral contraceptives. Indeed, this latter notion has been ascertained in a previous study (19), albeit using a different method for assessment (i.e., single radial immunodiffusion), and was associated with reduced VDBP levels. Our study did not find VDBP levels to be associated with age in female subjects, including the window of time when oral contraceptives would be a presumed (i.e., potential) activity, but, as noted, future efforts should examine this issue directly. Unfortunately, given our study design, study questionnaires reflecting contraception use and determining its contribution to our studies were not possible on this occasion.
Overall, our findings warrant additional investigation into the role of VDBP, as well as the contribution of other vitamin D pathway components, in type 1 diabetes. The various contributions of this pathway to innate immune function are the topic of much discussion and may be multifaceted, with each individual’s risk being the sum of different pathways to disease, hence the reason for the historical difficulty in describing a specific mechanism for the involvement of vitamin D in the pathogenesis of type 1 diabetes.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported, in part, by funds obtained from the National Institutes of Health, the Keene Professorship, and the Brehm Coalition for Type 1 Diabetes Research.
No potential conflicts of interest relevant to this article were reported.
D.B., Z.H., L.B., M.V.P.L.-R, and H.W. performed the research, interpreted data, and reviewed and edited the manuscript. M.H., D.S., and J.-X.S. contributed to the study design, interpreted the data, and reviewed and edited the manuscript. M.C.-S. contributed to the study design. C.M. and C.W. interpreted data and reviewed and edited the manuscript. M.A. contributed to the study design, interpreted data, and wrote the manuscript.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0576/-/DC1.
REFERENCES
- 1.Alizadeh BZ, Koeleman BP. Genetic polymorphisms in susceptibility to type 1 diabetes. Clin Chim Acta 2008;387:9–17 [DOI] [PubMed] [Google Scholar]
- 2.Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia 2005;48:1247–1257 [DOI] [PubMed] [Google Scholar]
- 3.Bailey R, Cooper JD, Zeitels L, et al. Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes 2007;56:2616–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pozzilli P, Manfrini S, Crinò A, et al. ; IMDIAB Group Low levels of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in patients with newly diagnosed type 1 diabetes. Horm Metab Res 2005;37:680–683 [DOI] [PubMed] [Google Scholar]
- 5.Bierschenk L, Alexander J, Wasserfall C, Haller M, Schatz D, Atkinson M. Vitamin D levels in subjects with and without type 1 diabetes residing in a solar rich environment. Diabetes Care 2009;32:1977–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–281 [DOI] [PubMed] [Google Scholar]
- 7.Driver JP, Foreman O, Mathieu C, van Etten E, Serreze DV. Comparative therapeutic effects of orally administered 1,25-dihydroxyvitamin D(3) and 1alpha-hydroxyvitamin D(3) on type-1 diabetes in non-obese diabetic mice fed a normal-calcaemic diet. Clin Exp Immunol 2008;151:76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zella JB, McCary LC, DeLuca HF. Oral administration of 1,25-dihydroxyvitamin D3 completely protects NOD mice from insulin-dependent diabetes mellitus. Arch Biochem Biophys 2003;417:77–80 [DOI] [PubMed] [Google Scholar]
- 9.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child 2008;93:512–517 [DOI] [PubMed] [Google Scholar]
- 10.Mohr SB, Garland CF, Gorham ED, Garland FC. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia 2008;51:1391–1398 [DOI] [PubMed] [Google Scholar]
- 11.Gomme PT, Bertolini J. Therapeutic potential of vitamin D-binding protein. Trends Biotechnol 2004;22:340–345 [DOI] [PubMed] [Google Scholar]
- 12.Ongagna JC, Kaltenbacher MC, Sapin R, Pinget M, Belcourt A. The HLA-DQB alleles and amino acid variants of the vitamin D-binding protein in diabetic patients in Alsace. Clin Biochem 2001;34:59–63 [DOI] [PubMed] [Google Scholar]
- 13.Ongagna JC, Pinget M, Belcourt A. Vitamin D-binding protein gene polymorphism association with IA-2 autoantibodies in type 1 diabetes. Clin Biochem 2005;38:415–419 [DOI] [PubMed] [Google Scholar]
- 14.de Kok JB, Wiegerinck ET, Giesendorf BA, Swinkels DW. Rapid genotyping of single nucleotide polymorphisms using novel minor groove binding DNA oligonucleotides (MGB probes). Hum Mutat 2002;19:554–559 [DOI] [PubMed] [Google Scholar]
- 15.Bolland MJ, Grey AB, Ames RW, et al. Age-, gender-, and weight-related effects on levels of 25-hydroxyvitamin D are not mediated by vitamin D binding protein. Clin Endocrinol (Oxf) 2007;67:259–264 [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Wang H, Jin Y, et al. IFIH1 polymorphisms are significantly associated with type 1 diabetes and IFIH1 gene expression in peripheral blood mononuclear cells. Hum Mol Genet 2009;18:358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooke NE, Haddad JG. Vitamin D binding protein (Gc-globulin). Endocr Rev 1989;10:294–307 [DOI] [PubMed] [Google Scholar]
- 18.Thrailkill KM, Jo CH, Cockrell GE, Moreau CS, Fowlkes JL. Enhanced excretion of vitamin D binding protein in type 1 diabetes: a role in vitamin D deficiency? J Clin Endocrinol Metab 2011;96:142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouillon R, van Baelen H, de Moor P. The measurement of the vitamin D-binding protein in human serum. J Clin Endocrinol Metab 1977;45:225–231 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.